Four Different Finger Positions and Their Effects on Hemodynamic Changes during Chest Compression in Asphyxiated Neonatal Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. Hemodynamic Parameters
2.3. Force Measurement
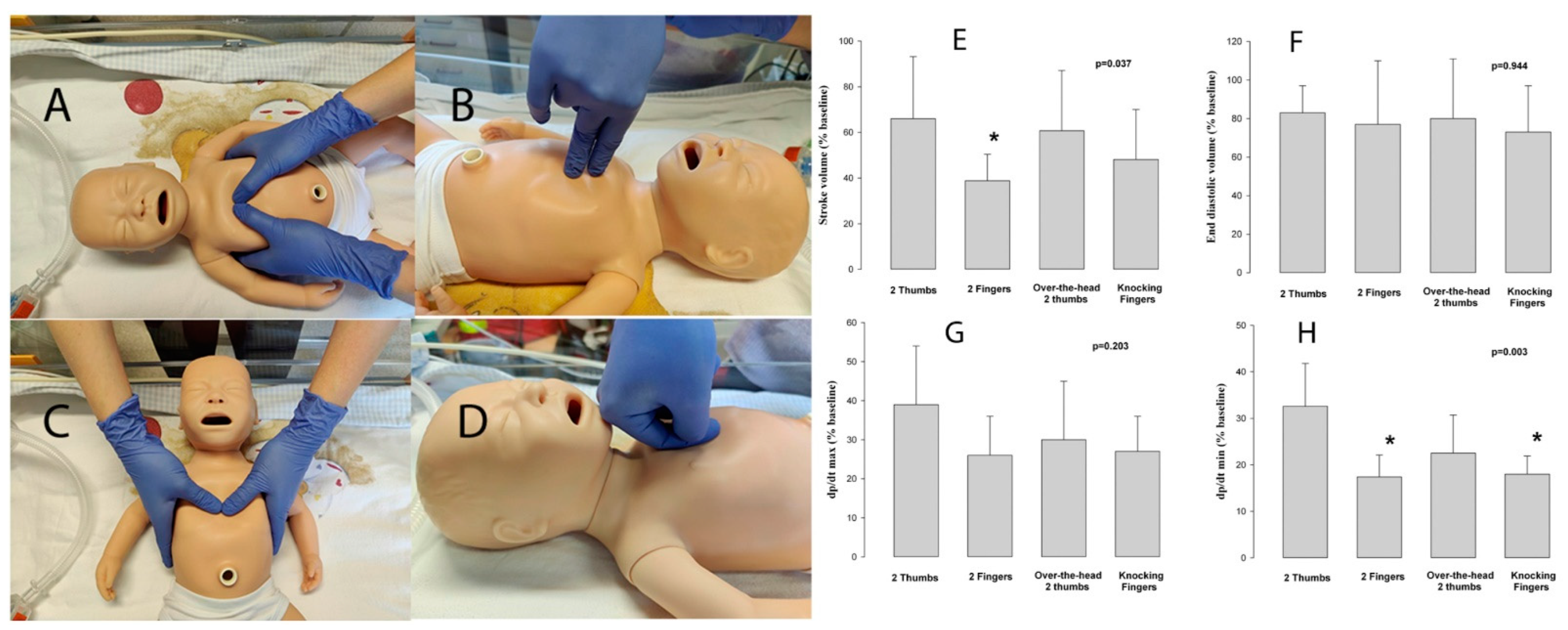
2.4. Experimental Protocol
2.5. Data Collection and Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CC | Chest compression |
| AP | Anterior-posterior chest diameters |
| dp/dtmax | Maximal rate of rise of left ventricular pressure |
| dp/dtmin | Minimum rate of change of ventricular pressure |
References
- Wyckoff, M.H.; Wyllie, J.; Aziz, K.; De Almeida, M.F.; Fabres, J.; Fawke, J.; Guinsburg, R.; Hosono, S.; Isayama, T.; Kapadia, V.S.; et al. Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2020, 142, S185–S221. [Google Scholar] [CrossRef]
- Aziz, K.; Lee, H.C.; Escobedo, M.B.; Hoover, A.V.; Kamath-Rayne, B.D.; Kapadia, V.S.; Magid, D.J.; Niermeyer, S.; Schmölzer, G.M.; Szyld, E.; et al. Part 5: Neonatal Resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S524–S550. [Google Scholar] [CrossRef]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rüdiger, M.; Skåre, C.; Szczapa, T.; Pas, A.T.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef]
- Martin, P.S.; Kemp, A.M.; Theobald, P.S.; A Maguire, S.; Jones, M.D. Do chest compressions during simulated infant CPR comply with international recommendations? Arch. Dis. Child. 2012, 98, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, J.L.; Bogumil, D.; Epstein, J.L.; Burke, R.V. Two-thumb-encircling advantageous for lay responder infant CPR: A randomised manikin study. Arch. Dis. Child. 2018, 104, 530–534. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, Y.; Shi, W.; Zhu, Y.; Tao, R.; Jiang, Y.; Lu, Y.; Tong, J. Two-thumb–encircling hands technique is more advisable than 2-finger technique when lone rescuer performs cardiopulmonary resuscitation on infant manikin. Am. J. Emerg. Med. 2015, 33, 531–534. [Google Scholar] [CrossRef]
- Udassi, S.; Udassi, J.P.; Lamb, M.A.; Theriaque, D.W.; Shuster, J.J.; Zaritsky, A.L.; Haque, I.U. Two-thumb technique is superior to two-finger technique during lone rescuer infant manikin CPR. Resuscitation 2010, 81, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.K.; Hemway, R.J.; Perlman, J.M. The Two-Thumb Technique Using an Elevated Surface is Preferable for Teaching Infant Cardiopulmonary Resuscitation. J. Pediatr. 2012, 161, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Kim, D.K.; Chang, I.; Jung, J.Y.; Paek, S.H.; Park, J.W.; Lee, J.H.; Kwak, Y.H. Two-Thumb Encircling Technique With a Novel Compression Assist Device Provides Safe and Effective Chest Compressions in Infants. Pediatr. Emerg. Care 2020, 36, e700–e703. [Google Scholar] [CrossRef]
- Dorfsman, M.L.; Menegazzi, J.J.; Wadas, R.J.; Auble, T.E. Two-thumb vs Two-finger Chest Compression in an Infant Model of Prolonged Cardiopulmonary Resuscitation. Acad. Emerg. Med. 2000, 7, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Christman, C.; Hemway, R.J.; Wyckoff, M.H.; Perlman, J.M. The two-thumb is superior to the two-finger method for administering chest compressions in a manikin model of neonatal resuscitation. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 96, F99–F101. [Google Scholar] [CrossRef]
- Saini, S.S.; Gupta, N.; Kumar, P.; Bhalla, A.K.; Kaur, H. A comparison of two-fingers technique and two-thumbs encircling hands technique of chest compression in neonates. J. Perinatol. 2011, 32, 690–694. [Google Scholar] [CrossRef]
- Zaichkin, W. Textbook of Neonatal Resuscitation (NRP), 7th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2016. [Google Scholar] [CrossRef]
- Ramachandran, S.; Bruckner, M.; Wyckoff, M.H.; Schmölzer, G.M. Chest compressions in newborn infants: A scoping review. Arch. Dis. Child. Fetal Neonatal Ed. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Na, J.U.; Choi, P.C.; Lee, H.J.; Shin, D.H.; Han, S.K.; Cho, J.H. A vertical two-thumb technique is superior to the two-thumb encircling technique for infant cardiopulmonary resuscitation. Acta Paediatr. 2015, 104, e70–e75. [Google Scholar] [CrossRef] [PubMed]
- Paek, S.H.; Kim, D.K.; Lee, J.H.; Kwak, Y.H. Comparison of standard and alternative methods for chest compressions in a single rescuer infant CPR: A prospective simulation study. PLoS ONE 2019, 14, e0226632. [Google Scholar] [CrossRef] [PubMed]
- Fakhraddin, B.Z.; Shimizu, N.; Kurosawa, S.; Sakai, H.; Miyasaka, K.; Mizutani, S. New method of chest compression for infants in a single rescuer situation: Thumb-index finger technique. J. Med. Dent. Sci. 2011, 58, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Oh, J.H.; Kim, C.W.; Kim, S.E.; Lee, D.H.; Hong, J.Y. Which Fingers Should We Perform Two-Finger Chest Compression Technique with When Performing Cardiopulmonary Resuscitation on an Infant in Cardiac Arrest? J. Korean Med. Sci. 2016, 31, 997–1002. [Google Scholar] [CrossRef]
- Yang, D.; Kim, K.H.; Oh, J.H.; Son, S.; Cho, J.; Seo, K.M. Development and Evaluation of a New Chest Compression Technique for Cardiopulmonary Resuscitation in Infants. Pediatr. Cardiol. 2019, 40, 1217–1223. [Google Scholar] [CrossRef]
- Ladny, J.R.; Smereka, J.; Rodríguez-Núñez, A.; Leung, S.; Ruetzler, K.; Szarpak, L. Is there any alternative to standard chest compression techniques in infants? A randomized manikin trial of the new “2-thumb-fist” option. Medicine 2018, 97, e9386. [Google Scholar] [CrossRef]
- Jung, W.J.; Hwang, S.O.; Kim, H.I.; Cha, Y.S.; Kim, O.H.; Kim, H.; Lee, K.H.; Cha, K.-C. ‘Knocking-fingers’ chest compression technique in infant cardiac arrest. Eur. J. Emerg. Med. 2019, 26, 261–265. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, E.; Martínez-Puga, A.; Carballo-Fazanes, A.; Abelairas-Gómez, C.; Rodríguez-Nuñez, A. Two new chest compression methods might challenge the standard in a simulated infant model. Eur. J. Pediatr. 2019, 178, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Houri, P.K.; Frank, L.R.; Menegazzi, J.J.; Taylor, R. A randomized, controlled trial of two-thumb vs two-finger chest compression in a swine infant model of cardiac arrest. Prehosp. Emerg. Care 2009, 1, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Strech, D.; Dirnagl, U. 3Rs missing: Animal research without scientific value is unethical. BMJ Open Sci. 2019, 3, e000035. [Google Scholar] [CrossRef]
- Bruckner, M.; Neset, M.; Garcia-Hidalgo, C.; Lee, T.-F.; O’Reilly, M.; Cheung, P.-Y.; Schmölzer, G.M. Chest Compression Rates of 90/min versus 180/min during Neonatal Cardiopulmonary Resuscitation: A Randomized Controlled Animal Trial. Children 2022, 9, 1838. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, M.; Kim, S.Y.; Shim, G.H.; Neset, M.; Garcia-Hidalgo, C.; Lee, T.-F.; O’Reilly, M.; Cheung, P.-Y.; Schmölzer, G.M. Assessment of optimal chest compression depth during neonatal cardiopulmonary resuscitation: A randomised controlled animal trial. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 107, 262–268. [Google Scholar] [CrossRef]
- Bruckner, M.; O’Reilly, M.; Lee, T.-F.; Neset, M.; Cheung, P.-Y.; Schmölzer, G.M. Effects of varying chest compression depths on carotid blood flow and blood pressure in asphyxiated piglets. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 553–556. [Google Scholar] [CrossRef]
- Schmölzer, G.M.; O’Reilly, M.; LaBossiere, J.; Lee, T.-F.; Cowan, S.; Qin, S.; Bigam, D.L.; Cheung, P.-Y. Cardiopulmonary resuscitation with chest compressions during sustained inflations: A new technique of neonatal resuscitation that improves recovery and survival in a neonatal porcine model. Circulation 2013, 128, 2495–2503. [Google Scholar] [CrossRef]
- Schmölzer, G.M. Chest Compressions During Sustained Inflation During Cardiopulmonary Resuscitation in Newborn Infants Translating Evidence From Animal Studies to the Bedside. JACC Basic Transl. Sci. 2019, 4, 116–121. [Google Scholar] [CrossRef]
- Garcia-Hidalgo, C.; Cheung, P.-Y.; Solevåg, A.L.; Vento, M.; O’Reilly, M.; Saugstad, O.; Schmölzer, G.M. A Review of Oxygen Use During Chest Compressions in Newborns—A Meta-Analysis of Animal Data. Front. Pediatr. 2018, 6, 400. [Google Scholar] [CrossRef]
- Menegazzi, J.J.; E Auble, T.; A Nicklas, K.; Hosack, G.M.; Rack, L.; Goode, J.S. Two-thumb versus two-finger chest compression during CPR in a swine infant model of cardiac arrest. Ann. Emerg. Med. 1993, 22, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.-Y.; Huang, H.; Xu, C.; Liu, J.-Q.; Ting, J.Y.; Wong, R.; Lee, W.; Xue, Y.; Yi, Y. Comparing the Quality of Cardiopulmonary Resuscitation Performed at the Over-the-Head Position and Lateral Position of Neonatal Manikin. Front. Pediatr. 2020, 7, 559. [Google Scholar] [CrossRef]
- Jo, C.H.; Cho, G.C.; Lee, C.H. Two-Thumb Encircling Technique Over the Head of Patients in the Setting of Lone Rescuer Infant CPR Occurred During Ambulance Transfer. Pediatr. Emerg. Care 2017, 33, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Perlman, J.M.; Wyllie, J.; Kattwinkel, J.; Wyckoff, M.H.; Aziz, K.; Guinsburg, R.; Kim, H.-S.; Liley, H.G.; Mildenhall, L.; Simon, W.M.; et al. Part 7: Neonatal Resuscitation 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2015, 132, S204–S241. [Google Scholar] [CrossRef] [PubMed]
- Schmölzer, G.M.; O Reilly, M.; Fray, C.; van Os, S.; Cheung, P.-Y. Chest compression during sustained inflation versus 3:1 chest compression:ventilation ratio during neonatal cardiopulmonary resuscitation: A randomised feasibility trial. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 103, F455–F460. [Google Scholar] [CrossRef]
- Schmölzer, G.M.; Pichler, G.; Solevåg, A.L.; Fray, C.; van Os, S.; Cheung, P.-Y. The SURV1VE trial—Sustained inflation and chest compression versus 3:1 chest compression-to-ventilation ratio during cardiopulmonary resuscitation of asphyxiated newborns: Study protocol for a cluster randomized controlled trial. Trials 2019, 20, 139. [Google Scholar] [CrossRef]
- Dannevig, I.; Solevåg, A.L.; Sonerud, T.; Saugstad, O.D.; Nakstad, B. Brain inflammation induced by severe asphyxia in newborn pigs and the impact of alternative resuscitation strategies on the newborn central nervous system. Pediatr. Res. 2012, 73, 163–170. [Google Scholar] [CrossRef]
- Solevåg, A.L.; Schmölzer, G.M.; O’Reilly, M.; Lu, M.; Lee, T.-F.; Hornberger, L.K.; Nakstad, B.; Cheung, P.-Y. Myocardial perfusion and oxidative stress after 21% vs. 100% oxygen ventilation and uninterrupted chest compressions in severely asphyxiated piglets. Resuscitation 2016, 106, 7–13. [Google Scholar] [CrossRef]
- Hooper, S.B.; Pas, A.T.; Polglase, G.R.; Wyckoff, M. Animal models in neonatal resuscitation research: What can they teach us? Semin. Fetal Neonatal Med. 2018, 23, 300–305. [Google Scholar] [CrossRef]
- Solevåg, A.L.; Cheung, P.-Y.; Lie, H.; O’Reilly, M.; Aziz, K.; Nakstad, B.; Schmölzer, G.M. Chest compressions in newborn animal models: A review. Resuscitation 2015, 96, 151–155. [Google Scholar] [CrossRef]
| Baseline Parameters | 2-Thumb-Technique (n = 7) | 2-Finger-Technique (n = 7) | Over-the-Head 2-Thumb-Technique (n = 7) | Knocking-Fingers-Technique (n = 7) | p-Value | |
|---|---|---|---|---|---|---|
| Applied Force (kg) | 1.30 (0.54) | 1.23 (0.62) | 1.04 (0.51) | 1.29 (0.66) | 0.594 | |
| Applied CC Depth (cm) | 3.3 (1.0) | 2.3 (1.0) | 3.3 (1.6) | 2.4 (1.1) | 0.325 | |
| Anterior-posterior CC depth (%) | 38 (0)% | 25 (0)% | 38 (0)% | 26 (0)% | ||
| Hemodynamic Parameter | ||||||
| Carotid blood flow (mL/kg/min) | 0 (0) | 10 (6) | 5 (3) | 6 (5) | 4 (3) | 0.13 |
| Slope rise of carotid blood flow (mL/min/s) | 0 (0) | 118 (45) | 75 (48) | 121 (46) | 71 (67) | 0.001 |
| Mean arterial blood pressure (mmHg) | 0 (0) | 19 (9) | 10 (5) | 12 (5) | 12 (7) | 0.12 |
| Diastolic blood pressure (mmHg) | 0 (0) | 9 (4) | 8 (2) | 8 (2) | 8 (2) | 0.67 |
| Stroke volume (mL/kg) | 0 (0) | 0.8 (0.3) | 0.5 (0.2) * | 0.8 (0.3) | 0.6 (0.3) | 0.12 |
| End diastolic volume (mL/kg) | 0 (0) | 2.6 (1.4) | 2.3 (1.3) | 2.4 (1.5) | 2.1 (1.2) | 0.94 |
| dp/dtmax (mmHg/s) | 0 (0) | 1128 (405) | 790 (398) | 877 (478) | 796 (357) | 0.40 |
| dp/dtmin (mmHg/s) | 0 (0) | −1052 (369) | −568 (229) * | −711 (310) | −578 (180) * | 0.012 |
| Respiratory Parameter | ||||||
| Tidal volume (mL/kg) | 9.5 (3.6) | 8.5 (3.5) | 7.6 (1.9) | 7.8 (2.4) | 0.611 | |
| Minute Ventilation (mL/kg/min) | 855 (320) | 763 (313) | 680 (172) | 702 (2019) | 0.432 | |
| Peak Inspiratory Flow (L/min) | 5.7 (1.6) | 5.5 (0.8) | 4.7 (0.3) | 5.0 (0.9) | 0.291 | |
| Peak Expiration Flow (L/min) | −9.1 (1.5) | −8.7 (3.0) | −7.4 (1.7) | −9.3 (1.8) | 0.337 | |
| Peak Inflation Pressure (cm H2O) | 29 (10) | 30 (13) | 28 (12) | 30 (11) | 0.996 | |
| End-tidal CO2 (mmHg) | 2.6 (2.8) | 1.6 (1.2) | 1.9 (1.7) | 1.6 (1.0) | 0.676 | |
| Rate (/min) | 90 (1) | 90 (1) | 90 (1) | 90 (1) | 1.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruckner, M.; Neset, M.; O’Reilly, M.; Lee, T.-F.; Cheung, P.-Y.; Schmölzer, G.M. Four Different Finger Positions and Their Effects on Hemodynamic Changes during Chest Compression in Asphyxiated Neonatal Piglets. Children 2023, 10, 283. https://doi.org/10.3390/children10020283
Bruckner M, Neset M, O’Reilly M, Lee T-F, Cheung P-Y, Schmölzer GM. Four Different Finger Positions and Their Effects on Hemodynamic Changes during Chest Compression in Asphyxiated Neonatal Piglets. Children. 2023; 10(2):283. https://doi.org/10.3390/children10020283
Chicago/Turabian StyleBruckner, Marlies, Mattias Neset, Megan O’Reilly, Tze-Fun Lee, Po-Yin Cheung, and Georg M. Schmölzer. 2023. "Four Different Finger Positions and Their Effects on Hemodynamic Changes during Chest Compression in Asphyxiated Neonatal Piglets" Children 10, no. 2: 283. https://doi.org/10.3390/children10020283
APA StyleBruckner, M., Neset, M., O’Reilly, M., Lee, T.-F., Cheung, P.-Y., & Schmölzer, G. M. (2023). Four Different Finger Positions and Their Effects on Hemodynamic Changes during Chest Compression in Asphyxiated Neonatal Piglets. Children, 10(2), 283. https://doi.org/10.3390/children10020283






