Should Perirectal Swab Culture Be Performed in Cases Admitted to the Neonatal Intensive Care Unit? Lessons Learned from the Neonatal Intensive Care Unit
Abstract
1. Introduction
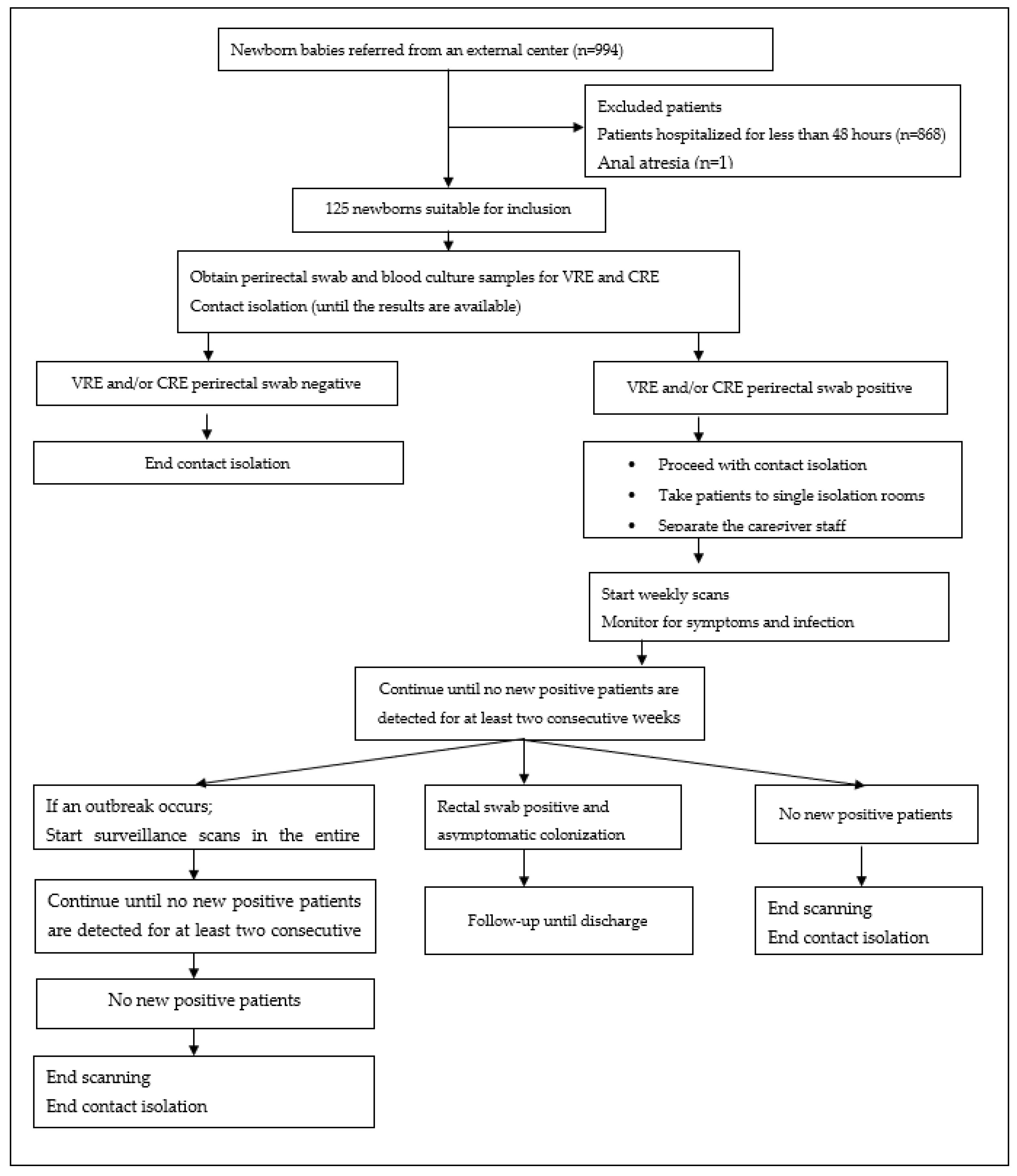
2. Materials and Methods
2.1. Study Design, Setting, and Population
2.1.1. Case Definition and Inclusion Criteria
2.1.2. Data Collection
2.1.3. Outcomes
2.2. Microbiological Evaluation
2.2.1. Isolation of Bacterial Isolates
2.2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiotos, K.; Han, J.H.; Tamma, P.D. Carbapenem-Resistant Enterobacteriaceae Infections in Children. Curr. Infect. Dis. Rep. 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.J.; Murphy, M.; McCallion, N.; Brennan, M.; Cunney, R.; Drew, R.J. Outbreaks of extended spectrum beta-lactamase-producing Enterobacteriaceae in neonatal intensive care units: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F72–F78. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.; Banerjee, T.; Anupurba, S.; Kumar, A. Vancomycin-resistant enterococci in neonatal stool as a cause of septicemia: Challenges for infection control practices. Indian J. Pathol. Microbiol. 2016, 59, 548–550. [Google Scholar] [CrossRef]
- Dong, Y.; Basmaci, R.; Titomanlio, L.; Sun, B.; Mercier, J.C. Neonatal sepsis: Within and beyond China. Chin. Med. J. (Engl.) 2020, 133, 2219–2228. [Google Scholar] [CrossRef]
- Flannery, D.D.; Chiotos, K.; Gerber, J.S.; Puopolo, K.M. Neonatal multidrug-resistant gram-negative infection: Epidemiology, mechanisms of resistance, and management. Pediatr. Res. 2022, 91, 380–391. [Google Scholar] [CrossRef]
- Saporito, L.; Graziano, G.; Mescolo, F.; Amodio, E.; Insinga, V.; Rinaudo, G.; Aleo, A.; Bonura, C.; Vitaliti, M.; Corsello, G.; et al. Efficacy of a coordinated strategy for containment of multidrug-resistant Gram-negative bacteria carriage in a Neonatal Intensive Care Unit in the context of an active surveillance program. Antimicrob. Resist. Infect. Control 2021, 10, 30. [Google Scholar] [CrossRef]
- Capasso, L.; Borrelli, A.C.; Ferrara, T.; Albachiara, R.; Coppola, C.; Raimondi, F. Adjuvant therapy in septic neonates with immunoglobulin preparations containing Ig isotypes in addition to IgG: A critical review of current literature. Curr. Pediatr. Res. 2017, 21, 535–540. [Google Scholar]
- Seidel, J.; Haller, S.; Eckmanns, T.; Harder, T. Routine screening for colonization by Gram-negative bacteria in neonates at intensive care units for the prediction of sepsis: Systematic review and meta-analysis. J. Hosp. Infect. 2018, 99, 367–380. [Google Scholar] [CrossRef]
- Cohen, B.; Saiman, L.; Cimiotti, J.; Larson, E. Factors associated with hand hygiene practices in two neonatal intensive care units. Pediatr. Infect. Dis. J. 2003, 22, 494–499. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Prevention Control of Carbapenem—Resistant Enterobactericea. In Acinetobacter baumannii and Pseudomonas Aeruginosa in Healt Care Facilities; WHO Guidelines Approved by the Guidelines Review Committee: Geneva, Switzerland, 2017. [Google Scholar]
- Chambers, H.F.; Fowler, V.G., Jr. Confronting antimicrobial resistance together. Am. J. Physiol. Lung Cell Mol. Physiol. 2022, 323, L643–L645. [Google Scholar] [CrossRef] [PubMed]
- Eichel, V.M.; Boutin, S.; Frank, U.; Weigand, M.A.; Heininger, A.; Mutters, N.T.; Büchler, M.W.; Heeg, K.; Nurjadi, D. Impact of discontinuing contact precautions and enforcement of basic hygiene measures on nosocomial vancomycin- resistant Enterococcus faecium transmission. J. Hosp. Infect. 2022, 121, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Reyes, K.; Bardossy, A.C.; Zervos, M. Vancomycin-Resistant Enterococci: Epidemiology, Infection Prevention, and Control. Infect. Dis. Clin. N. Am. 2016, 30, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Härtel, C.; Faust, K.; Fortmann, I.; Humberg, A.; Pagel, J.; Haug, C.; Kühl, R.; Bohnhorst, B.; Pirr, S.; Viemann, D.; et al. Sepsis related mortality of extremely low gestational age newborns after the introduction of colonization screening for multi-drug resistant organisms. Antimicrob. Resist. Infect. Control 2020, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Ulu-Kilic, A.; Özhan, E.; Altun, D.; Perçin, D.; Güneş, T.; Alp, E. Is it worth screening for vancomycin-resistant Enterococcus faecium colonization?: Financial burden of screening in a developing country. Am. J. Infect. Control 2016, 44, e45–e49. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Agyeman, P.K.A.; Stocker, M.; Posfay-Barbe, K.M.; Heininger, U.; Spycher, B.D.; Bernhard-Stirnemann, S.; Niederer-Loher, A.; Kahlert, C.R.; Donas, A.; et al. Neonatal Sepsis of Early Onset, and Hospital-Acquired and Community-Acquired Late Onset: A Prospective Population-Based Cohort Study. J. Pediatr. 2018, 201, 106–114.e104. [Google Scholar] [CrossRef]
- Berglund, B.; Hoang, N.T.B.; Lundberg, L.; Le, N.K.; Tärnberg, M.; Nilsson, M.; Bornefall, E.; Khu, D.T.K.; Welander, J.; Le, H.T.; et al. Clonal spread of carbapenem-resistant Klebsiella pneumoniae among patients at admission and discharge at a Vietnamese neonatal intensive care unit. Antimicrob. Resist. Infect. Control 2021, 10, 162. [Google Scholar] [CrossRef]
- Zakir, A.; Regasa Dadi, B.; Aklilu, A.; Oumer, Y. Investigation of Extended-Spectrum β-Lactamase and Carbapenemase Producing Gram-Negative Bacilli in Rectal Swabs Collected from Neonates and Their Associated Factors in Neonatal Intensive Care Units of Southern Ethiopia. Infect. Drug Resist. 2021, 14, 3907–3917. [Google Scholar] [CrossRef]
- Arhoune, B.; Oumokhtar, B.; Hmami, F.; Barguigua, A.; Timinouni, M.; El Fakir, S.; Chami, F.; Bouharrou, A. Rectal carriage of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae among hospitalised neonates in a neonatal intensive care unit in Fez, Morocco. J. Glob. Antimicrob. Resist. 2017, 8, 90–96. [Google Scholar] [CrossRef]
- Mairi, A.; Touati, A.; Ait Bessai, S.; Boutabtoub, Y.; Khelifi, F.; Sotto, A.; Lavigne, J.P.; Pantel, A. Carbapenemase-producing Enterobacteriaceae among pregnant women and newborns in Algeria: Prevalence, molecular characterization, maternal-neonatal transmission, and risk factors for carriage. Am. J. Infect. Control 2019, 47, 105–108. [Google Scholar] [CrossRef]
- Almeida, T.L.; Mendo, T.; Costa, R.; Novais., C.; Marçal, M.; Martins, F.; Tuna, M. Carbapenemase-Producing Enterobacteriaceae (CPE) Newborn Colonization in a Portuguese Neonatal Intensive Care Unit (NICU): Epidemiology and Infection Prevention and Control Measures. Infect. Dis. Rep. 2021, 13, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Decembrino, L.; Maini, A.; Decembrino, N.; Maggi, I.; Lacerenza, S. Management of outbreaks in neonatal intensive care units. Early Hum. Dev. 2014, 90, S54–S56. [Google Scholar] [CrossRef] [PubMed]
- Haytoğlu, Z.; Gündeşlioğlu, Ö.; Yıldızdaş, D.; Kocabaş, E.; Alabaz, D.; Horoz, Ö.Ö. Carbapenem and colistin resistance in children with Enterobacteriaceae infections. Turk. J. Pediatr. 2020, 62, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Crivaro, V.; Bagattini, M.; Salza, M.F.; Raimondi, F.; Rossano, F.; Triassi, M.; Zarrilli, R. Risk factors for extended-spectrum beta-lactamase-producing Serratia marcescens and Klebsiella pneumoniae acquisition in a neonatal intensive care unit. J. Hosp. Infect. 2007, 67, 135–141. [Google Scholar] [CrossRef]
- Clock, S.A.; Ferng, Y.H.; Tabibi, S.; Alba, L.; Patel, S.J.; Jia, H.; DeLaMora, P.; Perlman, J.M.; Paul, D.A.; Zaoutis, T.; et al. Colonization with Antimicrobial-Resistant Gram-Negative Bacilli at Neonatal Intensive Care Unit Discharge. J. Pediatr. Infect. Dis. Soc. 2017, 6, 219–226. [Google Scholar] [CrossRef]
- Chiotos, K.; Tamma, P.D.; Flett, K.B.; Naumann, M.; Karandikar, M.V.; Bilker, W.B.; Zaoutis, T.; Han, J.H. Multicenter Study of the Risk Factors for Colonization or Infection with Carbapenem-Resistant Enterobacteriaceae in Children. Antimicrob. Agents Chemother. 2017, 61, e01440-17. [Google Scholar] [CrossRef]
- Seesahai, J.; Church, P.T.; Asztalos, E.; Eng-Chong, M.; Arbus, J.; Banihani, R. Neonates with Maternal Colonization of Carbapenemase-Producing, Carbapenem-Resistant Enterobacteriaceae: A Mini-Review and a Suggested Guide for Preventing Neonatal Infection. Children 2021, 8, 399. [Google Scholar] [CrossRef]
- Jiménez-Rámila, C.; López-Cerero, L.; Martín, M.A.; Martín, C.V.; Serrano, L.; Pascual, Á.; Rodríguez-Baño, J. Vagino-rectal colonization and maternal–neonatal transmission of Enterobacteriaceae producing extended-spectrum β-lactamases or carbapenemases: A cross-sectional study. J. Hosp. Infect. 2019, 101, 167–174. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Singh, N.; Léger, M.M.; Campbell, J.; Short, B.; Campos, J.M. Control of vancomycin-resistant enterococci in the neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 2005, 26, 646–649. [Google Scholar] [CrossRef]
- Shrestha, S.; Kharel, S.; Homagain, S.; Aryal, R.; Mishra, S.K. Prevalence of vancomycin-resistant enterococci in Asia-A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2021, 46, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Akturk, H.; Sutcu, M.; Somer, A.; Acar, M.; Akgun Karapınar, B.; Aydin, D.; Cihan, R.; Ince, Z.; Çoban, A.; Salman, N. Vancomycin-resistant enterococci colonization in a neonatal intensive care unit: Who will be infected? J. Matern.-Fetal Neonatal Med. 2016, 29, 3478–3482. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, R.; Saffar, M.J.; Monfared, F.T.; Larijani, L.V.; Kenari, S.A.; Charati, J.Y. Prevalence, risk factors and molecular analysis of vancomycin-resistant Enterococci colonization in a referral neonatal intensive care unit: A prospective study in northern Iran. J. Glob. Antimicrob. Resist. 2022, 30, 474–479. [Google Scholar] [CrossRef]
- Iosifidis, E.; Evdoridou, I.; Agakidou, E.; Chochliourou, E.; Protonotariou, E.; Karakoula, K.; Stathis, I.; Sofianou, D.; Drossou-Agakidou, V.; Pournaras, S.; et al. Vancomycin-resistant Enterococcus outbreak in a neonatal intensive care unit: Epidemiology, molecular analysis and risk factors. Am. J. Infect. Control 2013, 41, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Kotsanas, D.; Tan, K.; Scott, C.; Baade, B.; Cheng, M.H.L.; Tan, Z.V.; Taylor, J.E.; Kwong, J.C.; Seemann, T.; Coombs, G.W.; et al. A nonclonal outbreak of vancomycin-sensitive Enterococcus faecalis bacteremia in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 2019, 40, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, M.; Liese, C.; Loescher, C.; Kunze, M.; Proempeler, H.; Berner, R.; Krueger, M. Enterococcal colonization of infants in a neonatal intensive care unit: Associated predictors, risk factors and seasonal patterns. BMC Infect. Dis. 2007, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Benzer, D.; Yavuzcan Öztürk, D.; Gürsoy, T.; Ocalmaz, M.S.; Karatekin, G.; Ovalı, H.F. Vancomycin-resistant enterococcus colonization in neonatal intensive care unit: Prevention and eradication experience. Mikrobiyol. Bul. 2012, 46, 682–688. [Google Scholar]
- Camacho-Gonzalez, A.; Spearman, P.W.; Stoll, B.J. Neonatal infectious diseases: Evaluation of neonatal sepsis. Pediatr. Clin. 2013, 60, 367–389. [Google Scholar]
- Folgori, L.; Tersigni, C.; Hsia, Y.; Kortsalioudaki, C.; Heath, P.; Sharland, M.; Bielicki, J. The relationship between Gram-negative colonization and bloodstream infections in neonates: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2018, 24, 251–257. [Google Scholar] [CrossRef]
- Harder, T.; Seidel, J.; Eckmanns, T.; Weiss, B.; Haller, S. Predicting late-onset sepsis by routine neonatal screening for colonisation by gram-negative bacteria in neonates at intensive care units: A protocol for a systematic review. BMJ Open 2017, 7, e014986. [Google Scholar] [CrossRef]
- Capasso, L.; Maddaluno, S.; Coppola, C.; Dolce, P.; di Cola, G.S.; Sierchio, E.; Borrrelli, A.C.; Bagattini, M.; Esposito, E.P.; Zarrilli, R.; et al. Do isolates from pharyngeal and rectal swabs match blood culture bacterial pathogens in septic VLBW infants? A pilot, cross-sectional study. Eur. J. Pediatr. 2021, 180, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Marom, R.; Mandel, D.; Haham, A.; Berger, I.; Ovental, A.; Raskind, C.; Grisaru-Soen, G.; Adler, A.; Lellouche, J.; Schwartz, D.; et al. A silent outbreak of vancomycin-resistant Enterococcus faecium in a neonatal intensive care unit. Antimicrob. Resist. Infect. Control 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Saiman, L.; Zhou, J.; Della-Latta, P.; Jia, H.; Graham, P.L., 3rd. Concordance of Gastrointestinal Tract Colonization and Subsequent Bloodstream Infections with Gram-negative Bacilli in Very Low Birth Weight Infants in the Neonatal Intensive Care Unit. Pediatr. Infect. Dis. J. 2010, 29, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Singh, A.K.; Pal, T.; Dasgupta, S.; Ramamurthy, T.; Basu, S. Colonization of the gut with Gram-negative bacilli, its association with neonatal sepsis and its clinical relevance in a developing country. J. Med. Microbiol. 2011, 60, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Kim, H.M.; Chung, D.R.; Choi, J.R.; Lee, M.A.; Huh, H.J.; Lee, N.Y.; Huh, K.; Kang, C.I.; Peck, K.R. The impact of vancomycin-resistant Enterococcus (VRE) screening policy change on the incidence of healthcare-associated VRE bacteremia. Infect. Control Hosp. Epidemiol. 2022, 43, 603–608. [Google Scholar] [CrossRef]
- Wang, J.; Lv, Y.; Yang, W.; Zhao, P.; Yin, C. Epidemiology and clinical characteristics of infection/colonization due to carbapenemase-producing Enterobacterales in neonatal patients. BMC Microbiol. 2022, 22, 177. [Google Scholar] [CrossRef]
| CRE (Carbapenem-Resistant Enterobacterales) | VRE (Vancomycin-Resistant Enterococci) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive n (%) | Negative n (%) | Total n (%) | p < 0.05 | Positive n (%) | Negative n (%) | Total n (%) | p < 0.05 | ||
| Sex | 125 (100) | 0.436 | 125 (100) | 0.240 | |||||
| Female | 12 (35.3) | 41 (45.1) | 53 (42.4) | 1 (16.7) | 52 (43.7) | 53 (42.4) | |||
| Male | 22 (64.7) | 50 (54.9) | 72 (57.6) | 5 (83.3) | 67 (56.3) | 72 (57.6) | |||
| Gestational age | 0.932 | 0.408 | |||||||
| <32 weeks | 5 (14.7) | 19 (20.9) | 24 (19.2) | 1 (16.7) | 23 (19.3) | 24 (19.2) | |||
| 32–36 weeks | 15 (44.1) | 17 (18.7) | 32 (25.6) | 2 (33.3) | 30 (25.2) | 32 (25.6) | |||
| 37 weeks and over | 14 (41.2) | 55 (60.4) | 69 (55.2) | 3 (50) | 66 (55.5) | 69 (55.2) | |||
| * Birth weight (g) (min-max) | 2732.5 (2200–3100) | 2900 (2240–3400) | - | 0.313 | 2900 (2200–3270) | 2780 (2200–3300) | - | 0.849 | |
| * Hospitalization weight (g) | 2745 (2220–3110) | 2910 (2100–3370) | - | 0.504 | 2730 (2010–3110) | 2850 (2200–3300) | - | 0.742 | |
| * Hospitalization age—days (min-max) | 14 (7–21) | 9 (4–18) | - | 0.110 | 7.5 (5–14) | 11 (4–20) | - | 0.431 | |
| Name of Healthcare Center | 125 (100) | 0.777 | 125 (100) | 1.000 | |||||
| City of Mersin | 28 (82.4) | 71 (78) | 99 (79.2) | 5 (83.3) | 94 (79) | 99 (79.2) | |||
| Outside the city | 6 (17.6) | 20 (22) | 26 (20.8) | 1 (16.7) | 25 (21) | 26 (20.8) | |||
| Mode of delivery | 125 (100) | 0.552 | 125 (100) | 0.176 | |||||
| Normal delivery | 9 (26.5) | 31 (34.1) | 40 (32.0) | 0 (0) | 40 (33.6) | 40 (32.0) | |||
| Cesarean | 25 (73.5) | 60 (65.9) | 85 (68.0) | 6 (100) | 79 (66.4) | 85 (68) | |||
| External center antibiotherapy | 125 (100) | 0.165 | 125 (100) | 0.867 | |||||
| No antibiotic treatment | 9 (26.5) | 18 (19.8) | 27 (21.6) | 1 (16.7) | 26 (21.8) | 27 (21.6) | |||
| Empirical (ampicillin- gentamicin) | 13 (38.2) | 49 (53.8) | 62 (49.6) | 3 (50) | 59 (49.6) | 62 (49.6) | |||
| # Broad-spectrum antibiotic | 12 (35.3) | 24 (26.4) | 36 (28.8) | 2 (33.3) | 34 (28.6) | 36 (28.8) | |||
| Externalcenter antibiotherapy duration | 125 (100) | 0.764 | 125 (100) | 0.634 | |||||
| 0 days | 9 (26.5) | 17 (18.7) | 26 (20.8) | 9 (25) | 18 (20.2) | 27 (21.6) | |||
| 1–10 days | 19 (55.9) | 68 (74.7) | 87 (69.6) | 21 (58.3) | 65 (73.0) | 86 (68.8) | |||
| >10 days | 6 (17.6) | 6 (6.6) | 12 (9.6) | 6 (16.7) | 6 (6.7) | 12 (9.6) | |||
| CRE (Carbapenem-Resistant Enterobacterales) | VRE (Vancomycin-Resistant Enterococci) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive n (%) | Negative n (%) | Total n (%) | p < 0.05 | Positive n (%) | Negative n (%) | Total n (%) | p < 0.05 | ||
| Duration of hospitalization after referral | 125 (100) | 0.089 | 125 (100) | 0.039 | |||||
| 1–10 days | 7 (20.6) | 28 (30.8) | 35 (28.0) | 3 (50) | 32 (26.9) | 35 (28.0) | |||
| 11–20 days | 6 (17.6) | 24 (26.4) | 30 (24.0) | 3 (50) | 27 (22.7) | 30 (24.0) | |||
| >20 days | 21 (61.8) | 39 (42.9) | 60 (48.0) | 0 (0) | 60 (50.4) | 60 (48.0) | |||
| Mechanical ventilator support | 125 (100) | 1.000 | 125 (100) | 0.401 | |||||
| Yes | 15 (44.1) | 39 (42.9) | 54 (43.2) | 4 (66.7) | 50 (42) | 54 (43.2) | |||
| No | 19 (55.9) | 52 (57.1) | 71 (41.6) | 2 (33.3) | 69 (58) | 71 (56.8) | |||
| Sepsis | 125 (100) | 0.818 | 125 (100) | 0.895 | |||||
| None | 16 (47.1) | 41 (45.1) | 57 (45.6) | 2 (33.3) | 55 (46.2) | 57 (45.6) | |||
| Clinical sepsis | 11 (32.4) | 32 (35.2) | 43 (34.4) | 3 (50) | 40 (33.6) | 43 (34.4) | |||
| Proven sepsis | 3 (8.8) | 4 (4.4) | 7 (5.6) | 1 (16.7) | 6 (5) | 7 (5.6) | |||
| Suspicious sepsis | 4 (11.8) | 14 (15.4) | 18 (14.4) | 0 (0) | 18 (15.1) | 18 (14.4) | |||
| * Laboratory | |||||||||
| White blood cell (mm3) | 11,510 (8800–16,290) | 10,770 (8340–14,200) | - | 0.451 | 9220 (8870–147,20) | 11,430 (8350–14,550) | - | 0.817 | |
| C-reactive protein (mg/L) | 1.7 (0.5–12.5) | 2.7 (0.7–15.5) | - | 0.564 | 7 (1.4–12) | 2.5 (0.6–15.5) | - | 0.564 | |
| Procalcitonin (ng/dL) | 2.4 (0.8–3.4) | 2 (0.7–3.5) | - | 0.814 | 3.5 (2.6–3.8) | 2 (0.7–3.2) | - | 0.039 | |
| Band-neutrophil ratio | 0.2 (0.1–0.2) | 0.1 (0.1–0.2) | - | 0.619 | 0.2 (0.2–0.3) | 0.1 (0.1–0.2) | - | 0.087 | |
| Blood culture factor | 125 (100) | 0.323 | 125 (100) | 0.038 | |||||
| No growth | 31 (91.2) | 87 (95.6) | 118 (94.4) | 5 (83.3) | 113 (95) | 118 (94.4) | |||
| MRCNS | 2 (5.9) | 3 (3.3) | 5 (4.0) | 0 (0) | 5 (4.2) | 5 (4.0) | |||
| K. pneumonia | 1 (2.9) | 1 (1.1) | 2 (1.6) | 1 (16.7) | 1 (0.8) | 2 (1.6) | |||
| Mortality | 125 (100) | 0.206 | 125 (100) | 0.461 | |||||
| No | 33 (97.1) | 82 (90.1) | 115 (92.0) | 6 (100) | 109 (91.6) | 115 (92.0) | |||
| Yes | 1 (2.9) | 9 (9.9) | 10 (8.0) | 0 (0.0) | 10 (8.1) | 10 (8.0) | |||
| Definite Diagnosis | Rectal Swab | ||
|---|---|---|---|
| Positive n (%) | Negative n (%) | p < 0.05 | |
| Respiratory distress | 5 (13.9) | 26 (29.2) | 0.391 |
| Metabolic causes | 7 (19.4) | 16 (18.0) | |
| Surgical causes | 9 (25) | 14 (15.7) | |
| Asphyxia/Neonatal convulsion | 7 (19.4) | 14 (15.7) | |
| Sepsis | 6 (16.7) | 10 (11.2) | |
| Hematological/oncological causes | 2 (5.6) | 9 (10.1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orman, A.; Celik, Y.; Evik, G.; Ersöz, G.; Kuyucu, N.; Ozmen, B.O. Should Perirectal Swab Culture Be Performed in Cases Admitted to the Neonatal Intensive Care Unit? Lessons Learned from the Neonatal Intensive Care Unit. Children 2023, 10, 187. https://doi.org/10.3390/children10020187
Orman A, Celik Y, Evik G, Ersöz G, Kuyucu N, Ozmen BO. Should Perirectal Swab Culture Be Performed in Cases Admitted to the Neonatal Intensive Care Unit? Lessons Learned from the Neonatal Intensive Care Unit. Children. 2023; 10(2):187. https://doi.org/10.3390/children10020187
Chicago/Turabian StyleOrman, Aysen, Yalcin Celik, Guliz Evik, Gulden Ersöz, Necdet Kuyucu, and Berfin Ozgokce Ozmen. 2023. "Should Perirectal Swab Culture Be Performed in Cases Admitted to the Neonatal Intensive Care Unit? Lessons Learned from the Neonatal Intensive Care Unit" Children 10, no. 2: 187. https://doi.org/10.3390/children10020187
APA StyleOrman, A., Celik, Y., Evik, G., Ersöz, G., Kuyucu, N., & Ozmen, B. O. (2023). Should Perirectal Swab Culture Be Performed in Cases Admitted to the Neonatal Intensive Care Unit? Lessons Learned from the Neonatal Intensive Care Unit. Children, 10(2), 187. https://doi.org/10.3390/children10020187





