The Impact of 131I-Metaiodobenzylguanidine as a Conditioning Regimen of Tandem High-Dose Chemotherapy and Autologous Stem Cell Transplantation for High-Risk Neuroblastoma †
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Assessment of Disease Extent and Response Criteria
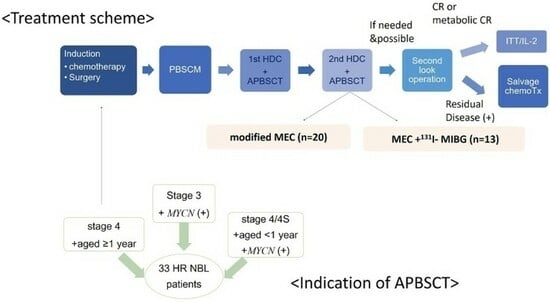
2.3. Pretransplant Treatment
2.4. Tandem HDC/ASCT
2.5. Post-HDC/ASCT
2.6. Evaluation of Adverse Effects
2.7. Survival Analysis and Statistics
3. Results
3.1. Patients’ Characteristics
3.2. Pretransplant Chemotherapy
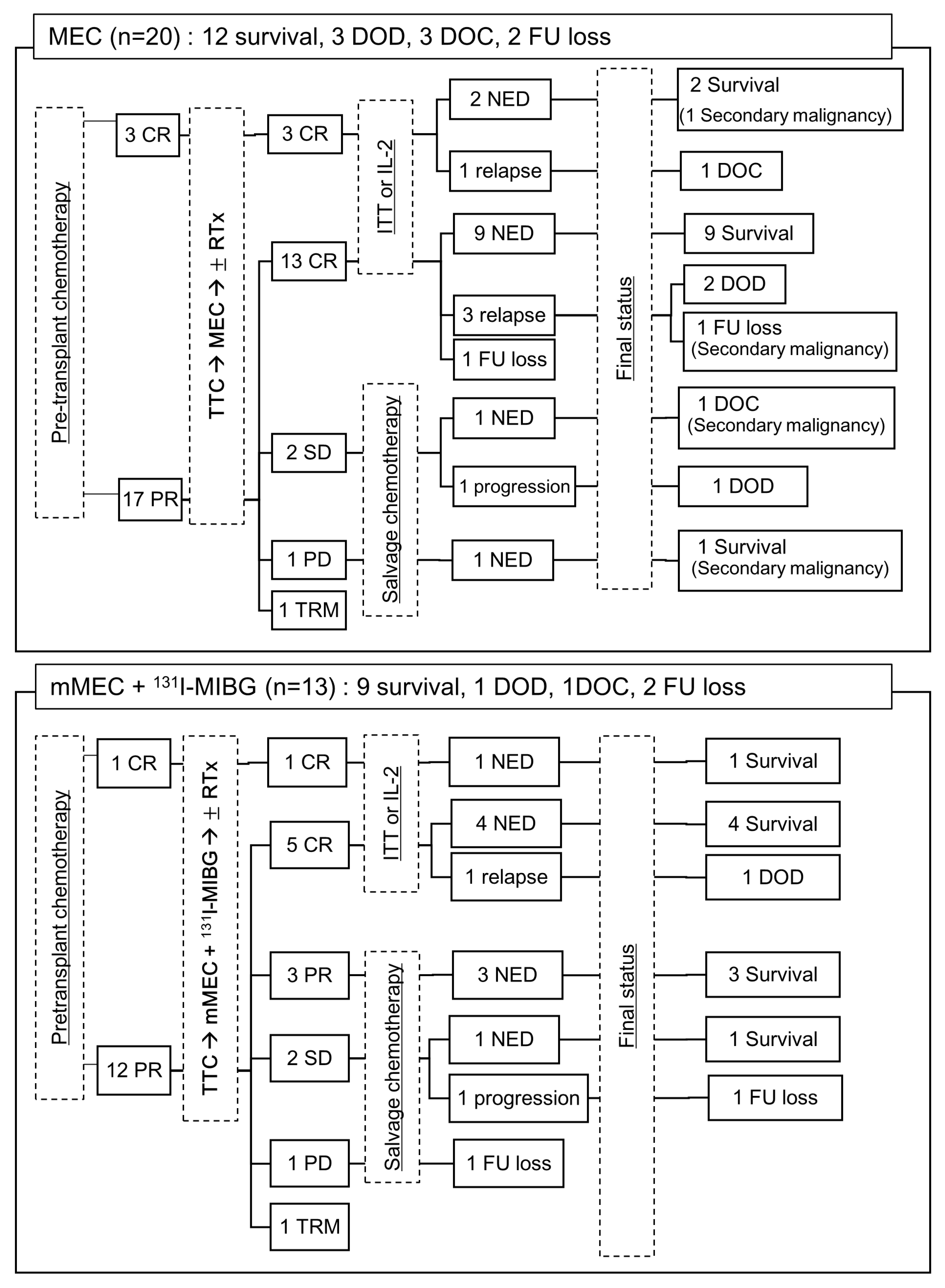
3.3. Tandem HDC/ASCT
3.4. Post-Consolidation Therapy
3.5. Toxicity and Complications
3.6. Relapse/Progression and Secondary Malignancy
3.7. Tumor Response and Survival
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sokol, E.; Desai, A.V. The Evolution of Risk Classification for Neuroblastoma. Children 2019, 6, 27. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Foster, J. High-Risk Neuroblastoma Treatment Review. Children 2018, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Colon, N.C.; Chung, D.H. Neuroblastoma. Adv. Pediatr. 2011, 58, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Whittle, S.B.; Smith, V.; Doherty, E.; Zhao, S.; Mccarty, S.; Zage, P.E. Overview and recent advances in the treatment of neuroblastoma. Expert Rev. Anticancer Ther. 2017, 17, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, B.; Kremer, L.C.; van Dalen, E.C. High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma. Cochrane Database Syst. Rev. 2015, 2015, CD006301. [Google Scholar] [CrossRef] [PubMed]
- Berthold, F.; Boos, J.; Burdach, S.; Erttmann, R.; Henze, G.; Hermann, J.; Klingebiel, T.; Kremens, B.; Schilling, F.H.; Schrappe, M.; et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: A randomised controlled trial. Lancet Oncol. 2005, 6, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Kreissman, S.G.; London, W.B.; Naranjo, A.; Cohn, S.L.; Hogarty, M.D.; Tenney, S.C.; Haas-Kogan, D.; Shaw, P.J.; Kraveka, J.M.; et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients with High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA 2019, 322, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Pötschger, U.; Pearson, A.D.J.; Brock, P.; Luksch, R.; Castel, V.; Yaniv, I.; Papadakis, V.; Laureys, G.; Malis, J.; et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. Apr. 2017, 18, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, C.; Dufour, C.; Goma, G.; Raquin, M.-A.; Lapierre, V.; Valteau-Couanet, D. Tandem high-dose chemotherapy with thiotepa and busulfan–melphalan and autologous stem cell transplantation in very high-risk neuroblastoma patients. Bone Marrow Transplant. 2016, 51, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Kraal, K.C.; van Dalen, E.C.; Tytgat, G.A.; Van Eck-Smit, B.L. Iodine-131-meta-iodobenzylguanidine therapy for patients with newly diagnosed high-risk neuroblastoma. Cochrane Database Syst. Rev. 2017, 2017, CD010349. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hero, B.; Simon, T. I-131-mIBG therapy in neuroblastoma: Established role and prospective applications. Clin. Transl. Imaging 2016, 4, 87–101. [Google Scholar] [CrossRef]
- Hamidieh, A.A.; Beiki, D.; Paragomi, P.; Fallahi, B.; Behfar, M.; Fard-Esfahani, A.; Hosseini, A.S.; Shamshiri, A.; Eftekhari, M.; Ghavamzadeh, A. The potential role of pretransplant MIBG diagnostic scintigraphy in targeted administration of 131I-MIBG accompanied by ASCT for high-risk and relapsed neuroblastoma: A pilot study. Pediatr. Transplant. 2014, 18, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Castel, V.; García-Miguel, P.; Cañete, A.; Melero, C.; Navajas, A.; Ruíz-Jiménez, J.; Navarro, S.; Badal, M. Prospective evaluation of the International Neuroblastoma Staging System (INSS) and the International Neuroblastoma Response Criteria (INRC) in a multicentre setting. Eur. J. Cancer Apr. 1999, 35, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Bagatell, R.; Cohn, S.L.; Pearson, A.D.; Villablanca, J.G.; Berthold, F.; Burchill, S.; Boubaker, A.; McHugh, K.; Nuchtern, J.G.; et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol. 2017, 35, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Stram, D.; Matthay, K.K.; O’Leary, M.; Reynolds, C.P.; Haase, G.M.; Atkinson, J.B.; Brodeur, G.M.; Seeger, R.C. Consolidation chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neuroblastoma: A report of two concurrent Children’s Cancer Group studies. J. Clin. Oncol. 1996, 14, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Yi, E.S.; Son, M.H.; Hyun, J.K.; Cho, H.W.; Ju, H.Y.; Lee, J.W.; Yoo, K.H.; Sung, K.W.; Koo, H.H. Predictors of survival in patients with high-risk neuroblastoma who failed tandem high-dose chemotherapy and autologous stem cell transplantation. Pediatr. Blood Cancer 2020, 67, e28066. [Google Scholar] [CrossRef]
- Simon, T.; Längler, A.; Harnischmacher, U.; Frühwald, M.C.; Jorch, N.; Claviez, A.; Berthold, F.; Hero, B. Topotecan, cyclophosphamide, and etoposide (TCE) in the treatment of high-risk neuroblastoma. Results of a phase-II trial. J. Cancer Res. Clin. Oncol. 2007, 133, 653–661. [Google Scholar] [CrossRef]
- Miano, M.; Faraci, M.; Dini, G.; Bordigoni, P.; EBMT Paediatric Working Party. Early complications following haematopoietic SCT in children. Bone Marrow Transplant. 2008, 41, S39–S42. [Google Scholar] [CrossRef][Green Version]
- Matthay, K.K.; Reynolds, C.P.; Seeger, R.C.; Shimada, H.; Adkins, E.S.; Haas-Kogan, D.; Gerbing, R.B.; London, W.B.; Villablanca, J.G. Long-Term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children’s oncology group study. J. Clin. Oncol. 2009, 27, 1007–1013. [Google Scholar] [CrossRef]
- Pritchard, J.; Cotterill, S.J.; Germond, S.M.; Imeson, J.; de Kraker, J.; Jones, D.R. High dose melphalan in the treatment of advanced neuroblastoma: Results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr. Blood Cancer 2004, 44, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Heneghan, M.B.; Li, Y.; Bunin, N.J.; A Grupp, S.; Bagatell, R.; E Seif, A. Toxicities of busulfan/melphalan versus carboplatin/etoposide/melphalan for high-dose chemotherapy with stem cell rescue for high-risk neuroblastoma. Bone Marrow Transplant. 2016, 51, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Seif, A.E.; Li, Y.; Getz, K.; Fisher, B.T.; Huang, V.; Mante, A.; Aplenc, R.; Bagatell, R. Resource Utilization and Toxicities After Carboplatin/Etoposide/Melphalan and Busulfan/Melphalan for Autologous Stem Cell Rescue in High-Risk Neuroblastoma Using a National Administrative Database. Pediatr. Blood Cancer 2016, 63, 901–907. [Google Scholar] [CrossRef]
- Suh, J.K.; Koh, K.; Min, S.Y.; Kim, Y.S.; Kim, H.; Im, H.J.; Namgoong, J.; Kim, D.Y.; Ahn, S.D.; Lee, J.J.; et al. Feasibility and effectiveness of treatment strategy of tandem high-dose chemotherapy and autologous stem cell transplantation in combi-nation with 131I-MIBG therapy for high-risk neuroblastoma. Pediatr. Transplant. 2020, 24, e13658. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, S.; Cho, H.W.; Ma, Y.; Yoo, K.H.; Sung, K.W.; Koo, H.H.; Cho, E.J.; Lee, S.-K.; Lim, D.H. Incorporation of high-dose (131)I-metaiodobenzylguanidine treatment into tandem high-dose chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma: Results of the SMC NB-2009 study. J. Hematol. Oncol. 2017, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.D.; Yanik, G.; Naranjo, A.; Zhang, F.F.; Fitzgerald, W.; Shulkin, B.L.; Parisi, M.T.; Russell, H.; Grupp, S.; Pater, L.; et al. A safety and feasibility trial of (131)I-MIBG in newly diagnosed high-risk neuroblastoma: A Children’s Oncology Group study. Pediatr. Blood Cancer 2021, 68, e29117. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; McGlynn, B.; Saggio, J.; Baniewicz, D.; Zhuang, H.; Maris, J.M.; Mosse, Y.P. Safety and efficacy of tandem 131I-metaiodobenzylguanidine infusions in relapsed/refractory neuroblastoma. Pediatr. Blood Cancer 2011, 57, 1124–1129. [Google Scholar] [CrossRef]
- Kayano, D.; Kinuya, S. Iodine-131 metaiodobenzylguanidine therapy for neuroblastoma: Reports so far and future perspec-tive. Sci. World J. 2015, 2015, 189135. [Google Scholar] [CrossRef]
- Matthay, K.K.; Tan, J.C.; Villablanca, J.G.; Yanik, G.A.; Veatch, J.; Franc, B.; Twomey, E.; Horn, B.; Reynolds, C.P.; Groshen, S.; et al. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: A new approaches to Neuroblastoma Therapy Consortium Study. J. Clin. Oncol. 2006, 24, 500–506. [Google Scholar] [CrossRef]
- French, S.; DuBois, S.G.; Horn, B.; Granger, M.; Hawkins, R.; Pass, A.; Plummer, E.; Matthay, K. 131I-MIBG followed by con-solidation with busulfan, melphalan and autologous stem cell transplantation for refractory neuroblastoma. Pediatr. Blood Cancer 2013, 60, 879–884. [Google Scholar] [CrossRef]
- Sung, K.W.; Son, M.H.; Yoo, K.H.; Koo, H.H.; Kim, J.Y.; Cho, E.J.; Lee, S.K.; Choi, Y.S.; Lim, D.H.; Kim, D.W. Tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk neuroblastoma: Results of SMC NB-2004 study. Bone Marrow Transplant. 2013, 48, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, V.P.; Dvorak, C.C.; Golden, C.; Vissa, M.; El-Haj, N.; Perwad, F.; Matthay, K.K.; Vo, K.T. Risk Factors for Trans-plant-Associated Thrombotic Microangiopathy after Autologous Hematopoietic Cell Transplant in High-Risk Neuroblas-toma. Biol. Blood Marrow Transplant. 2019, 25, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Jodele, S.; Dandoy, C.E.; Myers, K.; Wallace, G.; Lane, A.; Teusink-Cross, A.; Weiss, B.; Davies, S.M. High-dose Car-boplatin/Etoposide/Melphalan increases risk of thrombotic microangiopathy and organ injury after autologous stem cell transplantation in patients with neuroblastoma. Bone Marrow Transplant. 2018, 53, 1311–1318. [Google Scholar] [CrossRef] [PubMed]

| Regimen | Drug | Dose | Schedule | Total Dose |
|---|---|---|---|---|
| Pretransplant chemotherapy | ||||
| CCG 321P2 | Cisplatin | 60 mg/m2/day | Day 0 | 60 mg/m2 |
| Etoposide | 100 mg/m2/day | Day 2, 5 | 200 mg/m2 | |
| Adriamycin | 30 mg/m2/day | Day 2 | 30 mg/m2 | |
| Cyclophosphamide | 30 mg/kg/day | Day 3, 4 | 60 mg/kg | |
| Modified CCG-ICE | Ifosfamide | 1200 mg/m2/day | Day 0, 1, 2, 3, 4 | 6000 mg/m2 |
| Carboplatin | 350 mg/m2/day | Day 0, 1 | 700 mg/m2 | |
| Etoposide | 100 mg/m2/day | Day 0, 1, 2, 3 | 400 mg/m2 | |
| TCE | Topotecan | 1 mg/m2/day | Day 0, 1, 2, 3, 4 | 5 mg/m2 |
| Cyclophosphamide | 250 mg/m2/day | Day 0, 1, 2, 3, 4 | 1250 mg/m2 | |
| Etoposide | 100 mg/m2/day | Day 0, 1, 2 | 300 mg/m2 | |
| PBSCM | ||||
| CPM + VP | Cyclophosphamide | 1000 mg/m2/day | Day 0, 1, 2 | 3000 mg/m2 |
| Etoposide | 150 mg/m2/day | Day 0, 1, 2 | 450 mg/m2 | |
| G-CSF | 10 μg/kg | Day 7 to end of PBSCM | ||
| 1st HDC | ||||
| TTC | Topotecan | 2 mg/m2/day | Day -8, -7, -6, -5, -4 | 10 mg/m2 |
| Thiotepa | 300 mg/m2/day | Day -8, -7, -6 | 900 mg/m2 | |
| Carboplatin | 500 mg/m2/day | Day -5, -4, -3 | 1500 mg/m2 | |
| 2nd HDC | ||||
| MEC | Melphalan | 140 mg/m2/day (d-7) 70 mg/m2/day (d-6) | Day -7, -6 | 210 mg/m2 |
| Etoposide | 200 mg/m2/day | Day -8, -7, -6, -5 | 800 mg/m2 | |
| Carboplatin | 350 mg/m2/day | Day -8, -7, -6, -5 | 1400 mg/m2 | |
| mMEC + 131I-MIBG | 131I-MIBG | 12 mCi/kg (11–16.5 mCi/kg) | Day -21 | |
| Melphalan | 140 mg/m2/day (d-7) 70 mg/m2/day (d-6) | Day -7, -6 | 210 mg/m2 | |
| Etoposide | 200 mg/m2/day | Day -8, -7, -6, -5 | 800 mg/m2 | |
| Carboplatin | 300 mg/m2/day | Day -8, -7, -6, -5 | 1200 mg/m2 | |
| Characteristics | MEC (n = 20) | mMEC + MIBG (n = 13) | p-Value | Total (n = 33) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 11 (55.0) | 9 (69.2) | 0.485 | 20 (60.6) |
| Female | 9 (45.0) | 4 (30.8) | 13 (39.4) | |
| Age, m, median (range) at diagnosis | 38 (4–129) | 43 (16–163) | 0.984 | 43 (4–163) |
| INSS stage, n (%) | ||||
| Stage 3 | 5 (25.0) | 2 (15.4) | 0.676 | 7 (21.2) |
| Stage 4 | 14 (70.0) | 11 (84.6) | 25 (75.8) | |
| Stage 4S (+MYCN amplification) | 1 (5.0) | 0 (0.0) | 1 (3.0) | |
| MYCN amplification, n (%) | 6 (30.0) | 3 (23.1) | 1.000 | 9 (29.0) |
| INPC, n (%) | ||||
| Unfavorable | 7 (35.0) | 9 (69.2) | 0.156 | 16 (48.5) |
| favorable | 7 (35.0) | 2 (15.4) | 9 (27.3) | |
| Unknown | 6 (30.0) | 2 (15.4) | 8 (24.2) | |
| 123I-MIBG avidity, n (%) | ||||
| Yes | 5 (25.0) | 11 (84.6) | 0.004 | 16 (48.5) |
| No | 1 (5.0) | 0 (0.0) | 1 (3.0) | |
| Unknown | 14 (70.0) | 2 (15.4) | 16 (48.5) | |
| Primary site, n (%) | ||||
| Retroperitoneum | 16 (80.0) | 13 (100.0) | 0.136 | 29 (87.9) |
| Others | 4 (20.0) | 0 (0.0) | 4 (12.1) | |
| Disease status before ASCT, n (%) | ||||
| CR | 3 (15.0) | 1 (7.7) | 1.000 | 4 (12.1) |
| PR | 17 (85.0) | 12 (92.3) | 29 (87.9) | |
| Local RTx, n (%) | 17 (85.0) | 9 (69.2) | 0.393 | 26 (78.8) |
| Second-look surgery, n (%) | 0 (0.0) | 8 (57.1) | <0.001 | 8 (23.5) |
| Maintenance therapy total, n (%) | 19 (95.0) | 11 (78.6) | 0.283 | 30 (90.9) |
| Salvage chemotherapy, n (%) | 3 (15.0) | 5 (38.5) | 0.522 | 8 (24.2) |
| ITT, n (%) | 2 (10) | 1 (7.7) | 1.000 | 3 (9.1) |
| IL-2 + ITT, n (%) | 14 (70.0) | 5 (38.5) | 0.073 | 19 (57.6) |
| Follow-up duration, m, median (range) | 114.0 (18–150) | 61.0 (13–83) | 0.001 | 76 (13–150) |
| Complication | 1st HDC/ASCT | 2nd HDC/ASCT | |||
|---|---|---|---|---|---|
| TTC (n = 33) | MEC (n = 20) | mMEC + 131I-MIBG (n = 13) | p-Value | Total (n = 33) | |
| TRM (%) | 1 (5.0) | 1 (7.7) | 1.00 | 2 (6.1) | |
| VOD (%) | 0 (0.0) | 1 (5.0) | 0 (0.0) | 1.00 | 1 (3.0) |
| TMA (%) | 0 (0.0) | 1 (5.0) | 1 (7.7) | 1.00 | 2 (6.1) |
| Acute toxicity, CTCAE Grade 3/4 | |||||
| Febrile neutropenia (%) | 33 (100) | 19 (95.0) | 13 (100) | 1.00 | 33 (97.0) |
| Pericardial effusion (%) | 0 (0.0) | 1 (5.0) | 0 (0.0) | 1.00 | 1 (3.0) |
| Diarrhea (%) | 13 (39.4) | 3 (15.0) | 1 (7.7) | 1.00 | 4 (12.1) |
| Vomiting (%) | 10 (30.3) | 3 (15.0) | 1 (7.7) | 1.00 | 4 (12.1) |
| Oral mucositis (%) | 22 (66.7) | 8 (40.0) | 7 (53.8) | 0.435 | 15 (45.5) |
| Total bilirubin (%) | 0 (0.0) | 1 (5.0) | 1 (7.7) | 1.00 | 2 (6.1) |
| LFT elevation (%) | 15 (45.5) | 19 (95.0) | 1 (7.7) | <0.001 | 20 (60.6) |
| AKI (%) | 0 (0.0) | 1 (5.0) | 1 (7.7) | 1.00 | 2 (6.1) |
| Creatinine (%) | 0 (0.0) | 2 (10.0) | 1 (7.7) | 1.00 | 3 (9.1) |
| Proteinuria (%) | 0 (0.0) | 3 (15.0) | 1 (7.7) | 1.00 | 4 (12.1) |
| Hematuria (%) | 2 (6.1) | 0 (0.0) | 1 (7.7) | 0.394 | 1 (3.0) |
| Long-term toxicity (n = 26) | (n = 17) | (n = 9) | (n = 26) | ||
| Secondary malignancy (%) | 4 (23.5) | 0 (0.0) | 0.263 | 4 (15.4) | |
| Hypothyroidism (%) | 2 (11.8) | 2 (22.2) | 0.591 | 4 (15.4) | |
| Growth failure (%) | 6 (35.3) | 2 (22.2) | 0.667 | 8 (30.8) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.J.; Choi, J.Y.; Kim, B.K.; Hong, K.T.; Kim, H.-Y.; Kim, I.H.; Cheon, G.J.; Cheon, J.-E.; Park, S.-H.; Kang, H.J. The Impact of 131I-Metaiodobenzylguanidine as a Conditioning Regimen of Tandem High-Dose Chemotherapy and Autologous Stem Cell Transplantation for High-Risk Neuroblastoma. Children 2023, 10, 1936. https://doi.org/10.3390/children10121936
Park HJ, Choi JY, Kim BK, Hong KT, Kim H-Y, Kim IH, Cheon GJ, Cheon J-E, Park S-H, Kang HJ. The Impact of 131I-Metaiodobenzylguanidine as a Conditioning Regimen of Tandem High-Dose Chemotherapy and Autologous Stem Cell Transplantation for High-Risk Neuroblastoma. Children. 2023; 10(12):1936. https://doi.org/10.3390/children10121936
Chicago/Turabian StylePark, Hyun Jin, Jung Yoon Choi, Bo Kyung Kim, Kyung Taek Hong, Hyun-Young Kim, Il Han Kim, Gi Jeong Cheon, Jung-Eun Cheon, Sung-Hye Park, and Hyoung Jin Kang. 2023. "The Impact of 131I-Metaiodobenzylguanidine as a Conditioning Regimen of Tandem High-Dose Chemotherapy and Autologous Stem Cell Transplantation for High-Risk Neuroblastoma" Children 10, no. 12: 1936. https://doi.org/10.3390/children10121936
APA StylePark, H. J., Choi, J. Y., Kim, B. K., Hong, K. T., Kim, H.-Y., Kim, I. H., Cheon, G. J., Cheon, J.-E., Park, S.-H., & Kang, H. J. (2023). The Impact of 131I-Metaiodobenzylguanidine as a Conditioning Regimen of Tandem High-Dose Chemotherapy and Autologous Stem Cell Transplantation for High-Risk Neuroblastoma. Children, 10(12), 1936. https://doi.org/10.3390/children10121936