Does Foveal Hypoplasia Affect Emmetropization in Patients with Albinism?
Abstract
1. Introduction
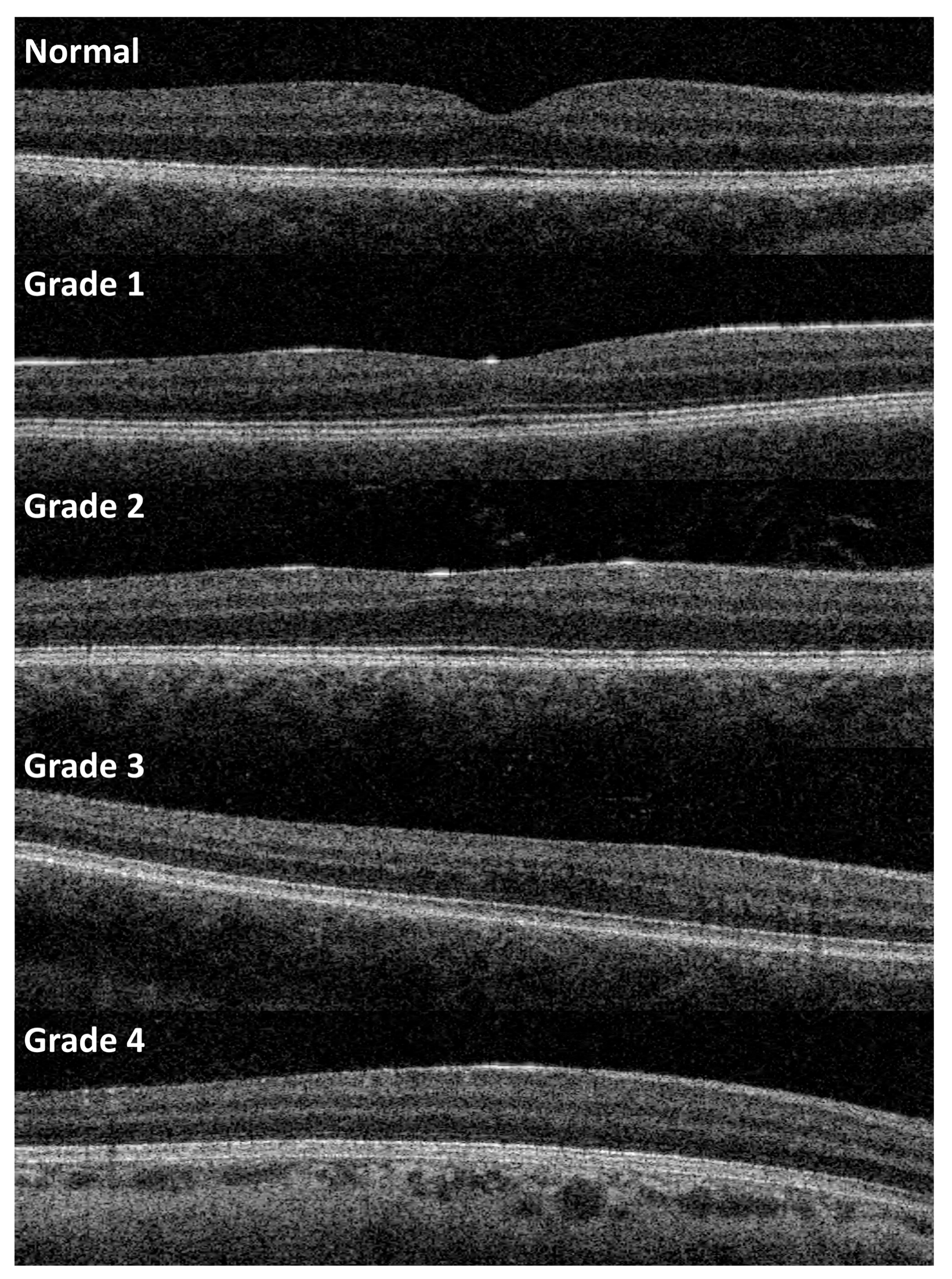
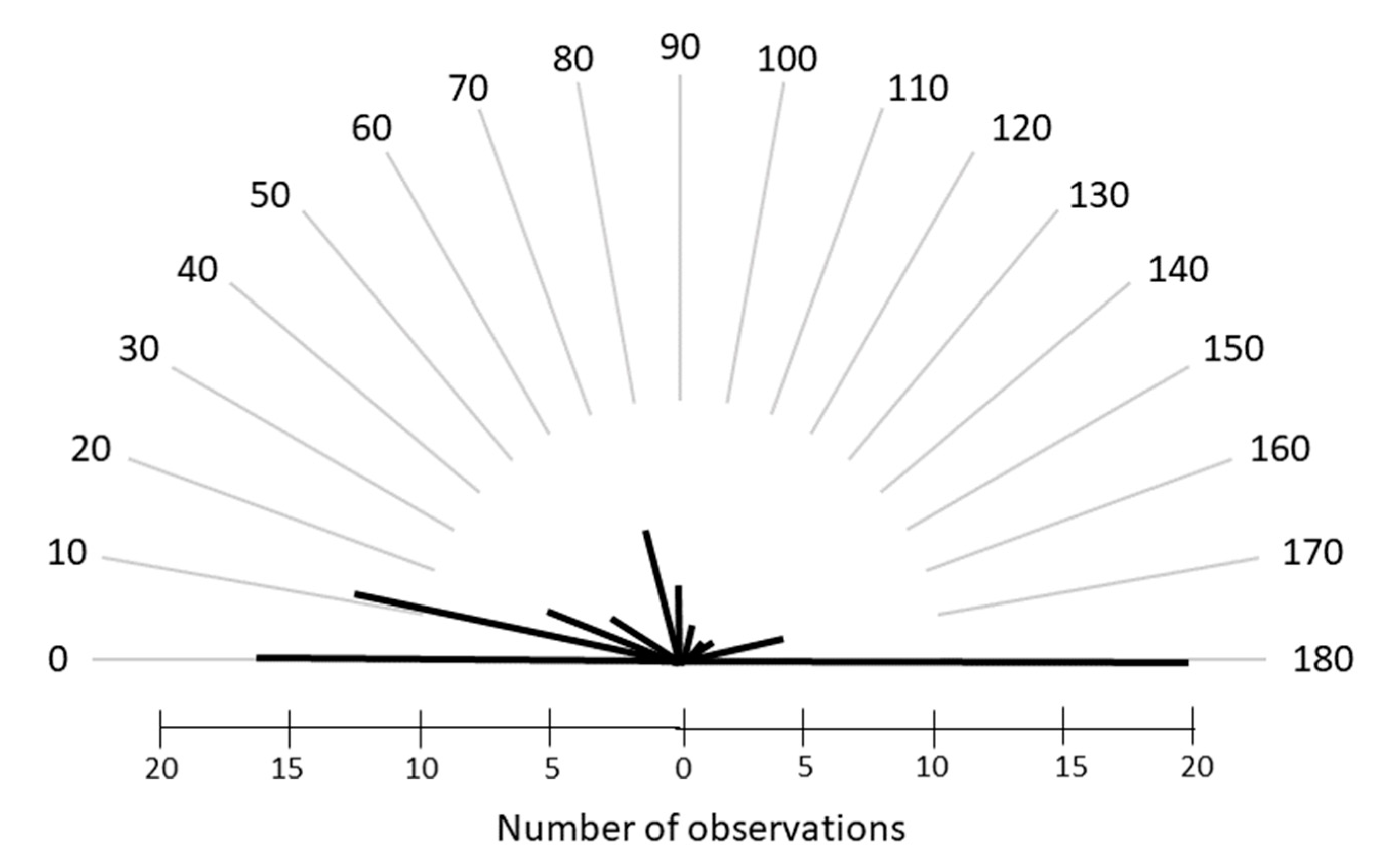
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ather, S.; Proudlock, F.A.; Welton, T.; Morgan, P.S.; Sheth, V.; Gottlob, I.; Dineen, R.A. Aberrant Visual Pathway Development in Albinism: From Retina to Cortex. Hum. Brain Mapp. 2019, 40, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Louis, S.J.; Fethiere, M.; Dure, D.; Rosen, J.; Morrison, B.W. The Prevalence of Nonmelanoma Skin Cancer in a Population of Patients with Oculocutaneous Albinism in Haiti. Int. J. Dermatol. 2022, 61, 867–871. [Google Scholar] [CrossRef]
- Aquaron, R. Oculocutaneous Albinism in Cameroon. A 15-Year Follow-up Study. Ophthalmic Paediatr. Genet. 1990, 11, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Rodríguez, M.C.; Reyes-Figueredo, L.S.; Velandia-Rodríguez, L.Á.; Méndez-Ruiz, O.D.; Gómez-Rodríguez, M.A.; Esguerra-Ochoa, L.T.; García-Lozada, D. Causes of Low Vision in Children: A Systematic Review. Arch. Soc. Esp. Oftalmol. 2023, 98, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Hvid, K.; Nissen, K.R.K.R.; Bayat, A.; Roos, L.; Grønskov, K.; Kessel, L. Prevalence and Causes of Infantile Nystagmus in a Large Population-Based Danish Cohort. Acta Ophthalmol. 2020, 98, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Wildsoet, C.F.; Oswald, P.J.; Clark, S. Albinism: Its Implications for Refractive Development. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1–7. [Google Scholar]
- Sayed, K.M.; Abdellah, M.M.; Kamel, A.G. Analysis of the Refractive Profile of Children with Oculocutaneous Albinism versus an Age-Matched Non-Albino Group. Clin. Ophthalmol. 2021, 15, 73–78. [Google Scholar] [CrossRef]
- Mayer, D.L.; Hansen, R.M.; Moore, B.D.; Kim, S.; Fulton, A.B. Cycloplegic Refractions in Healthy Children Aged 1 through 48 Months. Arch. Ophthalmol. 2001, 119, 1625–1628. [Google Scholar] [CrossRef]
- Troilo, D.; Smith, E.L.; Nickla, D.L.; Ashby, R.; Tkatchenko, A.V.; Ostrin, L.A.; Gawne, T.J.; Pardue, M.T.; Summers, J.A.; Kee, C.S.; et al. Imi—Report on Experimental Models of Emmetropization and Myopia. Investig. Ophthalmol. Vis. Sci. 2019, 60, M31–M88. [Google Scholar] [CrossRef]
- Brown, D.M.; Mazade, R.; Clarkson-Townsend, D.; Hogan, K.; Datta Roy, P.M.; Pardue, M.T. Candidate Pathways for Retina to Scleral Signaling in Refractive Eye Growth. Exp. Eye Res. 2022, 219, 109071. [Google Scholar] [CrossRef]
- Smith, E.L.; Ramamirtham, R.; Qiao-Grider, Y.; Hung, L.-F.; Huang, J.; Kee, C.; Coats, D.; Paysse, E. Effects of Foveal Ablation on Emmetropization and Form-Deprivation Myopia. Invest. Ophthalmol. Vis. Sci. 2007, 48, 3914–3922. [Google Scholar] [CrossRef] [PubMed]
- Healey, N.; McLoone, E.; Mahon, G.; Jackson, A.J.; Saunders, K.J.; McClelland, J.F. Investigating the Relationship between Foveal Morphology and Refractive Error in a Population with Infantile Nystagmus Syndrome. Invest. Ophthalmol. Vis. Sci. 2013, 54, 2934–2939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gottlob, I. Is Impaired Emmetropization Related to Foveal Hypoplasia or Is It Specific to Albinism? Investig. Ophthalmol. Vis. Sci. 2013, 54, 2940. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rymer, J.; Choh, V.; Bharadwaj, S.; Padmanabhan, V.; Modilevsky, L.; Jovanovich, E.; Yeh, B.; Zhang, Z.; Guan, H.; Payne, W.; et al. The Albino Chick as a Model for Studying Ocular Developmental Anomalies, Including Refractive Errors, Associated with Albinism. Exp. Eye Res. 2007, 85, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Long, K.; Schaeffel, F.; Zhang, S.; Zhou, X.; Lu, F.; Qu, J. Disruption of Emmetropization and High Susceptibility to Deprivation Myopia in Albino Guinea Pigs. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6124–6132. [Google Scholar] [CrossRef]
- Hansen, T.B.; Torner-Jordana, J.; Kessel, L. Photosensitivity and Filter Efficacy in Albinism. J. Optom. 2023, 16, 214–220. [Google Scholar] [CrossRef]
- Kessel, L.; Kjer, B.; Lei, U.; Duno, M.; Grønskov, K. Genotype-Phenotype Associations in Danish Patients with Ocular and Oculocutaneous Albinism. Ophthalmic Genet. 2021, 42, 230–238. [Google Scholar] [CrossRef]
- Thomas, M.G.; Kumar, A.; Mohammad, S.; Proudlock, F.A.; Engle, E.C.; Andrews, C.; Chan, W.-M.; Thomas, S.; Gottlob, I. Structural Grading of Foveal Hypoplasia Using Spectral-Domain Optical Coherence Tomography a Predictor of Visual Acuity? Ophthalmology 2011, 118, 1653–1660. [Google Scholar] [CrossRef]
- Fresina, M.; Benedetti, C.; Marinelli, F.; Versura, P.; Campos, E.C. Astigmatism in Patients with Idiopathic Congenital Nystagmus. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 1635–1639. [Google Scholar] [CrossRef]
- Wang, J.; Wyatt, L.M.; Felius, J.; Stager, D.R.; Stager, D.R.; Birch, E.E.; Bedell, H.E. Onset and Progression of With-the-Rule Astigmatism in Children with Infantile Nystagmus Syndrome. Investig. Ophthalmol. Vis. Sci. 2010, 51, 594–601. [Google Scholar] [CrossRef]
- Schweigert, A.; Lunos, S.; Connett, J.; Summers, C.G. Changes in Refractive Errors in Albinism: A Longitudinal Study over the First Decade of Life. J. AAPOS 2018, 22, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, A.R.; Kapetanakis, V.V.; Wathern, A.K.; Logan, N.S.; Gilmartin, B.; Whincup, P.H.; Cook, D.G.; Owen, C.G. Global Variations and Time Trends in the Prevalence of Childhood Myopia, a Systematic Review and Quantitative Meta-Analysis: Implications for Aetiology and Early Prevention. Br. J. Ophthalmol. 2016, 100, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Bullimore, M.A.; Ritchey, E.R.; Shah, S.; Leveziel, N.; Bourne, R.R.A.; Flitcroft, D.I. The Risks and Benefits of Myopia Control. Ophthalmology 2021, 128, 1561–1579. [Google Scholar] [CrossRef]
- Summers, J.A.; Schaeffel, F.; Marcos, S.; Wu, H.; Tkatchenko, A.V. Functional Integration of Eye Tissues and Refractive Eye Development: Mechanisms and Pathways. Exp. Eye Res. 2021, 209, 108693. [Google Scholar] [CrossRef]
- Irving, E.L.; Sivak, J.G.; Callender, M.G. Refractive Plasticity of the Developing Chick Eye: A Summary and Update. Ophthalmic Physiol. Opt. 2015, 35, 600–606. [Google Scholar] [CrossRef]
- Mutti, D.O.; Sinnott, L.T.; Lynn Mitchell, G.; Jordan, L.A.; Friedman, N.E.; Frane, S.L.; Lin, W.K. Ocular Component Development during Infancy and Early Childhood. Optom. Vis. Sci. 2018, 95, 976–985. [Google Scholar] [CrossRef]
- Larsen, J.S. The Sagittal Growth of the Eye. IV. Ultrasonic Measurement of the Axial Length of the Eye from Birth to Puberty. Acta Ophthalmol. 1971, 49, 873–886. [Google Scholar] [CrossRef]
- Verkicharla, P.; Thakur, S.; Kammari, P.; Dhakal, R.; Das, A.V. Refractive Development in Individuals with Ocular and Oculocutaneous Albinism. Int. Ophthalmol. 2022, 42, 2007–2015. [Google Scholar] [CrossRef]
- Rufai, S.R.; Thomas, M.G.; Purohit, R.; Bunce, C.; Lee, H.; Proudlock, F.A.; Gottlob, I. Can Structural Grading of Foveal Hypoplasia Predict Future Vision in Infantile Nystagmus?: A Longitudinal Study. Ophthalmology 2020, 127, 492–500. [Google Scholar] [CrossRef]
- Chakraborty, R.; Ostrin, L.A.; Benavente-Perez, A.; Verkicharla, P.K. Optical Mechanisms Regulating Emmetropisation and Refractive Errors: Evidence from Animal Models. Clin. Exp. Optom. 2020, 103, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Morgan, I.G.; Wu, P.C.; Ostrin, L.A.; Tideman, J.W.; Yam, J.C.; Lan, W.; Baraas, R.C.; He, X.; Sankaridurg, P.; Saw, S.M.; et al. IMI Risk Factors for Myopia. Investig. Ophthalmol. Vis. Sci. 2021, 62, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.K.G.; Kessel, L. Ametropia and Emmetropization in CNGB3 Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2021, 62, 10. [Google Scholar] [CrossRef] [PubMed]
- Kruijt, C.C.; de Wit, G.C.; Bergen, A.A.; Florijn, R.J.; Schalij-Delfos, N.E.; van Genderen, M.M. The Phenotypic Spectrum of Albinism. Ophthalmology 2018, 125, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Kuht, H.J.; Maconachie, G.D.E.; Han, J.; Kessel, L.; van Genderen, M.M.; McLean, R.J.; Hisaund, M.; Tu, Z.; Hertle, R.W.; Gronskov, K.; et al. Genotypic and Phenotypic Spectrum of Foveal Hypoplasia: A Multicenter Study. Ophthalmology 2022, 129, 708–718. [Google Scholar] [CrossRef]
- Grønskov, K.; Ek, J.; Sand, A.; Scheller, R.; Bygum, A.; Brixen, K.; Brondum-Nielsen, K.; Rosenberg, T. Birth Prevalence and Mutation Spectrum in Danish Patients with Autosomal Recessive Albinism. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, A.K.; Morse, C.L.; Hercinovic, A.; Cruz, O.A.; Sprunger, D.T.; Repka, M.X.; Lambert, S.R.; Wallace, D.K. American Academy of Ophthalmology Preferred Practice Pattern Pediatric Ophthalmology/Strabismus Panel Pediatric Eye Evaluations Preferred Practice Pattern. Ophthalmology 2023, 130, P222–P270. [Google Scholar] [CrossRef]
- Mutti, D.O. To Emmetropize or Not to Emmetropize? The Question for Hyperopic Development. Optom. Vis. Sci. 2007, 84, 97–102. [Google Scholar] [CrossRef]
- May, L.; Merrill, K.; Connett, J.E.; Summers, C.G. Does Early Glasses Wear Improve Visual Outcome in OCA1A? J. Binocul. Vis. Ocul. Motil. 2021, 71, 1–6. [Google Scholar] [CrossRef]


| 0–2 Years | 3–5 Years | 6–8 Years | 9–12 Years | 13–17 Years | ≥18 Years | |
|---|---|---|---|---|---|---|
| Spherical refraction (SER) | ||||||
| Grade 0–2 | 2.6 (1.5) [3] | 3.6 (1.3) [7] | 4.0 (1.5) [8] | 3.1 (149) [10] | 1.0 (4.4) [12] | 21.0(4.0) [18] |
| Grade 3 | 3.8 (4.0) [6] | 4.4 (2.9) [9] | 5.1 (2.2) [7] | 3.8 (2.7) [9] | 2.5 (3.0 [7] | 1.7 (1.7) [13] |
| Grade 4 | 4.6 (3.9) [21] | 4.1 (3.7) [37] | 4.2 (3.8) [38] | 3.7 (4.0) [40] | 3.9 (4.2) [35] | 3.5 (4.6) [45] |
| Cylinder refractive error | ||||||
| Grade 0–2 | −1.7 (1.4) [3] | −2.2 (1.2) [7] | −2.2 (1.0) [8] | −2.7 (1.5) [10] | −2.7 (1.5) [12] | −2.7 (1.7) [18] |
| Grade 3 | −1.7 (1.8) [6] | −1.4 (1.5) [9] | −2.0 (1.4) [7] | −3.0 (2.0) [9] | −2.4 (1.6) [7] | −2.4 (1.9) [13] |
| Grade 4 | −2.0 (0.8) [21] | −2.1 (1.2) [37] | −2.4 (1.1) [38] | −2.2 (1.6) [40] | −2.7 (1.2) [35] | −2.6 (1.3) [45] |
| Spherical equivalent refractive error (SEQ) | ||||||
| Grade 0–2 | 1.8 (1.4) [3] | 2.6 (1.3) [7] | 2.9(1.4) [8] | 1.8 (1.6) [10] | 1.8 (1.6) [12] | −0.3 (3.8) [18] |
| Grade 3 | 3.0 (3.9) [6] | 3.7 (1.7) [9] | 4.0 (1.8) [7] | 2.3 (2.7) [9] | 1.3 (3.1) [7] | 0.5 (1.2) [13] |
| Grade 4 | 3.6 (3.9) [21] | 3.0 (3.8) [37] | 3.05 (4.0) [38] | 2.6 (41) [40] | 2.6 (4.2) [35] | 3.9 (3.7) [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kessel, L.; Kjølholm, C.D.B.; Jordana, J.T. Does Foveal Hypoplasia Affect Emmetropization in Patients with Albinism? Children 2023, 10, 1910. https://doi.org/10.3390/children10121910
Kessel L, Kjølholm CDB, Jordana JT. Does Foveal Hypoplasia Affect Emmetropization in Patients with Albinism? Children. 2023; 10(12):1910. https://doi.org/10.3390/children10121910
Chicago/Turabian StyleKessel, Line, Christine Dahlgren Bohnsack Kjølholm, and Joaquim Torner Jordana. 2023. "Does Foveal Hypoplasia Affect Emmetropization in Patients with Albinism?" Children 10, no. 12: 1910. https://doi.org/10.3390/children10121910
APA StyleKessel, L., Kjølholm, C. D. B., & Jordana, J. T. (2023). Does Foveal Hypoplasia Affect Emmetropization in Patients with Albinism? Children, 10(12), 1910. https://doi.org/10.3390/children10121910







