Long-Term Effects of Child Early Surgical Ventricular Septal Defect Repair on Maternal Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedure
2.4. Measures
2.5. Statistical Analyses
3. Results
3.1. Stress Measures Intercorrelations and t1–t2 Stability
3.2. Long-Term Effects of Child Early Surgical VSD Repair on Maternal Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouma, B.J.; Mulder, B.J. Changing landscape of congenital heart disease. Circ. Res. 2017, 120, 908–922. [Google Scholar] [CrossRef]
- Zomer, A.C.; Vaartjes, I.; Grobbee, D.E.; Mulder, B.J.M. Adult congenital heart disease: New challenges. Int. J. Cardiol. 2013, 163, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Jortveit, J.; Leirgul, E.; Eskedal, L.; Greve, G.; Fomina, T.; Døhlen, G.; Tell, G.S.; Birkeland, S.; Øyen, N.; Holmstrøm, H. Mortality and complications in 3495 children with isolated ventricular septal defects. Arch. Dis. Child. 2016, 101, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Minette, M.S.; Sahn, D.J. Ventricular septal defects. Circ. Res. 2006, 114, 2190–2197. [Google Scholar] [CrossRef]
- Marino, B.S.; Lipkin, P.H.; Newburger, J.W.; Peacock, G.; Gerdes, M.; Gaynor, J.W.; Mussatto, K.A.; Uzark, K.; Goldberg, C.S.; Johnson, W.H.; et al. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management a scientific statement from the american heart association. Circ. Res. 2012, 126, 1143–1172. [Google Scholar] [CrossRef]
- Scully, B.B.; Morales, D.L.S.; Zafar, F.; McKenzie, E.D.; Fraser, C.D.; Heinle, J.S. Current Expectations for Surgical Repair of Isolated Ventricular Septal Defects. Ann. Thorac. Surg. 2010, 89, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Eichler, A.; Köhler-Jonas, N.; Stonawski, V.; Purbojo, A.; Moll, G.H.; Heinrich, H.; Cesnjevar, R.A.; Kratz, O. Child neurodevelopment and mental health after surgical ventricular septal defect repair: Risk and protective factors. Dev. Med. Child Neurol. 2019, 61, 152–160. [Google Scholar] [CrossRef]
- Lang, L.; Gerlach, J.; Plank, A.C.; Purbojo, A.; Cesnjevar, R.A.; Kratz, O.; Moll, G.H.; Eichler, A. Becoming a Teenager after Early Surgical Ventricular Septal Defect (VSD) Repair: Longitudinal Biopsychological Data on Mental Health and Maternal Involvement. J. Clin. Med. 2022, 11, 7242. [Google Scholar] [CrossRef]
- Ernst, M.M.; Marino, B.S.; Cassedy, A.; Piazza-Waggoner, C.; Franklin, R.C.; Brown, K.; Wray, J. Biopsychosocial predictors of quality of life outcomes in pediatric congenital heart disease. Pediatr. Cardiol. 2018, 39, 79–88. [Google Scholar] [CrossRef]
- Jaschinski, C.; Knetsch, V.; Parzer, P.; Meyr, J.; Schroeder, B.; Fonseca, E.; Karck, M.; Kaess, M.; Loukanov, T. Psychosocial impact of congenital heart diseases on patients and their families: A parent’s perspective. World J. Pediatr. Congenit. Heart Surg. 2022, 13, 9–15. [Google Scholar] [CrossRef]
- Ben-Amitay, G.; Kosov, I.; Reiss, A.; Toren, P.; Yoran-Hegesh, R.; Kotler, M.; Mozes, T. Is elective surgery traumatic for children and their parents? J. Paediatr. Child Health 2006, 42, 618–624. [Google Scholar] [CrossRef]
- Rattaz, V.; Puglisi, N.; Tissot, H.; Favez, N. Associations between parent–infant interactions, cortisol and vagal regulation in infants, and socioemotional outcomes: A systematic review. Infant Behav. Dev. 2022, 67, 101687. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.D.; Kazazian, V.; Ford, M.K.; Marini, D.; Miller, S.P.; Chau, V.; Seed, M.; Ly, L.G.; Williams, T.S.; Sananes, R. The association between parent stress, coping and mental health, and neurodevelopmental outcomes of infants with congenital heart disease. Clin. Neuropsychol. 2021, 35, 948–972. [Google Scholar] [CrossRef] [PubMed]
- Kolaitis, G.A.; Meentken, M.G.; Utens, E.M.W.J. Mental health problems in parents of children with congenital heart disease. Front. Pediatr. 2017, 5, 102. [Google Scholar] [CrossRef]
- Lisanti, A.J. Parental stress and resilience in CHD: A new frontier for health disparities research. Cardiol. Young 2018, 28, 1142–1150. [Google Scholar] [CrossRef]
- Tesson, S.; Butow, P.N.; Marshall, K.; Fonagy, P.; Kasparian, N.A. Parent-child bonding and attachment during pregnancy and early childhood following congenital heart disease diagnosis. Health Psychol. Rev. 2022, 16, 378–411. [Google Scholar] [CrossRef]
- McWhorter, L.G.; Christofferson, J.; Neely, T.; Hildenbrand, A.K.; Alderfer, M.A.; Randall, A.; Kazak, A.E.; Sood, E. Parental post-traumatic stress, overprotective parenting, and emotional and behavioural problems for children with critical congenital heart disease. Cardiol. Young 2022, 32, 738–745. [Google Scholar] [CrossRef]
- Gerlach, J.; Fößel, J.M.; Vierhaus, M.; Sann, A.; Eickhorst, A.; Zimmermann, P.; Spangler, G. Family risk and early attachment development: The differential role of parental sensitivity. Infant Ment. Health J. 2022, 43, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Mangin-Heimos, K.S.; Strube, M.; Taylor, K.; Galbraith, K.; O’Brien, E.; Rogers, C.; Lee, C.K.; Ortinau, C. Trajectories of Maternal and Paternal Psychological Distress After Fetal Diagnosis of Moderate–Severe Congenital Heart Disease. J. Pediatr. Psychol. 2022, 48, 305–316. [Google Scholar] [CrossRef]
- Clancy, T.; Jordan, B.; de Weerth, C.; Muscara, F. Early Emotional, Behavioural and Social Development of Infants and Young Children with Congenital Heart Disease: A Systematic Review. J. Clin. Psychol. Med. Settings 2020, 27, 686–703. [Google Scholar] [CrossRef]
- Woolf-King, S.E.; Arnold, E.; Weiss, S.; Teitel, D. “There’s no acknowledgement of what this does to people”: A qualitative exploration of mental health among parents of children with critical congenital heart defects. J. Clin. Nurs. 2018, 27, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Soulvie, M.A.; Desai, P.P.; White, C.P.; Sullivan, B.N. Psychological Distress Experienced by Parents of Young Children With Congenital Heart Defects: A Comprehensive Review of Literature. J. Soc. Serv. Res. 2012, 38, 484–502. [Google Scholar] [CrossRef]
- Carminati, M.; Butera, G.; Chessa, M.; De Giovanni, J.; Fisher, G.; Gewillig, M.; Peuster, M.; Piechaud, J.F.; Santoro, G.; Sievert, H.; et al. Transcatheter closure of congenital ventricular septal defects: Results of the European Registry. Eur. Heart J. 2007, 28, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Stonawski, V.; Vollmer, L.; Köhler-Jonas, N.; Rohleder, N.; Golub, Y.; Purbojo, A.; Moll, G.H.; Heinrich, H.; Cesnjevar, R.A.; Kratz, O.; et al. Long-term associations of an early corrected Ventricular septal defect and stress systems of child and mother at primary school age. Front. Pediatr. 2018, 5, 293. [Google Scholar] [CrossRef]
- Woolf-King, S.E.; Anger, A.; Arnold, E.A.; Weiss, S.J.; Teitel, D. Mental health among parents of children with critical Congenital Heart Defects: A systematic review. J. Am. Heart Assoc. 2017, 6, e004862. [Google Scholar] [CrossRef]
- Cohen, S.; Murphy, M.L.M.; Prather, A.A. Ten surprising facts about stressful life events and disease risk. Annu. Rev. Psychol. 2019, 70, 577–597. [Google Scholar] [CrossRef]
- Connor, J.A.; Kline, N.E.; Mott, S.; Harris, S.K.; Jenkins, K.J. The meaning of cost for families of children with congenital heart disease. J. Pediatr. Health Care 2010, 24, 318–325. [Google Scholar] [CrossRef]
- Watkins, S.; Isichei, O.; Gentles, T.L.; Brown, R.; Percival, T.; Sadler, L.; Gorinski, R.; Crengle, S.; Cloete, E.; de Laat, M.W.M.; et al. What is known about critical Congenital Heart Disease diagnosis and management experiences from the perspectives of family and healthcare providers? A systematic integrative literature review. Pediatr. Cardiol. 2022, 44, 280–296. [Google Scholar] [CrossRef]
- Potterton, J.; Ntsiea, V.; Brown, S.; Smith, R. Effect of cardiac surgery in young children with congenital heart disease on parenting stress in central South Africa: Initial outcomes. SA Heart 2017, 14, 162–169. [Google Scholar]
- Lloret, F.A.; Domínguez, S.G.; Merino, V.F.; Ferreiro, C.R.; Soto, A.M. Perioperative stress and anxiety in parents of children operated on for congenital heart disease. Enfermería Intensiv. (Engl. Ed.) 2023, in press. [Google Scholar] [CrossRef]
- Brosig, C.L.; Mussatto, K.; Kuhn, E.; Tweddell, J. Psychosocial outcomes for preschool children and families after surgery for complex congenital heart disease. Pediatr. Cardiol. 2007, 28, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wray, J.; Sensky, T. Psychological functioning in parents of children undergoing elective cardiac surgery. Cardiol. Young 2004, 14, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Menahem, S.; Poulakis, Z.; Prior, M. Children subjected to cardiac surgery for congenital heart disease. Part 2–parental emotional experiences. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Spijkerboer, A.W.; Helbing, W.A.; Bogers, A.J.; Van Domburg, R.T.; Verhulst, F.C.; Utens, E.M. Long-term psychological distress, and styles of coping, in parents of children and adolescents who underwent invasive treatment for congenital cardiac disease. Cardiol. Young 2007, 17, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Vrijmoet-Wiersma, C.J.; Ottenkamp, J.; van Roozendaal, M.; Grootenhuis, M.A.; Koopman, H.M. A multicentric study of disease-related stress, and perceived vulnerability, in parents of children with congenital cardiac disease. Cardiol. Young 2009, 19, 608–614. [Google Scholar] [CrossRef]
- Doherty, N.; McCusker, C.G.; Molloy, B.; Mulholland, C.; Rooney, N.; Craig, B.; Sands, A.; Stewart, M.; Casey, F. Predictors of psychological functioning in mothers and fathers of infants born with severe congenital heart disease. J. Reprod. Infant Psychol. 2009, 27, 390–400. [Google Scholar] [CrossRef]
- Lawoko, S.; Soares, J.J. Psychosocial morbidity among parents of children with congenital heart disease: A prospective longitudinal study. Heart Lung 2006, 35, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, M.R.; Smith, D.M.; Wittkowski, A. Coping in parents of children with congenital heart disease: A systematic review and meta-synthesis. J. Child Fam. Stud. 2019, 28, 1736–1753. [Google Scholar] [CrossRef]
- Wray, J.; Cassedy, A.; Ernst, M.M.; Franklin, R.C.; Brown, K.; Marino, B.S. Psychosocial functioning of parents of children with heart disease—Describing the landscape. Eur. J. Pediatr. 2018, 177, 1811–1821. [Google Scholar] [CrossRef]
- Franck, L.S.; Mcquillan, A.; Wray, J.; Grocott, M.P.; Goldman, A. Parent stress levels during children’s hospital recovery after congenital heart surgery. Pediatr. Cardiol. 2010, 31, 961–968. [Google Scholar] [CrossRef]
- O’Connor, D.B.; Thayer, J.F.; Vedhara, K. Stress and health: A review of psychobiological processes. Annu. Rev. Psychol. 2021, 72, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Chrousos, G.P. The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Mol. Psychiatry 2022, 27, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N. Stress and inflammation–The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 2019, 105, 164–171. [Google Scholar] [CrossRef]
- Dhabhar, F.S. The short-term stress response–Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocrinol. 2018, 49, 175–192. [Google Scholar] [CrossRef]
- Association, A.P. Stress Effects on the Body. 2018. Available online: https://www.apa.org/topics/stress/body (accessed on 28 September 2023).
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Renna, M.E.; Shrout, M.R.; Madison, A.A. Stress reactivity: What pushes us higher, faster, and longer—And why it matters. Curr. Dir. Psychol. Sci. 2020, 29, 492–498. [Google Scholar] [CrossRef]
- Gan, E.-H.; Quinton, R. Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones. Prog. Brain Res. 2010, 181, 111–126. [Google Scholar] [PubMed]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015, 48, 209. [Google Scholar] [CrossRef]
- Naughton, M.; Dinan, T.G.; Scott, L.V. Corticotropin-releasing hormone and the hypothalamic–pituitary–adrenal axis in psychiatric disease. Handb. Clin. Neurol. 2014, 124, 69–91. [Google Scholar]
- Ottaviani, C.; Thayer, J.F.; Verkuil, B.; Lonigro, A.; Medea, B.; Couyoumdjian, A.; Brosschot, J.F. Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychol. Bull. 2016, 142, 231. [Google Scholar] [CrossRef]
- Crosswell, A.D.; Lockwood, K.G. Best practices for stress measurement: How to measure psychological stress in health research. Health Psychol. Open 2020, 7, 2055102920933072. [Google Scholar] [CrossRef]
- Vives, A.H.; De Angel, V.; Papadopoulos, A.; Strawbridge, R.; Wise, T.; Young, A.; Arnone, D.; Cleare, A. The relationship between cortisol, stress and psychiatric illness: New insights using hair analysis. J. Psychiatr. Res. 2015, 70, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Raul, J.-S.; Cirimele, V.; Ludes, B.; Kintz, P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin. Biochem. 2004, 37, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Wennig, R. Potential problems with the interpretation of hair analysis results. Forensic Sci. Int. 2000, 107, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Steudte-Schmiedgen, S.; Alexander, N.; Klucken, T.; Vater, A.; Wichmann, S.; Kirschbaum, C.; Miller, R. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology 2017, 77, 261–274. [Google Scholar] [CrossRef]
- Malisiova, E.K.; Mourikis, I.; Darviri, C.; Nicolaides, N.C.; Zervas, I.M.; Papageorgiou, C.; Chrousos, G.P. Hair cortisol concentrations in mental disorders: A systematic review. Physiol. Behav. 2021, 229, 113244. [Google Scholar] [CrossRef]
- Caparros-Gonzalez, R.A.; Lynn, F.; Alderdice, F.; Peralta-Ramirez, M.I. Cortisol levels versus self-report stress measures during pregnancy as predictors of adverse infant outcomes: A systematic review. Stress 2022, 25, 189–212. [Google Scholar] [CrossRef] [PubMed]
- van Holland, B.J.; Frings-Dresen, M.H.; Sluiter, J.K. Measuring short-term and long-term physiological stress effects by cortisol reactivity in saliva and hair. Int. Arch. Occup. Environ. Health 2012, 85, 849–852. [Google Scholar] [CrossRef]
- Walker, F.R.; Pfingst, K.; Carnevali, L.; Sgoifo, A.; Nalivaiko, E. In the search for integrative biomarker of resilience to psychological stress. Neurosci. Biobehav. Rev. 2017, 74, 310–320. [Google Scholar] [CrossRef]
- Eichler, A.; Grunitz, J.; Grimm, J.; Walz, L.; Raabe, E.; Goecke, T.W.; Beckmann, M.W.; Kratz, O.; Heinrich, H.; Moll, G.H. Did you drink alcohol during pregnancy? Inaccuracy and discontinuity of women’s self-reports: On the way to establish meconium ethyl glucuronide (EtG) as a biomarker for alcohol consumption during pregnancy. Alcohol 2016, 54, 39–44. [Google Scholar] [CrossRef]
- Franke, G.H. Brief Symptom Inventory (BSI) von LR Derogatis:(Kurzform der SCL-90-R); Beltz Test: Göttingen, Germany, 2000. [Google Scholar]
- Jäkel, J.; Leyendecker, B. ESI-Everyday Stressors Index-Deutsche Fassung; Leibniz-Institut für Psychologie (ZPID): Trier, Germany, 2009. [Google Scholar]
- Tröster, H. Eltern-Belastungs-Inventar: EBI; Deutsche Version des Parenting Stress Index (PSI) von RR Abidin; Hogrefe: Göttingen, Germany, 2011. [Google Scholar]
- Derogatis, L.R.; Rickels, K.; Rock, A.F. The SCL-90 and the MMPI: A step in the validation of a new self-report scale. Br. J. Psychiatry 1976, 128, 280–289. [Google Scholar] [CrossRef]
- Hall, L.A. Social Supports, Everyday Stressors, and Maternal Mental Health; The University of North Carolina at Chapel Hill: Chapel Hill, NC, USA, 1983. [Google Scholar]
- Kanner, A.D.; Coyne, J.C.; Schaefer, C.; Lazarus, R.S. Comparison of two modes of stress measurement: Daily hassles and uplifts versus major life events. J. Behav. Med. 1981, 4, 1–39. [Google Scholar] [CrossRef]
- Grimm, J.; Stemmler, M.; Golub, Y.; Schwenke, E.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W.; Kratz, O.; Moll, G.H.; Kornhuber, J. The association between prenatal alcohol consumption and preschool child stress system disturbance. Dev. Psychobiol. 2021, 63, 687–697. [Google Scholar] [CrossRef]
- Frisch, N.; Eichler, A.; Plank, A.-C.; Golub, Y.; Moll, G.H.; Kratz, O. Exploring reference values for hair cortisol: Hair weight versus hair protein. Ther. Drug Monit. 2020. [Google Scholar] [CrossRef]
- Janson, J.; Rohleder, N. Distraction coping predicts better cortisol recovery after acute psychosocial stress. Biol. Psychol. 2017, 128, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Golub, Y.; Kuitunen-Paul, S.; Panaseth, K.; Stonawski, V.; Frey, S.; Steigleder, R.; Grimm, J.; Goecke, T.W.; Fasching, P.A.; Beckmann, M.W. Salivary and hair cortisol as biomarkers of emotional and behavioral symptoms in 6–9 year old children. Physiol. Behav. 2019, 209, 112584. [Google Scholar] [CrossRef] [PubMed]
- Bortz, J.; Schuster, C. Statistik für Human-und Sozialwissenschaftler: Limitierte Sonderausgabe; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Lawrence Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Park, C.L.; Cohen, L.H.; Murch, R.L. Assessment and prediction of stress-related growth. J. Personal. 1996, 64, 71–105. [Google Scholar] [CrossRef]
- Park, C.L.; Fenster, J.R. Stress-related growth: Predictors of occurrence and correlates with psychological adjustment. J. Soc. Clin. Psychol. 2004, 23, 195–215. [Google Scholar] [CrossRef]
- O’Toole, S.; Suarez, C.; Adair, P.; McAleese, A.; Willis, S.; McCormack, D. A systematic review of the factors associated with post-traumatic growth in parents following admission of their child to the intensive care unit. J. Clin. Psychol. Med. Settings 2022, 29, 509–537. [Google Scholar] [CrossRef]
- Golfenshtein, N.; Srulovici, E.; Medoff-Cooper, B. Investigating parenting stress across pediatric health conditions-a systematic review. Compr. Child Adolesc. Nurs. 2016, 39, 41–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-L.; Xu, N.; Huang, S.-T.; Lin, Z.-W.; Cao, H.; Chen, Q. WeChat-assisted health education and preoperative care improve the mental state of parents of children with ventricular septal defect. Psychol. Health Med. 2022, 27, 948–955. [Google Scholar] [CrossRef]
- Diener, E.; Pressman, S.D.; Hunter, J.; Delgadillo-Chase, D. If, why, and when subjective well-being influences health, and future needed research. Appl. Psychol. Health Well-Being 2017, 9, 133–167. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.E.; Enlow, M.B.; Plamondon, A.; Lyons-Ruth, K. The association between adversity and hair cortisol levels in humans: A meta-analysis. Psychoneuroendocrinology 2019, 103, 104–117. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.V.C. The Association between Hair Cortisol Concentrations and Mental Disorders in Adults with Traumatic Experiences: A Systematic Review; Universidade do Porto: Porto, Portugal, 2022. [Google Scholar]
- Staufenbiel, S.M.; Penninx, B.W.; de Rijke, Y.B.; van den Akker, E.L.; van Rossum, E.F. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology 2015, 60, 182–194. [Google Scholar] [CrossRef]
- Edwards, L.D.; Heyman, A.H.; Swidan, S. Hypocortisolism: An evidence-based review. Integr. Med. 2011, 10, 30–37. [Google Scholar]
- Fries, E.; Hesse, J.; Hellhammer, J.; Hellhammer, D.H. A new view on hypocortisolism. Psychoneuroendocrinology 2005, 30, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Sierau, S.; Glaesmer, H.; Klucken, T.; Stalder, T. Hair cortisol, lifetime traumatic experiences and psychopathology in unaccompanied refugee minors. Psychoneuroendocrinology 2019, 104, 191–194. [Google Scholar] [CrossRef]
- Yılmaz, A.A.; Üstündağ, M.F.; Yavuz, Y.; Şavluk, Ö.F.; Ceyran, H. Investigation of Anxiety, Depression and Perceived Caregiving Burden in Parents of Pediatric Patients Undergoing Open Heart Surgery and Being Followed Up in Intensive Care. Koşuyolu Heart J. 2021, 24, 40–46. [Google Scholar] [CrossRef]

| VSD (n = 24) | Controls (n = 24) | VSD vs. Controls | ||||
|---|---|---|---|---|---|---|
| Sociodemographic Characteristics | M (SD)/N (f(%)) | M (SD)/N (f(%)) | t/χ2 | p | d/φ | |
| Mother age at t1 | 36.82 (5.90) | 39.44 (4.64) | 1.68 | 0.10 | 0.50 | |
| Mother age at t2 | 41.89 (5.61) | 45.21 (4.70) | 2.15 | 0.04 * | 0.64 | |
| Child age at t1 | 7.31 (1.10) | 7.21 (0.69) | 0.41 | 0.69 | 0.12 | |
| Child age at t2 | 12.40 (0.93) | 13.19 (0.24) | 4.04 | <0.00 * | 1.17 | |
| Child gender | Female | 13 (54.2%) | 14 (58.3%) | 0.09 | 0.77 | 0.04 |
| Male | 11 (45.8%) | 10 (41.7%) | ||||
| Migration background | 2 (8.3%) | 2 (8.3%) | 0.00 | 1.00 | 0.00 | |
| SES | 11.08 (2.56) | 11.63 (2.50) | 0.74 | 0.46 | 0.21 | |
| Number children <18 y in the household | 1 | 5 (20.8%) | 5 (20.8%) | 0.51 | 0.62 | 0.15 |
| 2 | 12 (50.0%) | 14 (58.3%) | ||||
| 3 | 3 (12.5%) | 5 (20.8%) | ||||
| - a | 4 (16.7%) | - | ||||
| Mother working hours/week | 26.09 (11.58) | 26.23 (9.06) | 0.04 | 0.97 | 0.01 | |
| Mother mental health (self-report) | ||||||
| Psychiatric diagnosis | 4 (16.7%) | 3 (12.5%) | 0.17 | 0.68 | 0.06 | |
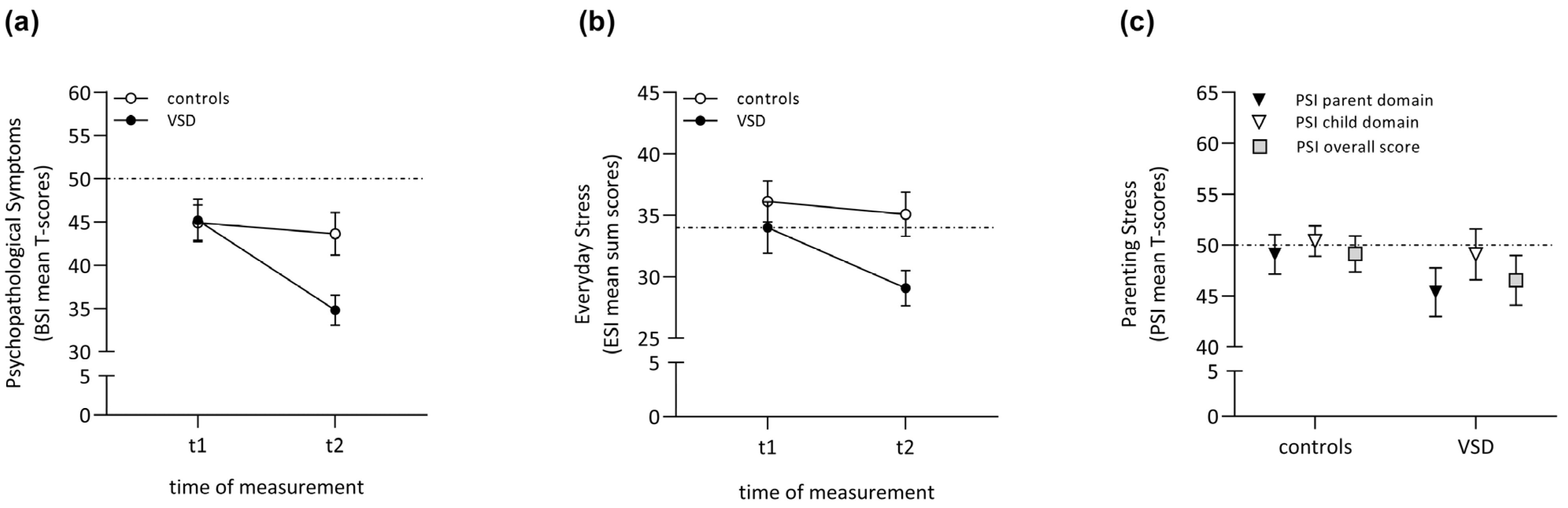
| Psychopathology t1 | 45.2 (11.8) | 44.9 (10.0) | 0.08 | 0.94 | 0.02 | |
| Psychopathology t2 | 34.8 (8.5) | 43.6 (12.0) | 2.93 | <0.00 * | 0.85 | |
| Everyday Stress t1 | 34.0 (10.0) | 36.1 (8.2) | 0.80 | 0.43 | 0.23 | |
| Everyday Stress t2 | 29.1 (7.0) | 35.1 (8.8) | 2.61 | 0.01 * | 0.75 | |
| Parenting Stress | Parent Child Overall | 45.4 (11.7) 49.1 (12.2) 46.5 (12.0) | 49.1 (9.5) 50.4 (7.3) 49.1 (8.6) | 0.86 1.21 0.45 | 0.40 0.23 0.66 | 0.25 0.35 0.13 |
| 1. | 2. | 3. | 4. | 5. | 6. | |
|---|---|---|---|---|---|---|
| Maternal self-reported psychological stress | ||||||
| 1. Psychopathology t1 | ||||||
| 2. Psychopathology t2 | 0.45 ** | |||||
| 3. Everyday stress t1 | 0.48 *** | <0.00 | ||||
| 4. Everyday stress t2 | 0.23 | 0.50 *** | 0.40 ** | |||
| 5. Parenting stress t2 | 0.39 ** | 0.54 *** | 0.30 * | 0.36 * | ||
| Maternal biophysiological stress | ||||||
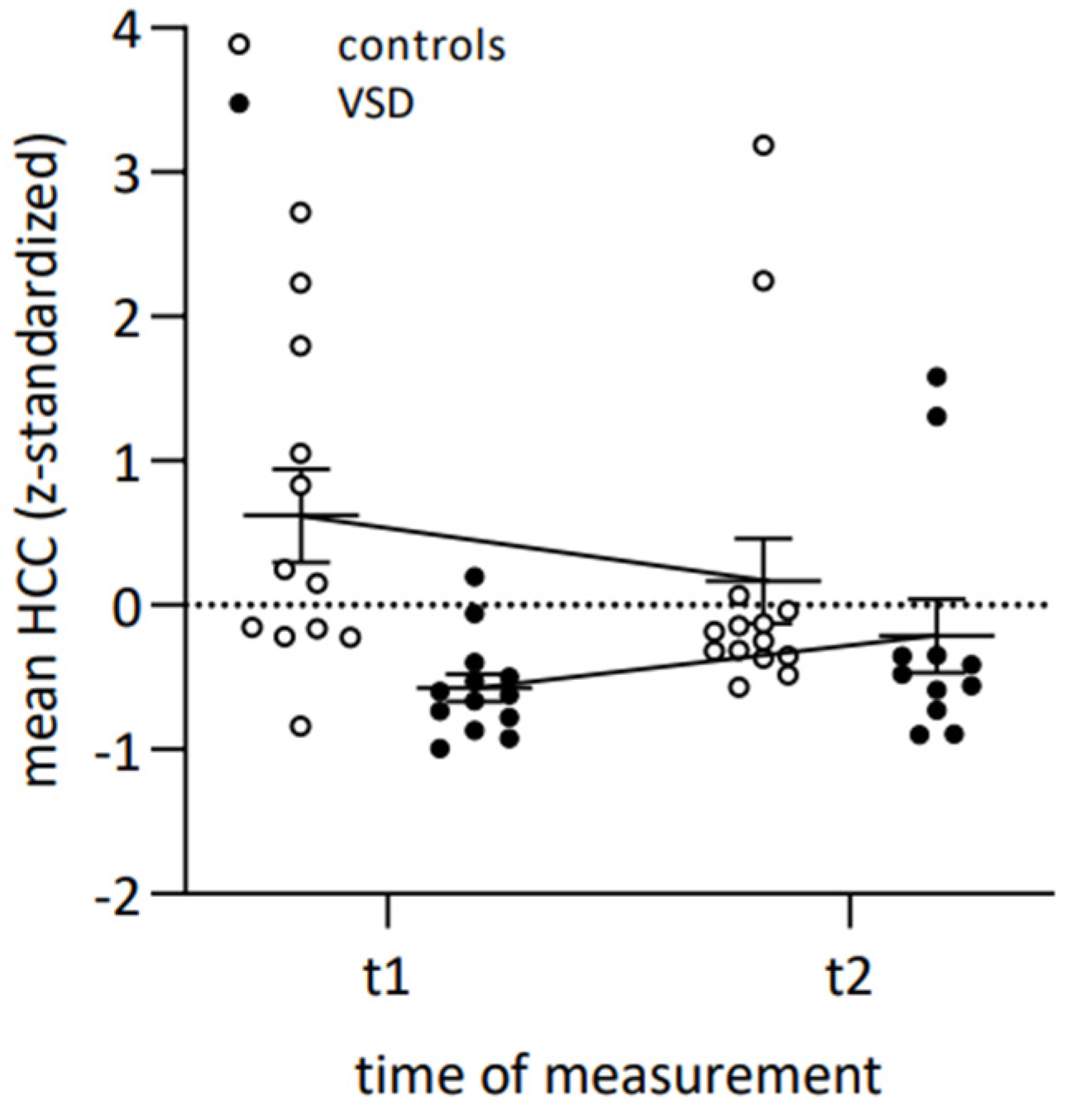
| 6. HCC t1 | −0.05 | 0.32 | <0.00 | 0.04 | −0.01 | |
| 7. HCC t2 | 0.22 | 0.14 | 0.31 | 0.04 | −0.01 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerlach, J.; Decker, E.S.; Plank, A.-C.; Mestermann, S.; Purbojo, A.; Cesnjevar, R.A.; Kratz, O.; Eichler, A. Long-Term Effects of Child Early Surgical Ventricular Septal Defect Repair on Maternal Stress. Children 2023, 10, 1832. https://doi.org/10.3390/children10121832
Gerlach J, Decker ES, Plank A-C, Mestermann S, Purbojo A, Cesnjevar RA, Kratz O, Eichler A. Long-Term Effects of Child Early Surgical Ventricular Septal Defect Repair on Maternal Stress. Children. 2023; 10(12):1832. https://doi.org/10.3390/children10121832
Chicago/Turabian StyleGerlach, Jennifer, Elena S. Decker, Anne-Christine Plank, Stefan Mestermann, Ariawan Purbojo, Robert A. Cesnjevar, Oliver Kratz, and Anna Eichler. 2023. "Long-Term Effects of Child Early Surgical Ventricular Septal Defect Repair on Maternal Stress" Children 10, no. 12: 1832. https://doi.org/10.3390/children10121832
APA StyleGerlach, J., Decker, E. S., Plank, A.-C., Mestermann, S., Purbojo, A., Cesnjevar, R. A., Kratz, O., & Eichler, A. (2023). Long-Term Effects of Child Early Surgical Ventricular Septal Defect Repair on Maternal Stress. Children, 10(12), 1832. https://doi.org/10.3390/children10121832






