Advances in the Diagnosis and Treatment of Enthesitis-Related Arthritis
Abstract
1. Introduction
2. Methods
3. Historic Evolution of ERA Definition and Classification
| Nomenclature | Enthesitis-Related Arthritis by ILAR Criteria | Enthesitis/Spondylitis-Related JIA by Provisional PRINTO Criteria |
|---|---|---|
| Inclusion criteria |
|
|
| Exclusion criteria |
| None |
4. Epidemiology
5. Clinical Spectrum of ERA
5.1. Arthritis
5.1.1. Peripheral Arthritis
5.1.2. Axial Involvement
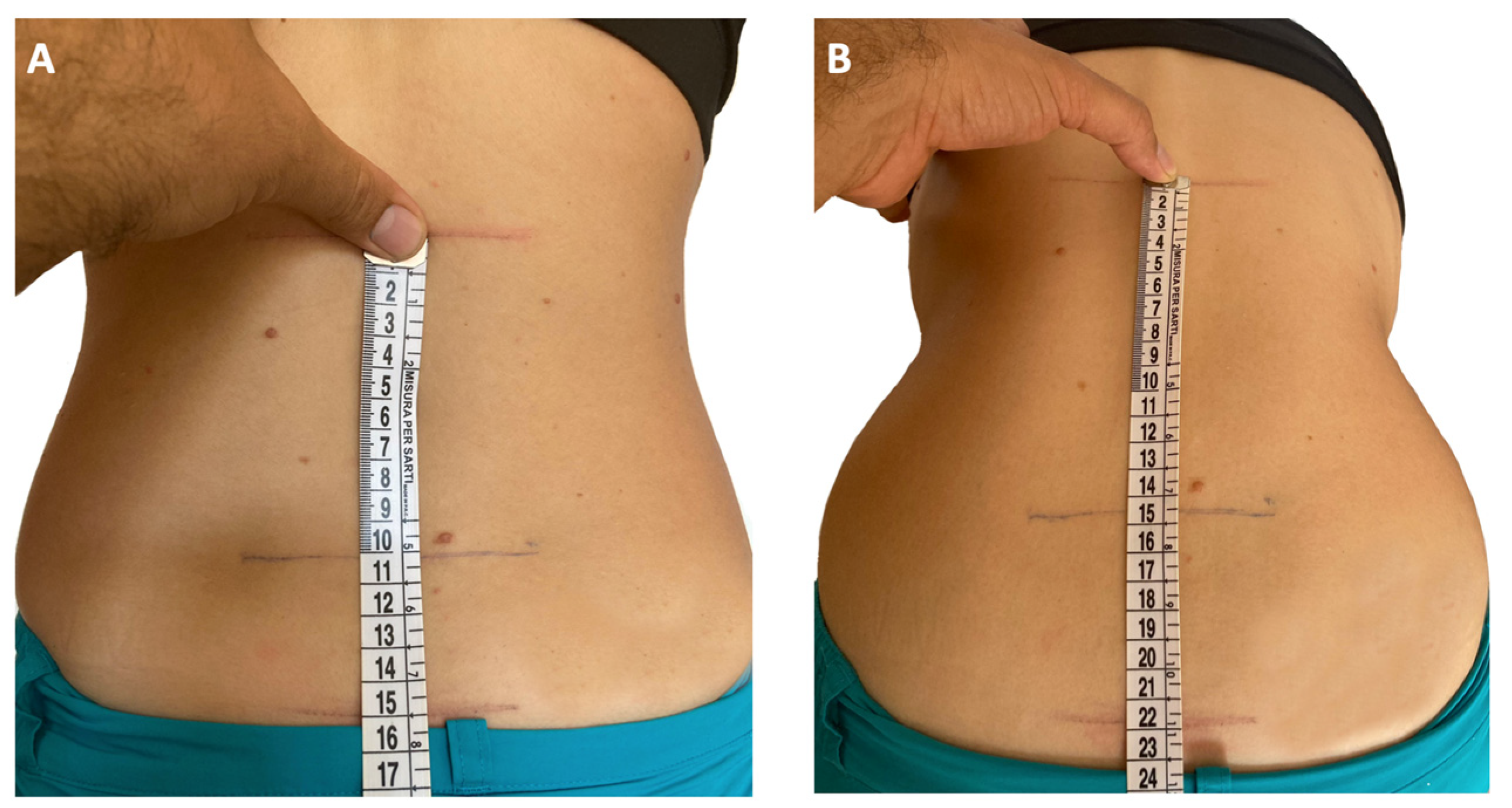
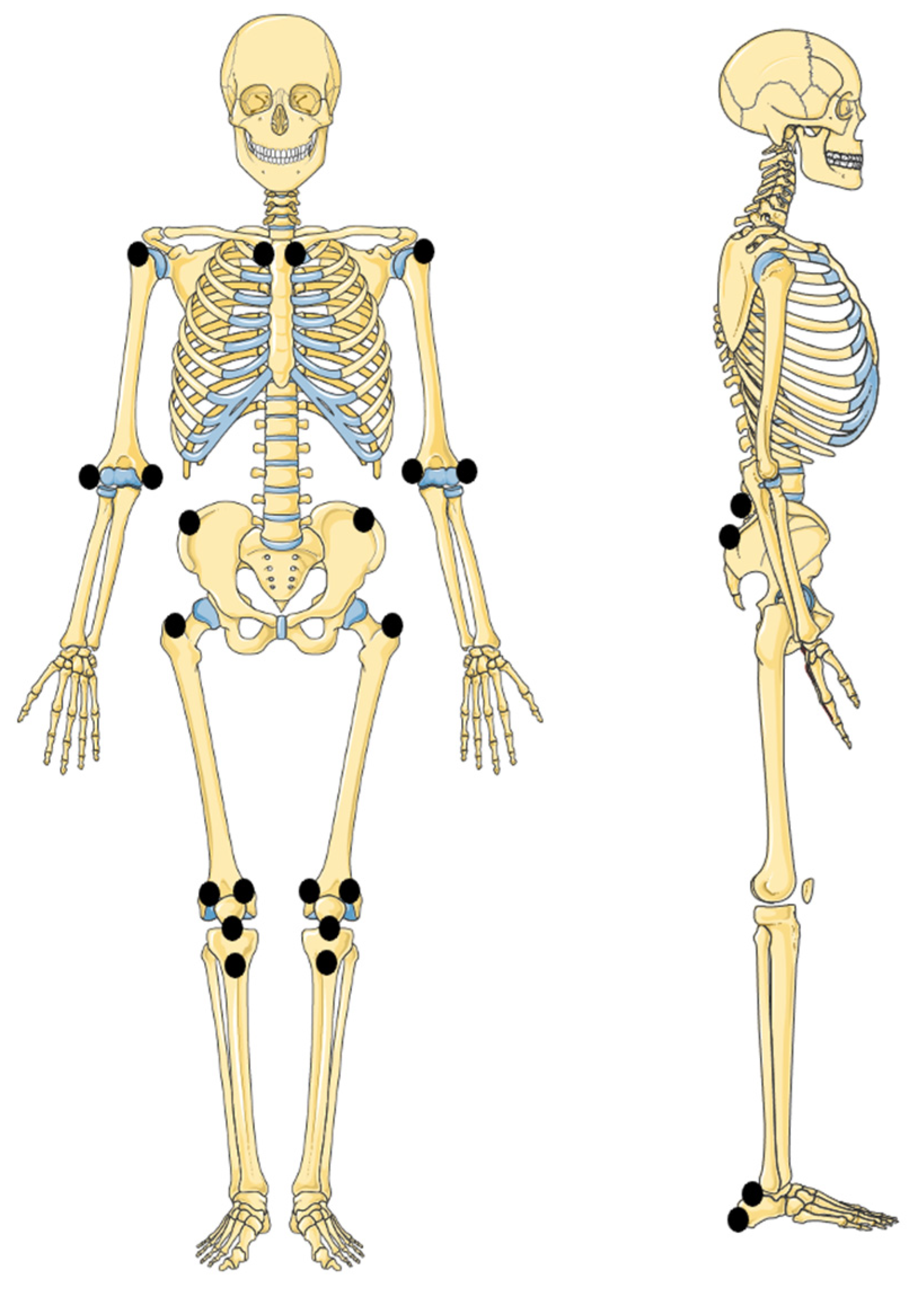
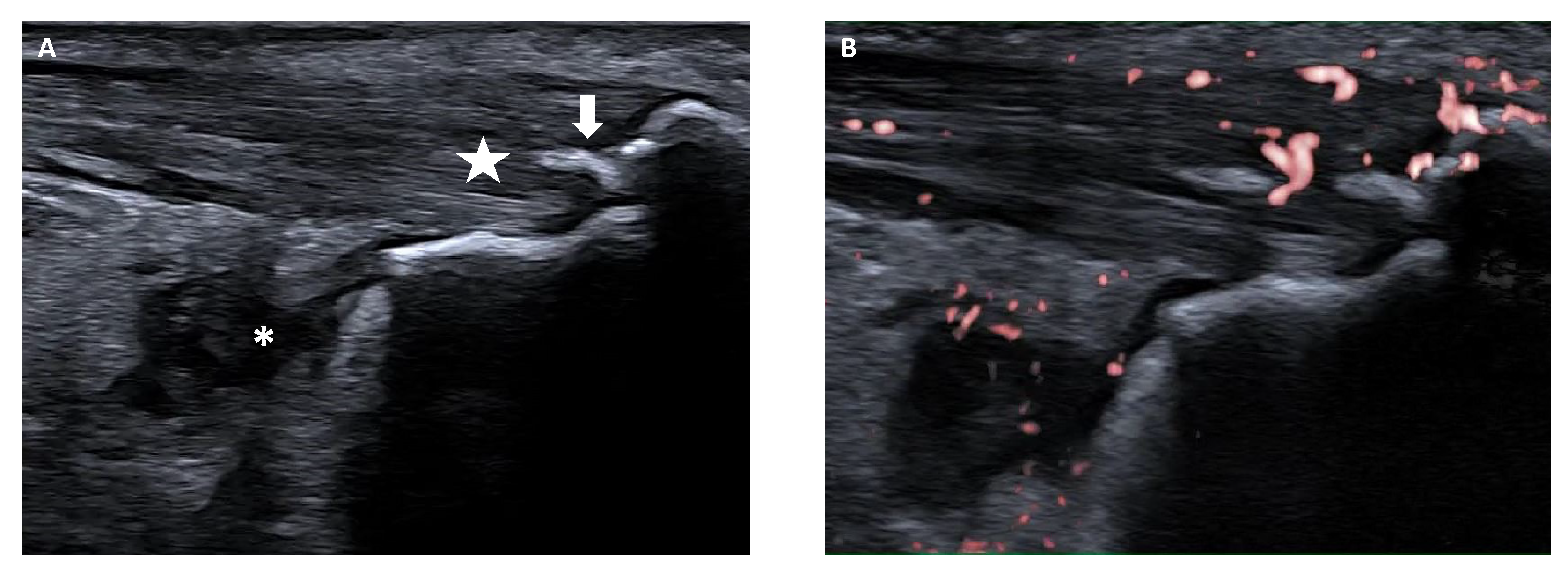
5.2. Enthesitis
5.3. Extra-Articular Manifestations
6. ERA Monitoring and Outcome
7. ERA Treatment
7.1. Current Therapeutic Recommendations
7.2. Emerging Biological Agents and Small Molecules
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravelli, A.; Martini, A. Juvenile idiopathic arthritis. Lancet 2007, 369, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Lovell, D.J.; Albani, S.; Brunner, H.I.; Hyrich, K.L.; Thompson, S.D.; Ruperto, N. Juvenile idiopathic arthritis. Nat. Rev. Dis. Primers 2022, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Thierry, S.; Fautrel, B.; Lemelle, I.; Guillemin, F. Prevalence and incidence of juvenile idiopathic arthritis: A systematic review. Jt. Bone Spine 2014, 81, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar] [PubMed]
- Consolaro, A.; Giancane, G.; Alongi, A.; van Dijkhuizen, E.H.P.; Aggarwal, A.; Al-Mayouf, S.M.; Bovis, F.; De Inocencio, J.; Demirkaya, E.; Flato, B.; et al. Phenotypic variability and disparities in treatment and outcomes of childhood arthritis throughout the world: An observational cohort study. Lancet Child. Adolesc. Health 2019, 3, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Moll, J.M.; Haslock, I.; Macrae, I.F.; Wright, V. Associations between ankylosing spondylitis, psoriatic arthritis, Reiter’s disease, the intestinal arthropathies, and Behcet’s syndrome. Medicine 1974, 53, 343–364. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, J.P.; Joyce-Shaikh, B.; Turner, S.P.; Chao, C.C.; Sathe, M.; Grein, J.; Gorman, D.M.; Bowman, E.P.; McClanahan, T.K.; Yearley, J.H.; et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat. Med. 2012, 18, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Moll, J.M.; Wright, V. New York clinical criteria for ankylosing spondylitis. A statistical evaluation. Ann. Rheum. Dis. 1973, 32, 354–363. [Google Scholar] [CrossRef]
- Amor, B.; Dougados, M.; Mijiyawa, M. Criteria of the classification of spondylarthropathies. Rev. Rhum. Mal. Osteoartic. 1990, 57, 85–89. [Google Scholar]
- Dougados, M.; van der Linden, S.; Juhlin, R.; Huitfeldt, B.; Amor, B.; Calin, A.; Cats, A.; Dijkmans, B.; Olivieri, I.; Pasero, G. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1991, 34, 1218–1227. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Landewé, R.; van der Heijde, D.; Listing, J.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Collantes-Estevez, E.; Davis, J.; Dijkmans, B.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): Classification of paper patients by expert opinion including uncertainty appraisal. Ann. Rheum. Dis. 2009, 68, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Akkoc, N.; Brandt, J.; Chou, C.T.; Dougados, M.; Huang, F.; Gu, J.; Kirazli, Y.; et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 2011, 70, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; Group, C.S. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Tse, S.M.; Colbert, R.A. Enthesitis related arthritis. In Textbook of Pediatric Rheumatology, 8th ed.; Petty, R.E., Laxer, R., Lindsley, C., Wedderburn, L., Mellins, E.D., Fuhlbrigge, R.C., Eds.; Elsevier: Philadelphia, PA, USA, 2021. [Google Scholar]
- Burgos-Vargas, R.; Pacheco-Tena, C.; Vázquez-Mellado, J. Juvenile-onset spondyloarthropathies. Rheum. Dis. Clin. N. Am. 1997, 23, 569–598. [Google Scholar] [CrossRef]
- Ansell, B.M. Juvenile spondylitis and related disorders. In Ankylosing Spondylitis; Moll, J.M.H., Ed.; Churchill Livingstone: Edinburgh, UK, 1980. [Google Scholar]
- Rosenberg, A.M.; Petty, R.E. A syndrome of seronegative enthesopathy and arthropathy in children. Arthritis Rheum. 1982, 25, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.; Levinson, J.; Bass, J.; Baum, J.; Brewer, E.; Fink, C.; Hanson, V.; Jacobs, J.; Masi, A.; Schaller, J.; et al. A study of classification criteria for a diagnosis of juvenile rheumatoid arthritis. Arthritis Rheum. 1986, 29, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Fink, C.W. Proposal for the development of classification criteria for idiopathic arthritides of childhood. J. Rheumatol. 1995, 22, 1566–1569. [Google Scholar]
- Petty, R.E.; Southwood, T.R.; Baum, J.; Bhettay, E.; Glass, D.N.; Manners, P.; Maldonado-Cocco, J.; Suarez-Almazor, M.; Orozco-Alcala, J.; Prieur, A.M. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J. Rheumatol. 1998, 25, 1991–1994. [Google Scholar]
- Colbert, R.A. Classification of juvenile spondyloarthritis: Enthesitis-related arthritis and beyond. Nat. Rev. Rheumatol. 2010, 6, 477–485. [Google Scholar] [CrossRef]
- Martini, A. Are the number of joints involved or the presence of psoriasis still useful tools to identify homogeneous disease entities in juvenile idiopathic arthritis? J. Rheumatol. 2003, 30, 1900–1903. [Google Scholar] [PubMed]
- Martini, A. It is time to rethink juvenile idiopathic arthritis classification and nomenclature. Ann. Rheum. Dis. 2012, 71, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Ravelli, A.; Avcin, T.; Beresford, M.W.; Burgos-Vargas, R.; Cuttica, R.; Ilowite, N.T.; Khubchandani, R.; Laxer, R.M.; Lovell, D.J.; et al. Toward new classification criteria for juvenile idiopathic arthritis: First steps, Pediatric Rheumatology International Trials Organization International Consensus. J. Rheumatol. 2019, 46, 190–197. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.G.; Bakker, P.A.; van der Heijde, D.; Weber, U.; Rudwaleit, M.; Hermann, K.G.; Sieper, J.; Baraliakos, X.; Bennett, A.; Braun, J.; et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: Update by the ASAS MRI working group. Ann. Rheum. Dis. 2016, 75, 1958–1963. [Google Scholar] [CrossRef]
- Sieper, J.; van der Heijde, D.; Landewé, R.; Brandt, J.; Burgos-Vagas, R.; Collantes-Estevez, E.; Dijkmans, B.; Dougados, M.; Khan, M.A.; Leirisalo-Repo, M.; et al. New criteria for inflammatory back pain in patients with chronic back pain: A real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann. Rheum. Dis. 2009, 68, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Kunjir, V.; Venugopalan, A.; Chopra, A. Profile of Indian patients with juvenile onset chronic inflammatory joint disease using the ILAR classification criteria for JIA: A community-based cohort study. J. Rheumatol. 2010, 37, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Saurenmann, R.K.; Rose, J.B.; Tyrrell, P.; Feldman, B.M.; Laxer, R.M.; Schneider, R.; Silverman, E.D. Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: Ethnicity as a risk factor. Arthritis Rheum. 2007, 56, 1974–1984. [Google Scholar] [CrossRef]
- Hyrich, K.L.; Lal, S.D.; Foster, H.E.; Thornton, J.; Adib, N.; Baildam, E.; Gardner-Medwin, J.; Wedderburn, L.R.; Chieng, A.; Davidson, J.; et al. Disease activity and disability in children with juvenile idiopathic arthritis one year following presentation to paediatric rheumatology. Results from the Childhood Arthritis Prospective Study. Rheumatology 2010, 49, 116–122. [Google Scholar] [CrossRef]
- Weiss, P.F.; Beukelman, T.; Schanberg, L.E.; Kimura, Y.; Colbert, R.A.; CARRA Registry Investigators. Enthesitis-related arthritis is associated with higher pain intensity and poorer health status in comparison with other categories of juvenile idiopathic arthritis: The Childhood Arthritis and Rheumatology Research Alliance Registry. J. Rheumatol. 2012, 39, 2341–2351. [Google Scholar] [CrossRef]
- Srivastava, R.; Phatak, S.; Yadav, A.; Bajpai, P.; Aggarwal, A. HLA B27 typing in 511 children with juvenile idiopathic arthritis from India. Rheumatol. Int. 2016, 36, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Gmuca, S.; Xiao, R.; Brandon, T.G.; Pagnini, I.; Wright, T.B.; Beukelman, T.; Morgan, E.M.; Weiss, P.F. Multicenter inception cohort of enthesitis-related arthritis: Variation in disease characteristics and treatment approaches. Arthritis Res. Ther. 2017, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Goirand, M.; Breton, S.; Chevallier, F.; Duong, N.P.; Uettwiller, F.; Melki, I.; Mouy, R.; Wouters, C.; Bader-Meunier, B.; Job-Deslandre, C.; et al. Clinical features of children with enthesitis-related juvenile idiopathic arthritis/juvenile spondyloarthritis followed in a French tertiary care pediatric rheumatology centre. Pediatr. Rheumatol. Online J. 2018, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Arkachaisri, T.; Teh, K.L.; Book, Y.X.; Hoh, S.F.; Gao, X.; Das, L. Enthesitis related arthritis in a longitudinal Southeast Asian registry: High prevalence of HLA-B27, different sacroiliitis risk factors and less common drug-free remission. J. Clin. Med. 2021, 10, 568. [Google Scholar] [CrossRef] [PubMed]
- Oen, K.; Duffy, C.M.; Tse, S.M.; Ramsey, S.; Ellsworth, J.; Chédeville, G.; Chetaille, A.L.; Saint-Cyr, C.; Cabral, D.A.; Spiegel, L.R.; et al. Early outcomes and improvement of patients with juvenile idiopathic arthritis enrolled in a Canadian multicenter inception cohort. Arthritis Care Res. 2010, 62, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Madrid, C.; Merino, R.; De Inocencio, J.; García-Consuegra, J. Tarsitis as an initial manifestation of juvenile spondyloarthropathy. Clin. Exp. Rheumatol. 2009, 27, 691–694. [Google Scholar] [PubMed]
- Phatak, S.; Mohindra, N.; Zanwar, A.; Aggarwal, A. Prominent midfoot involvement in children with enthesitis-related arthritis category of juvenile idiopathic arthritis. Clin. Rheumatol. 2017, 36, 1737–1745. [Google Scholar] [CrossRef]
- Tse, S.M.L.; Petty, R.E. Enthesitis related arthritis. In Textbook of Pediatric Rheumatology, 7th ed.; Petty, R.E., Laxer, R., Lindsley, C., Wedderburn, L., Eds.; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Bowyer, S. Hip contracture as the presenting sign in children with HLA-B27 arthritis. J. Rheumatol. 1995, 22, 165–167. [Google Scholar]
- Pagnini, I.; Savelli, S.; Matucci-Cerinic, M.; Fonda, C.; Cimaz, R.; Simonini, G. Early predictors of juvenile sacroiliitis in enthesitis-related arthritis. J. Rheumatol. 2010, 37, 2395–2401. [Google Scholar] [CrossRef]
- Bou Antoun, M.; Adamsbaum, C.; Semerano, L.; Koné-Paut, I.; Rossi-Semerano, L. Clinical predictors of magnetic resonance imaging-detected sacroiliitis in children with enthesitis related arthritis. Jt. Bone Spine 2017, 84, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Fang, Y.; Zhang, T.; Pan, Y.; Wang, P.; Fan, Z.; Yu, H. Axial involvement in enthesitis-related arthritis: Results from a single-center cohort. Pediatr. Rheumatol. Online J. 2023, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Chan, O.M.; Lai, B.M.; Leung, A.S.; Leung, T.F.; Ho, A.C. High prevalence of sacroiliitis and early structural changes in the sacroiliac joint in children with enthesitis-related arthritis: Findings from a tertiary centre in Hong Kong. Pediatr. Rheumatol. Online J. 2023, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.F.; Klink, A.J.; Behrens, E.M.; Sherry, D.D.; Finkel, T.H.; Feudtner, C.; Keren, R. Enthesitis in an inception cohort of enthesitis-related arthritis. Arthritis Care Res. 2011, 63, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.F.; Xiao, R.; Biko, D.M.; Chauvin, N.A. Assessment of sacroiliitis at diagnosis of juvenile spondyloarthritis by radiography, magnetic resonance imaging, and clinical examination. Arthritis Care Res. 2016, 68, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Vendhan, K.; Sen, D.; Fisher, C.; Ioannou, Y.; Hall-Craggs, M.A. Inflammatory changes of the lumbar spine in children and adolescents with enthesitis-related arthritis: Magnetic resonance imaging findings. Arthritis Care Res. 2014, 66, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Ergen, F.B.; Taydaş, O.; Sağ, E.; Bilginer, Y.; Aydıngöz, Ü.; Özen, S. Spinal involvement in juvenile idiopathic arthritis: What do we miss without imaging? Rheumatol. Int. 2022, 42, 519–527. [Google Scholar] [CrossRef] [PubMed]
- J P Bray, T.; Vendhan, K.; Ambrose, N.; Atkinson, D.; Punwani, S.; Fisher, C.; Sen, D.; Ioannou, Y.; Hall-Craggs, M.A. Diffusion-weighted imaging is a sensitive biomarker of response to biologic therapy in enthesitis-related arthritis. Rheumatology 2017, 56, 399–407. [Google Scholar] [CrossRef][Green Version]
- Macrae, I.F.; Wright, V. Measurement of back movement. Ann. Rheum. Dis. 1969, 28, 584–589. [Google Scholar] [CrossRef]
- Flatø, B.; Smerdel, A.; Johnston, V.; Lien, G.; Dale, K.; Vinje, O.; Egeland, T.; Sørskaar, D.; Førre, Ø. The influence of patient characteristics, disease variables, and HLA alleles on the development of radiographically evident sacroiliitis in juvenile idiopathic arthritis. Arthritis Rheum. 2002, 46, 986–994. [Google Scholar] [CrossRef]
- Flatø, B.; Hoffmann-Vold, A.M.; Reiff, A.; Førre, Ø.; Lien, G.; Vinje, O. Long-term outcome and prognostic factors in enthesitis-related arthritis: A case-control study. Arthritis Rheum. 2006, 54, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huang, S.; Guo, Y.; Jin, J.; Yan, W.; Wang, P.; Fang, Y.; Liu, Y.; Pan, Y.; Fan, Z.; et al. Effectiveness of tumor necrosis factor inhibitors in children with enthesitis-related arthritis: A single-center retrospective analysis. Front. Pediatr. 2023, 11, 1122233. [Google Scholar] [CrossRef] [PubMed]
- Rumsey, D.G.; Guzman, J.; Rosenberg, A.M.; Huber, A.M.; Scuccimarri, R.; Shiff, N.J.; Bruns, A.; Feldman, B.M.; Eurich, D.T.; Research in Arthritis in Canadian Children Emphasizing Outcomes Investigators. Characteristics and course of enthesitis in a juvenile idiopathic arthritis inception cohort. Arthritis Care Res. 2018, 70, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.F. Diagnosis and treatment of enthesitis-related arthritis. Adolesc. Health Med. Ther. 2012, 2012, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.F. Evaluation and Treatment of Enthesitis-Related Arthritis. Curr. Med. Lit. Rheumatol. 2013, 32, 33–41. [Google Scholar] [PubMed]
- Rumsey, D.G.; Guzman, J.; Rosenberg, A.M.; Huber, A.M.; Scuccimarri, R.; Shiff, N.J.; Bruns, A.; Feldman, B.M.; Eurich, D.T.; Research in Arthritis in Canadian Children Emphasizing Outcomes Investigators. Worse quality of life, function, and pain in children with enthesitis, irrespective of their juvenile arthritis category. Arthritis Care Res. 2020, 72, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.F.; Chauvin, N.A.; Klink, A.J.; Localio, R.; Feudtner, C.; Jaramillo, D.; Colbert, R.A.; Sherry, D.D.; Keren, R. Detection of enthesitis in children with enthesitis-related arthritis: Dolorimetry compared to ultrasonography. Arthritis Rheumatol. 2014, 66, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.F.; Colbert, R.A. Juvenile Spondyloarthritis: A distinct form of juvenile arthritis. Pediatr. Clin. N. Am. 2018, 65, 675–690. [Google Scholar] [CrossRef]
- Balint, P.V.; Terslev, L.; Aegerter, P.; Bruyn, G.A.W.; Chary-Valckenaere, I.; Gandjbakhch, F.; Iagnocco, A.; Jousse-Joulin, S.; Möller, I.; Naredo, E.; et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: An OMERACT US initiative. Ann. Rheum. Dis. 2018, 77, 1730–1735. [Google Scholar] [CrossRef]
- Rossi-Semerano, L.; Ravagnani, V.; Collado, P.; Vojinovic, J.; Roth, J.; Magni-Manzoni, S.; Naredo, E.; D’Agostino, M.A.; Jousse-Joulin, S. Validity of ultrasonography in detecting enthesitis in children: A systematic literature review. Jt. Bone Spine 2023, 90, 105538. [Google Scholar] [CrossRef]
- Ravelli, A.; Varnier, G.C.; Oliveira, S.; Castell, E.; Arguedas, O.; Magnani, A.; Pistorio, A.; Ruperto, N.; Magni-Manzoni, S.; Galasso, R.; et al. Antinuclear antibody-positive patients should be grouped as a separate category in the classification of juvenile idiopathic arthritis. Arthritis Rheum. 2011, 63, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.R.; Patro, P.; Agarwal, V.; Misra, D.P. Enthesitis-related arthritis: Current perspectives. Open Access Rheumatol. 2019, 11, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Walscheid, K.; Glandorf, K.; Rothaus, K.; Niewerth, M.; Klotsche, J.; Minden, K.; Heiligenhaus, A. Enthesitis-related arthritis: Prevalence and complications of associated uveitis in children and adolescents from a population-based nationwide study in Germany. J. Rheumatol. 2021, 48, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Jhaj, G.; Kopplin, L.J. Ocular features of the HLA-B27-positive seronegative spondyloarthropathies. Curr. Opin. Ophthalmol. 2018, 29, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Stoll, M.L.; Punaro, M.; Patel, A.S. Fecal calprotectin in children with the enthesitis-related arthritis subtype of juvenile idiopathic arthritis. J. Rheumatol. 2011, 38, 2274–2275. [Google Scholar] [CrossRef] [PubMed]
- Stoll, M.L.; Patel, A.S.; Punaro, M.; Dempsey-Robertson, M. MR enterography to evaluate sub-clinical intestinal inflammation in children with spondyloarthritis. Pediatr. Rheumatol. Online J. 2012, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- van Straalen, J.W.; Krol, R.M.; Giancane, G.; Panaviene, V.; Ailioaie, L.M.; Doležalová, P.; Cattalini, M.; Susic, G.; Sztajnbok, F.R.; Maritsi, D.; et al. Increased incidence of inflammatory bowel disease on etanercept in juvenile idiopathic arthritis regardless of concomitant methotrexate use. Rheumatology 2022, 61, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Consolaro, A.; Ruperto, N.; Bazso, A.; Pistorio, A.; Magni-Manzoni, S.; Filocamo, G.; Malattia, C.; Viola, S.; Martini, A.; Ravelli, A.; et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009, 61, 658–666. [Google Scholar] [CrossRef]
- McErlane, F.; Beresford, M.W.; Baildam, E.M.; Chieng, S.E.; Davidson, J.E.; Foster, H.E.; Gardner-Medwin, J.; Lunt, M.; Wedderburn, L.R.; Thomson, W.; et al. Validity of a three-variable Juvenile Arthritis Disease Activity Score in children with new-onset juvenile idiopathic arthritis. Ann. Rheum. Dis. 2013, 72, 1983–1988. [Google Scholar] [CrossRef]
- Consolaro, A.; Negro, G.; Gallo, M.C.; Bracciolini, G.; Ferrari, C.; Schiappapietra, B.; Pistorio, A.; Bovis, F.; Ruperto, N.; Martini, A.; et al. Defining criteria for disease activity states in nonsystemic juvenile idiopathic arthritis based on a three-variable juvenile arthritis disease activity score. Arthritis Care Res. 2014, 66, 1703–1709. [Google Scholar] [CrossRef]
- Weiss, P.F.; Colbert, R.A.; Xiao, R.; Feudtner, C.; Beukelman, T.; DeWitt, E.M.; Pagnini, I.; Wright, T.B.; Wallace, C.A. Development and retrospective validation of the juvenile spondyloarthritis disease activity index. Arthritis Care Res 2014, 66, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Srinivasalu, H.; Treemarcki, E.B.; Rumsey, D.G.; Weiss, P.F.; Colbert, R.A.; CARRA Spondyloarthritis Workgroup and the CARRA Registry Investigators. Modified Juvenile Spondyloarthritis Disease Activity Index in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. J. Rheumatol. 2023, 50, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Zanwar, A.; Phatak, S.; Aggarwal, A. Prospective validation of the Juvenile Spondyloarthritis Disease Activity Index in children with enthesitis-related arthritis. Rheumatology 2018, 57, 2167–2171. [Google Scholar] [CrossRef] [PubMed]
- Boiu, S.; Marniga, E.; Bader-Meunier, B.; Mouy, R.; Compeyrot-Lacassagne, S.; Quartier, P.; Wouters, C.H. Functional status in severe juvenile idiopathic arthritis in the biologic treatment era: An assessment in a French paediatric rheumatology referral centre. Rheumatology 2012, 51, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.K.; Misra, R.; Aggarwal, A. Outcome in patients with enthesitis related arthritis (ERA): Juvenile arthritis damage index (JADI) and functional status. Pediatr. Rheumatol. Online J. 2008, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Glerup, M.; Rypdal, V.; Arnstad, E.D.; Ekelund, M.; Peltoniemi, S.; Aalto, K.; Rygg, M.; Toftedal, P.; Nielsen, S.; Fasth, A.; et al. Long-term outcomes in juvenile idiopathic arthritis: Eighteen years of follow-up in the population-based Nordic juvenile idiopathic arthritis cohort. Arthritis Care Res. 2020, 72, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Minden, K.; Niewerth, M.; Listing, J.; Biedermann, T.; Bollow, M.; Schöntube, M.; Zink, A. Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002, 46, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Deligeorgakis, D.; Trachana, M.; Pratsidou-Gertsi, P.; Dimopoulou, D.; Haidich, A.B.; Garyfallos, A. Capturing the enthesitis related arthritis contemporary profile of Caucasian patients in the era of biologics. Rheumatol. Int. 2020, 40, 941–949. [Google Scholar] [CrossRef]
- Berntson, L.; Nordal, E.; Aalto, K.; Peltoniemi, S.; Herlin, T.; Zak, M.; Nielsen, S.; Rygg, M.; Rheumatology, Nordic Study Group of Paediatric Rheumatology. HLA-B27 predicts a more chronic disease course in an 8-year followup cohort of patients with juvenile idiopathic arthritis. J. Rheumatol. 2013, 40, 725–731. [Google Scholar] [CrossRef]
- Shih, Y.J.; Yang, Y.H.; Lin, C.Y.; Chang, C.L.; Chiang, B.L. Enthesitis-related arthritis is the most common category of juvenile idiopathic arthritis in Taiwan and presents persistent active disease. Pediatr. Rheumatol. Online J. 2019, 17, 58. [Google Scholar] [CrossRef]
- Naveen, R.; Mohindra, N.; Jain, N.; Majumder, S.; Aggarwal, A. Hip involvement in children with enthesitis related arthritis (ERA) is associated with poor outcomes in adulthood. Clin. Rheumatol. 2021, 40, 4619–4627. [Google Scholar] [CrossRef] [PubMed]
- Naveen, R.; Guleria, S.; Mohindra, N.; Aggarwal, A. Predictors of long-term functional outcomes of juvenile idiopathic arthritis-enthesitis related arthritis: A single centre experience. Rheumatology 2023, 62, 3110–3116. [Google Scholar] [CrossRef]
- Onel, K.B.; Horton, D.B.; Lovell, D.J.; Shenoi, S.; Cuello, C.A.; Angeles-Han, S.T.; Becker, M.L.; Cron, R.Q.; Feldman, B.M.; Ferguson, P.J.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Juvenile Idiopathic Arthritis: Recommendations for Nonpharmacologic Therapies, Medication Monitoring, Immunizations, and Imaging. Arthritis Rheumatol. 2022, 74, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Onel, K.B.; Horton, D.B.; Lovell, D.J.; Shenoi, S.; Cuello, C.A.; Angeles-Han, S.T.; Becker, M.L.; Cron, R.Q.; Feldman, B.M.; Ferguson, P.J.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Oligoarthritis, Temporomandibular Joint Arthritis, and Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2022, 74, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Ringold, S.; Angeles-Han, S.T.; Beukelman, T.; Lovell, D.; Cuello, C.A.; Becker, M.L.; Colbert, R.A.; Feldman, B.M.; Ferguson, P.J.; Gewanter, H.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Rheumatol. 2019, 71, 846–863. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.; Consolaro, A.; Horneff, G.; Laxer, R.M.; Lovell, D.J.; Wulffraat, N.M.; Akikusa, J.D.; Al-Mayouf, S.M.; Antón, J.; Avcin, T.; et al. Treating juvenile idiopathic arthritis to target: Recommendations of an international task force. Ann. Rheum. Dis. 2018, 77, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Kuntze, G.; Nesbitt, C.; Whittaker, J.L.; Nettel-Aguirre, A.; Toomey, C.; Esau, S.; Doyle-Baker, P.K.; Shank, J.; Brooks, J.; Benseler, S.; et al. Exercise therapy in juvenile idiopathic arthritis: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2018, 99, 178–193.e1. [Google Scholar] [CrossRef]
- Cimaz, R.; Maioli, G.; Calabrese, G. Current and emerging biologics for the treatment of juvenile idiopathic arthritis. Exp. Opin. Biol. Ther. 2020, 20, 725–740. [Google Scholar] [CrossRef]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 pathway in rheumatic diseases: An overview. Front. Immunol. 2021, 12, 637829. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Haibel, H.; Brandt, H.C.; Song, I.H.; Brandt, A.; Listing, J.; Rudwaleit, M.; Sieper, J. No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: A 16-week open-label trial. Ann. Rheum. Dis. 2007, 66, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, B.; Bintley-Bagot, S.; Bulgen, D.Y.; Thompson, R.N.; Tunn, E.J.; Moots, R.J. Is methotrexate effective in ankylosing spondylitis? Rheumatology 2002, 41, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.F.; Fuhlbrigge, R.C.; von Scheven, E.; Lovell, D.J.; Colbert, R.A.; Brunner, H.I.; PRCSG Advisory Council and the CARRA Executive Committee. Children with enthesitis-related arthritis and possible benefits from treatments for adults with spondyloarthritis. Arthritis Care Res 2022, 74, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.F.; Xiao, R.; Brandon, T.G.; Pagnini, I.; Wright, T.B.; Beukelman, T.; Morgan-DeWitt, E.; Feudtner, C. Comparative effectiveness of Tumor Necrosis Factor agents and disease-modifying antirheumatic therapy in children with enthesitis-related arthritis: The first year after diagnosis. J. Rheumatol. 2018, 45, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Foeldvari, I.; Constantin, T.; Vojinović, J.; Horneff, G.; Chasnyk, V.; Dehoorne, J.; Panaviene, V.; Sušić, G.; Stanevicha, V.; Kobusinska, K.; et al. Etanercept treatment for extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis, or psoriatic arthritis: 6-year efficacy and safety data from an open-label trial. Arthritis Res. Ther. 2019, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Constantin, T.; Foeldvari, I.; Vojinovic, J.; Horneff, G.; Burgos-Vargas, R.; Nikishina, I.; Akikusa, J.D.; Avcin, T.; Chaitow, J.; Koskova, E.; et al. Two-year efficacy and safety of etanercept in pediatric patients with extended oligoarthritis, enthesitis-related arthritis, or psoriatic arthritis. J. Rheumatol. 2016, 43, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Horneff, G.; Burgos-Vargas, R.; Constantin, T.; Foeldvari, I.; Vojinovic, J.; Chasnyk, V.G.; Dehoorne, J.; Panaviene, V.; Susic, G.; Stanevica, V.; et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: Part 1 (week 12) of the CLIPPER study. Ann. Rheum. Dis. 2014, 73, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Vargas, R.; Loyola-Sanchez, A.; Ramiro, S.; Reding-Bernal, A.; Alvarez-Hernandez, E.; van der Heijde, D.; Vázquez-Mellado, J. A randomized, double-blind, placebo-controlled 12-week trial of infliximab in patients with juvenile-onset spondyloarthritis. Arthritis Res. Ther. 2022, 24, 187. [Google Scholar] [CrossRef]
- Burgos-Vargas, R.; Tse, S.M.; Horneff, G.; Pangan, A.L.; Kalabic, J.; Goss, S.; Unnebrink, K.; Anderson, J.K. A randomized, double-blind, placebo-controlled multicenter study of adalimumab in pediatric patients with enthesitis-related arthritis. Arthritis Care Res. 2015, 67, 1503–1512. [Google Scholar] [CrossRef]
- Ruperto, N.; Brunner, H.I.; Pacheco-Tena, C.; Louw, I.; Vega-Cornejo, G.; Spindler, A.J.; Kingsbury, D.J.; Schmeling, H.; Borzutzky, A.; Cuttica, R.; et al. Open-label phase 3 study of intravenous golimumab in patients with polyarticular juvenile idiopathic arthritis. Rheumatology 2021, 60, 4495–4507. [Google Scholar] [CrossRef]
- de Jager, W.; Hoppenreijs, E.P.; Wulffraat, N.M.; Wedderburn, L.R.; Kuis, W.; Prakken, B.J. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: A cross-sectional study. Ann. Rheum. Dis. 2007, 66, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, I.D.; Griffin, P.; Michel, J.J.; Yano, H.; Gaffen, S.L.; Mueller, R.G.; Dvergsten, J.A.; Piganelli, J.D.; Rosenkranz, M.E.; Kietz, D.A.; et al. T Cell Receptor-independent, CD31/IL-17A-driven inflammatory axis shapes synovitis in juvenile idiopathic arthritis. Front. Immunol. 2018, 9, 1802. [Google Scholar] [CrossRef] [PubMed]
- Vijatov-Djuric, G.; Doronjski, A.; Mitic, I.; Brkic, S.; Barisic, N. Interleukin-17A levels increase in serum of children with juvenile idiopathic arthritis. Arch. Rheumatol. 2017, 32, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Misra, R.; Aggarwal, A. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J. Rheumatol. 2008, 35, 515–519. [Google Scholar] [PubMed]
- Mahendra, A.; Misra, R.; Aggarwal, A. Th1 and Th17 predominance in the enthesitis-related arthritis form of juvenile idiopathic arthritis. J. Rheumatol. 2009, 36, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Sieper, J.; Braun, J.; Baraliakos, X.; Dougados, M.; Emery, P.; Deodhar, A.; Porter, B.; Martin, R.; Andersson, M.; et al. Secukinumab, an Interleukin-17A inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 2015, 373, 2534–2548. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, A.; Blanco, R.; Dokoupilová, E.; Hall, S.; Kameda, H.; Kivitz, A.J.; Poddubnyy, D.; van de Sande, M.; Wiksten, A.S.; Porter, B.O.; et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with Secukinumab: Primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 2021, 73, 110–120. [Google Scholar] [CrossRef]
- Braun, J.; Kiltz, U.; Deodhar, A.; Tomita, T.; Dougados, M.; Bolce, R.; Sandoval, D.; Lin, C.Y.; Walsh, J. Efficacy and safety of ixekizumab treatment in patients with axial spondyloarthritis: 2-year results from COAST. RMD Open 2022, 8, e002165. [Google Scholar] [CrossRef]
- Brunner, H.I.; Foeldvari, I.; Alexeeva, E.; Ayaz, N.A.; Calvo Penades, I.; Kasapcopur, O.; Chasnyk, V.G.; Hufnagel, M.; Żuber, Z.; Schulert, G.; et al. Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: A randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial. Ann. Rheum. Dis. 2023, 82, 154–160. [Google Scholar] [CrossRef]
- Baer, J.; Klotsche, J.; Foeldvari, I. Secukinumab in the treatment for patients with juvenile enthesitis related arthritis non-responsive to anti-TNF treatment according the Juvenile Spondyloarthritis Disease Activity Index. Clin. Exp. Rheumatol. 2022, 40, 620–624. [Google Scholar] [CrossRef]
- Mannion, M.L.; McAllister, L.; Cron, R.Q.; Stoll, M.L. Ustekinumab as a therapeutic option for children with refractory enthesitis-related arthritis. J. Clin. Rheumatol. 2016, 22, 282–284. [Google Scholar] [CrossRef]
- Ruperto, N.; Brunner, H.I.; Synoverska, O.; Ting, T.V.; Mendoza, C.A.; Spindler, A.; Vyzhga, Y.; Marzan, K.; Grebenkina, L.; Tirosh, I.; et al. Tofacitinib in juvenile idiopathic arthritis: A double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet 2021, 398, 1984–1996. [Google Scholar] [CrossRef]
- Ramanan, A.V.; Quartier, P.; Okamoto, N.; Foeldvari, I.; Spindler, A.; Fingerhutová, Š.; Antón, J.; Wang, Z.; Meszaros, G.; Araújo, J.; et al. Baricitinib in juvenile idiopathic arthritis: An international, phase 3, randomised, double-blind, placebo-controlled, withdrawal, efficacy, and safety trial. Lancet 2023, 402, 555–570. [Google Scholar] [CrossRef]
| JIA Subtypes Defined by ILAR | Frequency 1 |
|---|---|
| Systemic arthritis | 4.2–33% |
| Oligoarthritis | 10.8–56.7% |
| Polyarthritis (Rheumatoid Factor positive) | 1.3–11.2% |
| Polyarthritis (Rheumatoid Factor negative) | 12.7–31.5% |
| Psoriatic arthritis | 1.3–7.1% |
| Enthesitis-related arthritis | 5.4–29.8% |
| Undifferentiated arthritis | 3.7–10.4% |
| Anatomic Region | Sites for Entheseal Assessment |
|---|---|
| Chest | First and seventh costosternal junctions |
| Upper extremity | Supraspinatus attachment into greater tuberosity of humerus |
| Common flexor attachment at medial epicondyle of humerus Common extensor attachment at lateral epicondyle of humerus | |
| Spine | Fifth lumbar spinous process |
| Pelvis | Abdominal muscle attachments to iliac crest |
| Sartorius attachment at anterior superior iliac spine | |
| Posterior superior iliac spine | |
| Hip extensor attachment at greater trochanter of femur | |
| Gracilis and adduction attachment to pubis symphysis | |
| Hamstrings attachment to ischial tuberosity | |
| Knee | Quadriceps tendon attachment to patella (2 and 10 o’clock) |
| Infrapatellar ligament attachment to patella (6 o’clock) and tibial tuberosity | |
| Foot and ankle | Achilles tendon attachment to calcaneus |
| Plantar fascia attachment to calcaneus | |
| Plantar fascia attachment to metatarsal heads | |
| Plantar fascia attachment to base of fifth metatarsal |
| bDMARDs | Composition and Action Mechanism |
|---|---|
| Etanercept | TNF-α receptor fusion protein able to bind circulating TNF-α |
| Adalimumab | Humanized monoclonal antibody binding both soluble and membrane-bound TNF-α |
| Infliximab | Chimeric monoclonal antibody binding both soluble and membrane-bound TNF-α |
| Golimumab | Human monoclonal antibody binding both soluble and membrane-bound TNF-α |
| Secukimumab | Human monoclonal antibody that binds IL-17A, avoiding the activation of IL-17 pathway |
| Ustekimumab | Human monoclonal antibody directed against the p40 subunit shared by IL-12 and IL-23, preventing the Th-17 cells activation |
| Ixekizumab | Humanized monoclonal antibody targeting IL-17A, avoiding the activation of IL-17 pathway |
| Tofacitinib | JAK1/JAK3 inhibitor able to prevent JAK-STAT cascade activation |
| Baricitinib | JAK1/JAK2 inhibitor able to prevent JAK-STAT cascade activation |
| Isolated Peripheral Arthritis (<5 Affected Joints) | Isolated Peripheral Arthritis (≥5 Affected Joints) * | Presence of Sacroiliitis * | Presence of Enthesitis * | |
|---|---|---|---|---|
| First-line therapy | IAGCs, trial of NSAIDs |
| NSAIDs | NSAIDs |
| Inadequate responseto first-line therapy | csDMARD, with MTX as preferred agent |
|
|
|
| Inadequate responseto second-line therapy | bDMARD | Switch to another bDMARD |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gennaro, S.; Di Matteo, G.; Stornaiuolo, G.; Anselmi, F.; Lastella, T.; Orlando, F.; Alessio, M.; Naddei, R. Advances in the Diagnosis and Treatment of Enthesitis-Related Arthritis. Children 2023, 10, 1647. https://doi.org/10.3390/children10101647
Di Gennaro S, Di Matteo G, Stornaiuolo G, Anselmi F, Lastella T, Orlando F, Alessio M, Naddei R. Advances in the Diagnosis and Treatment of Enthesitis-Related Arthritis. Children. 2023; 10(10):1647. https://doi.org/10.3390/children10101647
Chicago/Turabian StyleDi Gennaro, Simona, Gennaro Di Matteo, Gianmarco Stornaiuolo, Federica Anselmi, Teresa Lastella, Francesca Orlando, Maria Alessio, and Roberta Naddei. 2023. "Advances in the Diagnosis and Treatment of Enthesitis-Related Arthritis" Children 10, no. 10: 1647. https://doi.org/10.3390/children10101647
APA StyleDi Gennaro, S., Di Matteo, G., Stornaiuolo, G., Anselmi, F., Lastella, T., Orlando, F., Alessio, M., & Naddei, R. (2023). Advances in the Diagnosis and Treatment of Enthesitis-Related Arthritis. Children, 10(10), 1647. https://doi.org/10.3390/children10101647





