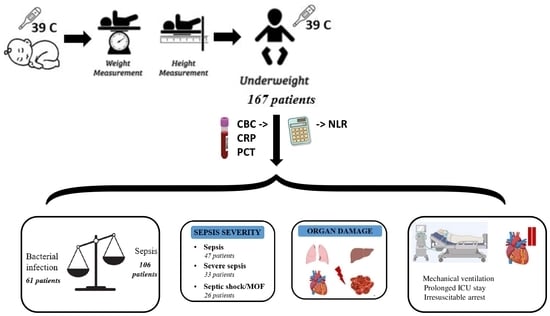
The Neutrophil-to-Lymphocyte Ratio (NLR) Can Predict Sepsis’s Presence and Severity in Malnourished Infants—A Single Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Clinical Assessments and Laboratory Measurements
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Statistical Analysis of Clinical Characteristics of Sepsis Infants
3.3. Correlation Analysis between NLR and Clinical Variables
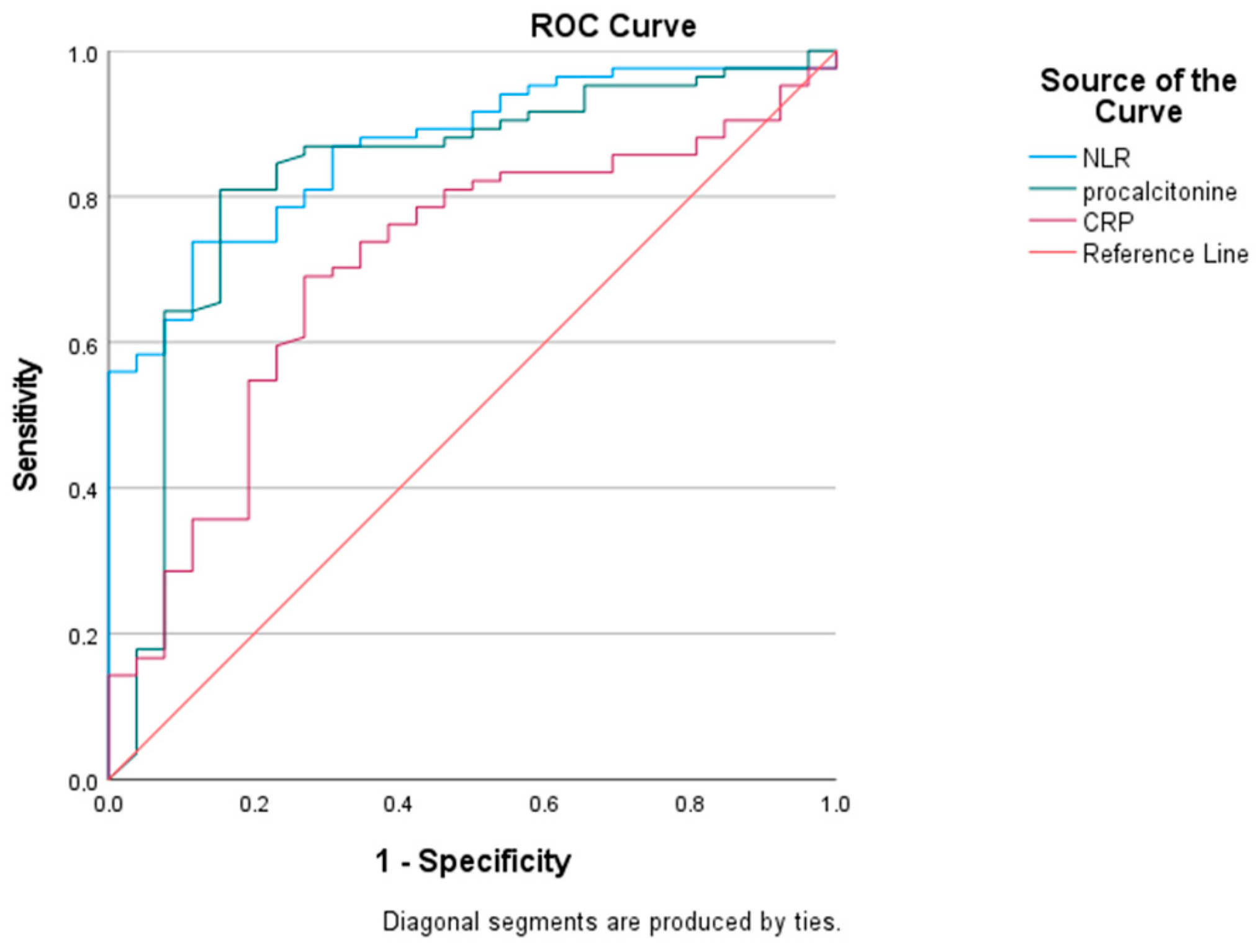
3.4. Diagnostic Performances of PCT, CRP, and NLR
3.5. Association between NLR Levels and Sepsis
4. Discussion
5. Limitations
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balamuth, F.; Weiss, S.L.; Neuman, M.I.; Scott, H.; Brady, P.W.; Paul, R.; Farris, R.W.; McClead, R.; Hayes, K.; Gaieski, D.; et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr. Crit. Care Med. 2014, 15, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Fitzgerald, J.C.; Pappachan, J.; Wheeler, D.; Jaramillo-Bustamante, J.C.; Salloo, A.; Singhi, S.C.; Erickson, S.; Roy, J.A.; Bush, J.L.; et al. Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am. J. Respir. Crit. Care Med. 2015, 191, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.J.; Straney, L.; Alexander, J.; MacLaren, G.; Festa, M.; Schibler, A.; Slater, A. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–2013: A multicentre retrospective cohort study. Lancet Infect. Dis. 2015, 15, 46–54. [Google Scholar] [CrossRef]
- Tamelytė, E.; Vaičekauskienė, G.; Dagys, A.; Lapinskas, T.; Jankauskaitė, L. Early Blood Biomarkers to Improve Sepsis/Bacteremia Diagnostics in Pediatric Emergency Settings. Medicina 2019, 55, 99. [Google Scholar] [CrossRef]
- Hermon, M.M.; Etmayr, T.; Brandt, J.B.; Sadeghi, K.; Burda, G.; Golej, J. Pediatric infection and sepsis in five age subgroups: Single-center registry. Wien. Med. Wochenschr. 2021, 171, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.D.; Berkley, J.A. Severe acute malnutrition and infection. Paediatr. Int. Child Health 2014, 34 (Suppl. S1), S1–S29. [Google Scholar] [CrossRef] [PubMed]
- Morlese, J.F.; Forrester, T.; Jahoor, F. Acute-phase protein response to infection in severe malnutrition. Am. J. Physiol. 1998, 275, E112–E117. [Google Scholar] [CrossRef]
- Dowton, S.B.; Colton, H.R. Acute phase reactants in inflammation and infection. Semin. Hematol. 1988, 25, 84–90. [Google Scholar]
- Fleck, A. Clinical and nutritional aspects of changes in acutephase proteins during inflammation. Proc. Nutr. Soc. 1989, 48, 347–354. [Google Scholar] [CrossRef]
- Djordjevic, D.; Rondovic, G.; Surbatovic, M.; Stanojevic, I.; Udovicic, I.; Andjelic, T.; Zeba, S.; Milosavljevic, S.; Stankovic, N.; Abazovic, D.; et al. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically ill and injured patients: Which ratio to choose to predict outcome and nature of bacteremia? Mediat. Inflamm. 2018, 2018, 3758068. [Google Scholar]
- Martins, E.C.; Silveira, L.D.F.; Viegas, K.; Beck, A.D.; Júnior, G.F.; Cremonese, R.V.; Lora, P.S. Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an intensive care unit: A case-control study. Rev. Bras. Ter. Intensiv. 2019, 31, 64–70. [Google Scholar] [CrossRef]
- de Pablo, R.; Monserrat, J.; Prieto, A.; Alvarez-Mon, M. Role of circulating lymphocytes in patients with sepsis. BioMed Res. Int. 2014, 2014, 67108. [Google Scholar]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [CrossRef]
- Doherty, J.F.; Golden, M.H.; Raynes, J.G.; Griffin, G.E.; McAdam, K.P. Acute-phase protein response is impaired in severely malnourished children. Clin. Sci. 1993, 84, 169–175. [Google Scholar] [CrossRef]
- Shahrin, L.; Chisti, M.J.; Brintz, B.; Islam, Z.; Shahid, A.S.; Hassan, Z.; Leung, D.T.; Chowdhury, F. Clinical and laboratory predictors of 30-day mortality in severe acute malnourished children with severe pneumonia. Trop. Med. Int. Health 2020, 25, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Page, A.L.; de Rekeneire, N.; Sayadi, S.; Aberrane, S.; Janssens, A.-C.; Dehoux, M.; Baron, E. Diagnostic and prognostic value of procalcitonin and C-reactive protein in malnourished children. Pediatrics 2014, 133, e363–e370. [Google Scholar] [CrossRef]
- Ekanem, E.E.; Umotong, A.B.; Raykundalia, C.; Catty, D. Serum C-reactive protein and C3 complement protein levels in severely malnourished Nigerian children with and without bacterial infections. Acta Paediatr. 1997, 86, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Malavé, I.; Vethencourt, M.A.; Pirela, M.; Cordero, R. Serum levels of thyroxine-binding prealbumin, C-reactive protein and interleukin-6 in protein-energy undernourished children and normal controls without or with associated clinical infections. J. Trop. Pediatr. 1998, 44, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.; Badaloo, A.; Forrester, T.; Morlese, J.F.; Heird, W.C.; Jahoor, F. The acute-phase protein response to infection in edematous and nonedematous protein-energy malnutrition. Am. J. Clin. Nutr. 2002, 76, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Rytter, M.J.; Kolte, L.; Briend, A.; Friis, H.; Christensen, V.B. The immune system in children with malnutrition—A systematic review. PLoS ONE 2014, 9, e105017. [Google Scholar]
- Fakhir, S.; Ahmad, P.; Faridi, M.A.; Rattan, A. Cell-mediated immune responses in malnourished host. J. Trop. Pediatr. 1989, 35, 175–178. [Google Scholar] [CrossRef]
- Bung, B.G.; Mahalanabis, D.; Mukherjee, K.L.; Bang, F.B. Tand B lymphocyte rosetting in under nourished children. Soc. Exp. Biol. Med. 1975, 149, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K.; Newberne, P.M. Cell-mediated immunity in nutritional imbalance. Fed. Proc. 1980, 39, 3088–3099. [Google Scholar] [PubMed]
- Puri, V.; Misra, P.K.; Saxena, K.C.; Saxena, P.N.; Saxena, R.K.; Agrawal, C.G. Immune status in malnutrition. Ind. Pediatr. 1980, 2, 127–133. [Google Scholar]
- World Health Organization. Training Course on Child Growth Assessment; WHO: Geneva, Switzerland, 2008; Available online: https://apps.who.int/iris/handle/10665/43601 (accessed on 5 June 2023).
- Goldstein, B.; Giroir, B.; Randolph, A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef]
- Walsh, S.R.; Cook, E.; Goulder, F.; Justin, T.; Keeling, N. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 2005, 91, 181–458. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhan, X.; Wang, W.-M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular car- 461 cinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer 464 after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar]
- Niseteo, T.; Hojsak, I.; Kolaček, S. Malnourished children acquire nosocomial infections more often and have significantly increased length of hospital stay. Clin. Nutr. 2020, 39, 1560–1563. [Google Scholar] [CrossRef]
- Hecht, C.; Weber, M.; Grote, V.; Daskalou, E.; Dell’Era, L.; Flynn, D.; Gerasimidis, K.; Gottrand, F.; Hartman, C.; Hulst, J.; et al. Disease associated malnutrition correlates with length of hospital stay in children. Clin. Nutr. 2015, 34, 53e9. [Google Scholar] [CrossRef]
- Pawellek, I.; Dokoupil, K.; Koletzko, B. Prevalence of malnutrition in paediatric hospital patients. Clin. Nutr. 2008, 27, 72e6. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mao, X.; Sheng, J.; Huang, J.; Wang, Y.; Tang, Q.; Cai, W. Development and validation of a pediatric nutritional screening score (PNSS) for hospitalized children. Asia Pac. J. Clin. Nutr. 2018, 27, 65–71. [Google Scholar]
- Prasadajudio, M.; Devaera, Y.; Noormanto, N.; Kuswiyanto, R.B.; Sudarmanto, B.; Andriastuti, M.; Sidiartha, I.G.L.; Sitorus, N.L.; Basrowi, R.W. Disease-Related Malnutrition in Pediatric Patients with Chronic Disease: A Developing Country Perspective. Curr. Dev. Nutr. 2022, 7, 100021. [Google Scholar] [CrossRef] [PubMed]
- Campanozzi, A.; Russo, M.; Catucci, A.; Rutigliano, I.; Canestrino, G.; Giardino, I.; Romondia, A.; Pettoello-Mantovani, M. Hospital-acquired malnutrition in children with mild clinical conditions. Nutrition 2009, 25, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef]
- Sofouli, G.A.; Tsintoni, A.; Fouzas, S.; Vervenioti, A.; Gkentzi, D.; Dimitriou, G. Early Diagnosis of Late-Onset Neonatal Sepsis Using a Sepsis Prediction Score. Microorganisms 2023, 11, 235. [Google Scholar] [CrossRef]
- Wilar, R. Diagnostic value of eosinopenia and neutrophil to lymphocyte ratio on early onset neonatal sepsis. Korean J. Pediatr. 2019, 62, 217–223. [Google Scholar] [CrossRef]
- Karabulut, B.; Alatas, S.O. Diagnostic Value of Neutrophil to Lymphocyte Ratio and Mean Platelet Volume on Early Onset Neonatal Sepsis on Term Neonate. J. Pediatr. Intensive Care 2021, 10, 143–147. [Google Scholar] [CrossRef]
- McAdam, K.P.W.J.; Elin, R.J.; Sipe, I.D.; Wolff, S.M. Changes in human serum amyloid A and C-reactive protein after etiocholanoloneinduced inflammation. J. Clin. Investig. 1978, 61, 390–394. [Google Scholar] [CrossRef]
- Delgado, A.F.; Okay, T.S.; Leone, C.; Nichols, B.; Del Negro, G.M.; Vaz, F.A. Hospital malnutrition and inflammatory response in critically ill children and adolescents admitted to a tertiary intensive care unit. Clinics 2008, 63, 357–362. [Google Scholar] [CrossRef]
- Manary, M.J.; Broadhead, R.L.; Yaresheski, K.E. Whole-body protein kinetics in marasmus and kwashiorkor during acute infection. Am. J. Clin. Nutr. 1998, 67, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Amesty-Valbuena, A.; Pereira, N.; Castillo, J.; García, D.; Nuñez, J.R.; Cayama, N.; Morán, A.; Parra, M.A.; Troconiz, C. Mediadores de inflamación (proteina C reactiva) enelniño con desnutriciónproteico-energética y enelniñoeutrófico. Investig. Clin. 2004, 45, 53–62. [Google Scholar]
- Manary, M.J.; Yarasheski, K.E.; Berger, R.; Abrams, E.T.; Hart, C.A.; Broadhead, R.L. Wholebody leucine kinetics and the acute phase response during acute infection in marasmic Malawian children. Pediatr. Res. 2004, 55, 940–946. [Google Scholar] [CrossRef]
- Li, T.; Dong, G.; Zhang, M.; Xu, Z.; Hu, Y.; Xie, B.; Wang, Y.; Xu, B. Association of Neutrophil-Lymphocyte Ratio and the Presence of Neonatal Sepsis. J. Immunol. Res. 2020, 2020, 7650713. [Google Scholar] [CrossRef] [PubMed]
- Alkan Ozdemir, S.; Arun Ozer, E.; Ilhan, O.; Sutcuoglu, S. Can neutrophil to lymphocyte ratio predict late-onset sepsis in preterm infants? J. Clin. Lab. Anal. 2018, 32, e22338. [Google Scholar] [CrossRef] [PubMed]
- Hamiel, U.; Bahat, H.; Kozer, E.; Hamiel, Y.; Ziv-Baran, T.; Goldman, M. Diagnostic markers of acute infections in infants aged 1 week to 3 months: A retrospective cohort study. BMJ Open 2018, 8, e018092. [Google Scholar] [CrossRef]
- Dursun, A.; Ozsoylu, S.; Akyildiz, B.N. Neutrophil-to-lymphocyte ratio and mean platelet volume can be useful markers to predict sepsis in children. Pak. J. Med. Sci. 2018, 34, 918–922. [Google Scholar] [CrossRef]
- de Jager, C.P.; Wever, P.C.; Gemen, E.F.; Kusters, R.; Van Gageldonk-Lafeber, A.B.; Van Der Poll, T.; Laheij, R.J.F. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS ONE 2012, 7, e46561. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.S.; Park, K.B.; Yoo, G.H.; Kim, S.S.; Lee, S.M. Prediction of renal cortical defect and scar using neutrophil-to-lymphocyte ratio in children with febrile urinary tract infection. Nuklearmedizin 2017, 56, 109–114. [Google Scholar] [CrossRef]
- Zhang, S.; Luan, X.; Zhang, W.; Jin, Z. Platelet-to-Lymphocyte and Neutrophil-to-Lymphocyte Ratio as Predictive Biomarkers for Early-onset Neonatal Sepsis. J. Coll. Physicians Surg. Pak. 2021, 30, 821–824. [Google Scholar]
- Ayala, A.; Herdon, C.D.; Lehman, D.L.; Ayala, C.; Chaudry, I. Differential induction of apoptosis in lymphoid tissues during sepsis: Variation in onset, frequency, and the nature of the mediators. Blood 1996, 87, 4261–4275. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Sun, K.H.; Choi, D.H.; Lee, Y.M.; Choi, S.W.; Kang, S.H.; Park, K.H.; Song, H. Prediction of long-term mortality based on neutrophil-lymphocyte ratio after percutaneous coronary intervention. Am. J. Med. Sci. 2016, 351, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Urgancı, N.; Polat, T.; Ozer, N.; Kayaalp, N. The presence of clinical signs in malnourished infants with acute lower respiratory tract infections. Paediatr. Child Health 2003, 8, 83–86. [Google Scholar] [CrossRef]
- Rosen, E.U.; Geefhuysen, J.; Anderson, R.; Joffe, M.; Rabson, A.R. Leucocyte function in children with kwashiorkor. Arch. Dis. Child. 1975, 50, 220–224. [Google Scholar] [CrossRef]
- Schopfer, K.; Douglas, S.D. Neutrophil function in children with kwashiorkor. J. Lab. Clin. Med. 1976, 88, 450–461. [Google Scholar]
- Blasquez, A.; Clouzeau, H.; Fayon, M.; Mouton, J.-B.; Thambo, J.-B.; Enaud, R.; Lamireau, T. Evaluation of nutritional status and support in children with congenital heart disease. Eur. J. Clin. Nutr. 2016, 70, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Groleau, V.; Thibault, M.; Doyon, M.; Brochu, E.E.; Roy, C.C.; Babakissa, C. Malnutrition in hospitalized children: Prevalence, impact, and management. Can. J. Diet. Pract. Res. 2014, 75, 29–34. [Google Scholar] [CrossRef]
- de Souza Menezes, F.; Leite, H.P.; Koch Nogueira, P.C. Malnutrition as an independent predictor of clinical outcome in critically illchildren. Nutrition 2012, 28, 267–270. [Google Scholar] [CrossRef]
- Corish, C.A.; Kennedy, N.P. Protein-energy undernutrition in hospital in-patients. Br. J. Nutr. 2000, 83, 575–591. [Google Scholar] [CrossRef]
- Santana e Meneses, J.F.; Leite, H.P.; de Carvalho, W.B.; Lopes, J.E. Hypophosphatemia in critically ill children: Prevalence and associated risk factors. Pediatr. Crit. Care Med. 2009, 10, 234–238. [Google Scholar] [CrossRef]
- Boles, J.M.; Bion, J.; Connors, A.; Herridge, M.; Marsh, B.; Melot, C.; Pearl, R.; Silverman, H.; Stanchina, M.; Vieillard-Baron, A.; et al. Weaning from mechanical ventilation. Eur. Respir. J. 2007, 29, 1033–1056. [Google Scholar] [CrossRef]
- Bagri, N.K.; Jose, B.; Shah, S.K.; Bhutia, T.D.; Kabra, S.K.; Lodha, R. Impact of Malnutrition on the Outcome of Critically Ill Children. Indian J. Pediatr. 2015, 82, 601–605. [Google Scholar] [CrossRef]
- Arora, N.S.; Rochester, D.F. Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. Am. Rev. Respir. Dis. 1982, 126, 5–8. [Google Scholar]
- McDonald, C.M.; Olofin, I.; Flaxman, S.; Fawzi, W.W.; Spiegelman, D.; Caulfield, L.E.; Black, R.E.; Ezzati, M.; Danaei, G.; on behalf of the Nutrition Impact Model Study. The effect of multiple anthropometric deficits on child mortality: Meta-analysis of individual data in 10 prospective studies from developing countries. Am. J. Clin. Nutr. 2013, 97, 896–901. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Hoboken, N.J. Applied Logistic Regression; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Goldberg, O.; Amitai, N.; Chodick, G.; Bromiker, R.; Scheuerman, O.; Ben-Zvi, H.; Klinger, G. Can we improve early identification of neonatal late-onset sepsis? A validated prediction model. J. Perinatol. 2020, 40, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.; Maaroof, A.; Mohammad, M.H.S.; Abdelwahab, A. Salivary C-reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. J. Pediatr. 2018, 94, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gong, P.; Jia, X.; Zhang, X.; Li, X.; Zhang, Y.; Zhou, H.; Kang, Y. Comparison of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for the diagnosis of neonatal sepsis: A systematic review and meta-analysis. BMC Pediatr. 2023, 23, 334. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Ma, A.; Zhang, Z.; Liu, Y.; Liang, G. Neutrophil-to-lymphocyte ratio as a predictive marker for severe pediatric sepsis. Transl. Pediatr. 2021, 10, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.K.; Zambruni, M.; Melby, C.L.; Melby, P.C. Impact of Childhood Malnutrition on Host Defense and Infection. Clin. Microbiol. Rev. 2017, 30, 919–971. [Google Scholar] [CrossRef]
- de Sousa, J.C.S.; de Carvalho, A.V.D.; Monte de Prada, L.C.; Marinho, A.P.; de Lima, K.F.; Macedo, S.K.O.; Santos, C.D.P.; da Câmara, S.M.A.; Barreto, A.C.D.N.G.; Pereira, S.A. Nutritional Factors Associated with Late-Onset Sepsis in Very Low Birth Weight Newborns. Nutrients 2021, 14, 196. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.H.; Roumiantsev, S.; Singh, R. PediTools Electronic Growth Chart Calculators: Applications in Clinical Care, Research, and Quality Improvement. J. Med. Internet Res. 2020, 22, e16204. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, A.; Allegaert, K. Catch-Up Growth in Former Preterm Neonates: No Time to Waste. Nutrients 2016, 8, 817. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Bacterial Infection (n = 61) | Sepsis (n = 106) | p-Value |
|---|---|---|---|
| Age (months) | 3 (2, 4) | 3 (2, 5) | 0.033 U |
| Males % (n) | 75.4 (46) | 51.88 (55) | 0.004 χ2 |
| Preterm birth % (n) | 70.49 (43) | 50 (53) | 0.035 χ2 |
| Anthropometric parameters | |||
| 3.52 (3.01, 4.13) | 3.86 (3.10, 5.00) | 0.082 U |
| 0.53 ± 0.04 | 0.57 ± 0.06 | <0.001 U |
| −2.09 (−3.07, −1.39) | −2.48 (−3.89, −1.86) | 0.011 U |
| −0.90 (−1.51, −0.35) | −0.82 (−1.45, −0.12) | 0.205 U |
| −1.98 (−3.01, −1.20) | −2.64 (−3.90, −1.77) | 0.008 U |
| Comorbidity % (n) | |||
| 31.14 (19) | 29.24 (31) | 0.637 χ2 |
| 3.27 (2) | 12.26 (13) | 0.174 χ2 |
| 14.75 (9) | 24.52 (26) | 0.181 χ2 |
| 16.39 (10) | 22.64 (24) | 0.226 χ2 |
| 31.14 (19) | 34.90 (37) | 0.340 χ2 |
| 9.83 (6) | 7.54 (8) | 0.975 χ2 |
| 22.95 (14) | 19.81 (22) | 0.824 χ2 |
| Laboratory parameters | |||
| 9.65 (0.83, 36.1) | 57.2 (17.11, 137) | <0.001 U |
| 0.57 (0.22, 1.47) | 8.81 (2.49, 23.9) | <0.001 U |
| 11.88 (9.94, 15.7) | 18.95 (13.77, 26.82) | <0.001 U |
| 436 (287, 516) | 332 (169, 484) | 0.004 U |
| 10.03 ± 1.02 | 10.04 ± 1.14 | 0.591 U |
| 0.40 (0.27, 0.92) | 0.70 (0.30, 2.42) | 0.075 U |
| 43.8 (16.1, 49.5) | 45.2 (37.7, 51.1) | 0.567 U |
| 1.10 (0.62, 1.64) | 3.21 (2.03, 5.27) | <0.001 U |
| 473 (243, 722) | 935 (429, 1757) | <0.001 U |
| 1.85 (0.97, 3.33) | 5.56 (2.96, 10.23) | <0.001 U |
| Parameters | Sepsis (n = 47) | Severe Sepsis (n = 33) | Septic Shock/MOF (n = 26) | p-Value |
|---|---|---|---|---|
| Age (months) | 3 (2, 5) | 3 (2, 4) | 3.5 (2, 5) | 0.508 |
| GA (weeks) | 37 (31, 39) | 38 (32, 40) | 37.5 (33.5, 39) | 0.633 |
| Weight for age (z) | −2.23 (−2.77, −1.41) | −2.71 (−4.03, −1.98) | −3.45 (−4.70, −2.27) | 0.002 |
| Height for age (z) | −0.79 (−1.31, −0.20) | −0.73 (−1.59, −0.15) | −0.84 (−1.85, −0.37) | 0.904 |
| Weight for height (z) | −2.38 (−3.11, −1.40) | −2.83 (−3.90, −2.09) | −3.47 (−5.08, −2.25) | 0.011 |
| Mechanical ventilation % (n) | 6.38 (3) | 24.2 (8) | 69.2 (18) | <0.001 |
| Prolonged ICU stay % (n) | 31.9 (15) | 48.5 (16) | 57.7 (15) | 0.032 |
| Irresuscitable arrest % (n) | 0 | 0 | 57.7 (15) | <0.001 |
| CRP (mg/L) | 65.5 (20.1, 154) | 47.1 (7.09, 88.5) | 94.1 (35.5, 51.1) | 0.293 |
| PCT (ng/mL) | 4.35 (1.83, 12.4) | 13.1 (4.37, 21.5) | 38.3 (6.73, 71.8) | <0.001 |
| WBC (×103 μL) | 18.9 (13.7, 24.2) | 17.9 (13.9, 29.9) | 19.3 (13.5, 28) | 0.877 |
| PLT (×103 μL) | 336 (179, 488) | 362 (204, 497) | 280 (90.7, 451) | 0.273 |
| MPV (fL) | 9.90 (9.10, 10.6) | 9.90 (9.20, 10.95) | 9.60 (9.10, 10.8) | 0.820 |
| IG (×103 μL) | 0.40 (0.27, 1.30) | 0.70 (0.30, 2.50) | 1.90 (0.40, 3.30) | 0.205 |
| RDW (%) | 46.1 (37.1, 51.6) | 44.5 (39.3, 53.2) | 45.8 (35.5, 51.1) | 0.231 |
| NLR | 2.39 (1.65, 3.10) | 4.80 (2.65, 5.65) | 5.02 (3.10, 8.56) | <0.001 |
| SII | 867 (269, 1396) | 1343 (511, 2300) | 1145 (344, 2725) | 0.061 |
| SIRI | 4.90 (2.46, 7.79) | 6.79 (3.95, 12.4) | 7.05 (3.41, 14.1) | 0.142 |
| NLR | CRP | PCT | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Prolonged ICU stay (>7 days) | 0.345 | <0.001 | −0.214 | 0.032 | −0.032 | 0.769 |
| Acute organ dysfunction | 0.201 | 0.038 | −0.109 | 0.280 | −0.021 | 0.847 |
| Mechanical ventilation | 0.529 | <0.001 | −0.038 | 0.706 | 0.133 | 0.222 |
| Nonresuscitable arrest | 0.405 | <0.001 | 0.001 | 0.992 | 0.109 | 0.319 |
| Sepsis severity | 0.470 | <0.001 | −0.015 | 0.879 | 0.429 | <0.001 |
| AUC | SE | 95%CI | Sensitivity | Specificity | Cut-Off | p-Value | |
|---|---|---|---|---|---|---|---|
| PCT | 0.828 | 0.051 | 0.79–0.93 | 0.82 | 0.76 | 1.56 | <0.001 |
| CRP | 0.704 | 0.057 | 0.59–0.81 | 0.70 | 0.65 | 28.3 | 0.002 |
| NLR | 0.867 | 0.033 | 0.80–0.91 | 0.85 | 0.69 | 1.43 | <0.001 |
| Multivariate Analysis | ||
|---|---|---|
| Variable | β (95% CI) | p-Value |
| PCT | 0.212 (0.001–0.007) | 0.016 |
| CRP | 0.238 (0–0.002) | 0.020 |
| NLR | 0.274 (0.010–0.045) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domnicu, A.E.; Boia, E.R.; Mogoi, M.; Manea, A.-M.; Marcovici, T.M.; Mărginean, O.; Boia, M. The Neutrophil-to-Lymphocyte Ratio (NLR) Can Predict Sepsis’s Presence and Severity in Malnourished Infants—A Single Center Experience. Children 2023, 10, 1616. https://doi.org/10.3390/children10101616
Domnicu AE, Boia ER, Mogoi M, Manea A-M, Marcovici TM, Mărginean O, Boia M. The Neutrophil-to-Lymphocyte Ratio (NLR) Can Predict Sepsis’s Presence and Severity in Malnourished Infants—A Single Center Experience. Children. 2023; 10(10):1616. https://doi.org/10.3390/children10101616
Chicago/Turabian StyleDomnicu, Alina Emilia, Eugen Radu Boia, Mirela Mogoi, Aniko-Maria Manea, Tamara Marcela Marcovici, Otilia Mărginean, and Marioara Boia. 2023. "The Neutrophil-to-Lymphocyte Ratio (NLR) Can Predict Sepsis’s Presence and Severity in Malnourished Infants—A Single Center Experience" Children 10, no. 10: 1616. https://doi.org/10.3390/children10101616
APA StyleDomnicu, A. E., Boia, E. R., Mogoi, M., Manea, A.-M., Marcovici, T. M., Mărginean, O., & Boia, M. (2023). The Neutrophil-to-Lymphocyte Ratio (NLR) Can Predict Sepsis’s Presence and Severity in Malnourished Infants—A Single Center Experience. Children, 10(10), 1616. https://doi.org/10.3390/children10101616