Association between the Clinical, Laboratory and Ultrasound Characteristics and the Etiology of Peripheral Lymphadenopathy in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Methods
2.3. Ethical Approval and Data Availability
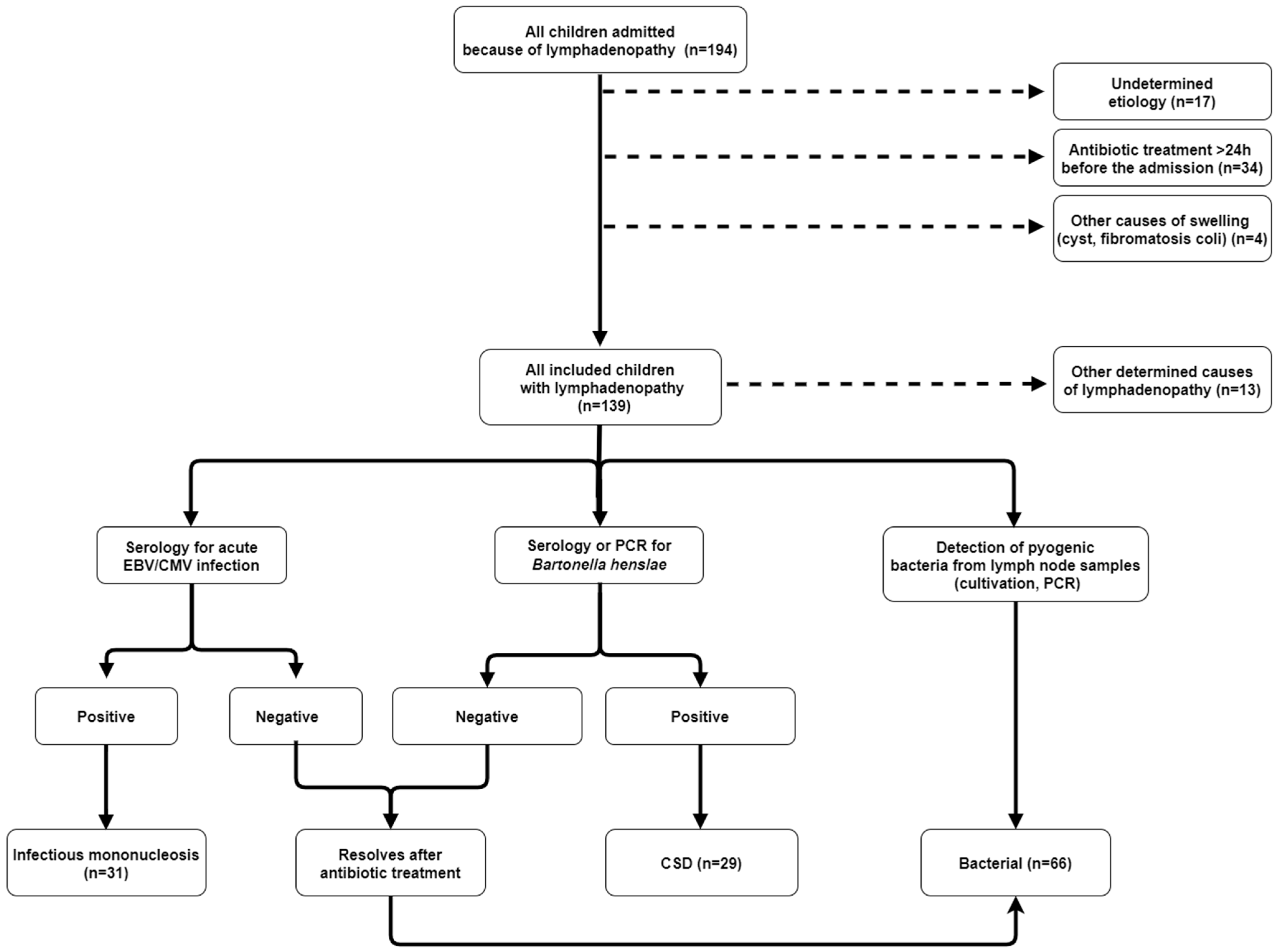
2.4. Stratification of Patients
2.5. Statistical Analysis
3. Results
3.1. Epidemiological, Clinical, Laboratory and Ultrasound Characteristics
3.2. Etiology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahai, S. Lymphadenopathy. Pediatr. Rev. 2013, 34, 216–227. [Google Scholar] [CrossRef]
- Chang, S.S.Y.; Xiong, M.; How, C.H.; Lee, D.M. An approach to cervical lymphadenopathy in children. Singap. Med. J. 2020, 61, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M.S.; Patel, N.A.; Smith, L.P. Pediatric cervical lymphadenopathy. Pediatr. Rev. 2018, 39, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Locke, R.; Comfort, R.; Kubba, H. When does an enlarged cervical lymph node in a child need excision? A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Williamson, H.A. Lymphadenopathy in a family practice: A descriptive study of 249 cases. J. Fam. Pract. 1985, 20, 449–452. [Google Scholar]
- Leung, A.K.C.; Davies, H.D. Cervical Lymphadenitis: Etiology, diagnosis, and management. Curr. Infect. Dis. Rep. 2009, 11, 183–189. [Google Scholar] [CrossRef]
- Ebell, M.H. Epstein-Barr Virus Infectious Mononucleosis. Am. Fam. Physician 2004, 70, 1279–1287. [Google Scholar]
- Howard-Jones, A.R.; Al Abdali, K.; Britton, P.N. Acute bacterial lymphadenitis in children: A retrospective, cross-sectional study. Eur. J. Pediatr. 2023, 182, 2325–2333. [Google Scholar] [CrossRef]
- Hamilton, W.; Pascoe, J.; John, J.; Coats, T.; Davies, S. Diagnosing groin lumps. BMJ 2021, 372, 578. [Google Scholar] [CrossRef]
- Celenk, F.; Gulsen, S.; Baysal, E.; Aytac, I.; Kul, S.; Kanlikama, M. Predictive factors for malignancy in patients with persistent cervical lymphadenopathy. Eur. Arch. Otorhinolaryngol. 2015, 73, 251–256. [Google Scholar] [CrossRef]
- Carithers, H.A. Cat-scratch disease: An overview based on a study of 1200 patients. Am. J. Dis. Child. 1985, 139, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Deosthali, A.; Donches, K.; DelVecchio, M.; Aronoff, S. Etiologies of pediatric cervical lymphadenopathy: A systematic review of 2687 subjects. Glob. Pediatr. Health 2019, 6, 2333794X19865440. [Google Scholar] [CrossRef]
- Sgro, J.M.; Campisi, E.S.; Selvam, S.; Greer, M.C.; Alexander, S.; Ngan, B.; Campisi, P. Cervical lymph node biopsies in the evaluation of children with suspected lymphoproliferative disorders; Experience in a tertiary pediatric setting. J. Pediatr. Surg. 2022, 57, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Oguz, A.; Karadeniz, C.; Temel, E.A.; Citak, E.C.; Visal, O.F. Evaluation of peripheral lymphadenopathy in children. Pediatr. Hematol. Oncol. 2006, 23, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Ling, R.E.; Capsomidis, A.; Patel, S.R. Urgent suspected cancer referrals for childhood lymphadenopathy. Arch. Dis. Child. 2015, 100, 1098–1099. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Krakovitz, P. Enlarged neck lymph nodes in children. Pediatr. Clin. N. Am. 2013, 60, 923–936. [Google Scholar] [CrossRef]
- Ataş, E.; Kesik, V.; Kürşat Fidanci, M.; Kismet, E.; Köseoǧlu, V. Evaluation of children with lymphadenopathy. Turk. Pediatr. Ars. 2014, 49, 30–35. [Google Scholar] [CrossRef]
- Simanovsky, N.; Hiller, N. Importance of sonographic detection of enlarged abdominal lymph nodes in children. J. Ultrasound Med. 2007, 26, 581–584. [Google Scholar] [CrossRef]
- Pattanayak, S.; Chatterjee, S.; Ravikumar, R.; Nijhawan, V.S.; Sharma, V.; Debnath, J. Ultrasound evaluation of cervical lymphadenopathy: Can it reduce the need of histopathology/cytopathology? Med. J. Armed Forces India 2018, 74, 227–234. [Google Scholar] [CrossRef]
- Farndon, S.; Behjati, S.; Jonas, N.; Messahel, B. How to use… lymph node biopsy in paediatrics. Arch. Dis. Child. Educ. Pract. Ed. 2017, 102, 244–248. [Google Scholar] [CrossRef]
- Spijkers, S.; Littooij, A.S.; Nievelstein, R.A.J. Measurements of cervical lymph nodes in children on computed tomography. Pediatr. Radiol. 2020, 50, 534–542. [Google Scholar] [CrossRef]
- Heiduk, M.; Päge, I.; Kliem, C.; Abicht, K.; Klein, G. Pediatric reference intervals determined in ambulatory and hospitalized children and juveniles. Clin. Chim. Acta 2009, 406, 156–161. [Google Scholar] [CrossRef]
- Ross, S.A.; Novak, Z.; Pati, S.; Boppana, S.B. Diagnosis of cytomegalovirus infections. Infect. Disord. Drug Targets 2011, 11, 466–474. [Google Scholar] [CrossRef]
- De Paschale, M.; Clerici, P. Serological diagnosis of Epstein-Barr virus infection: Problems and solutions. World J. Virol. 2012, 1, 31–43. [Google Scholar] [CrossRef]
- Allizond, V.; Costa, C.; Sidoti, F.; Scutera, S.; Bianco, G.; Sparti, R.; Banche, G.; Dalmasso, P.; Cuffini, A.M.; Cavallo, R.; et al. Serological and molecular detection of Bartonella henselae in specimens from patients with suspected cat scratch disease in Italy: A comparative study. PLoS ONE 2019, 14, e0211945. [Google Scholar] [CrossRef] [PubMed]
- Bozlak, S.; Varkal, M.A.; Yildiz, I.; Toprak, S.; Karaman, S.; Erol, O.B.; Yekeler, E.; Unuvar, A.; Kilic, A.; Oguz, F.; et al. Cervical lymphadenopathies in children: A prospective clinical cohort study. Int. J. Pediatr. Otorhinolaryngol. 2016, 82, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Dajani, A.S.; Garcia, R.E.; Wolinsky, E. Etiology of cervical lymphadenitis in children. N. Eng. J. Med. 1963, 24, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Barton, L.L.; Feigin, R.D. Childhood cervical lymphadenitis: A reappraisal. J. Pediatr. 1974, 84, 846–852. [Google Scholar] [CrossRef]
- De Corti, F.; Cecchetto, G.; Vendraminelli, R.; Mognato, G. Fine-needle aspiration cytology in children with superficial lymphadenopathy. Pediatr. Medica Chir. 2014, 36, 80–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaddey, H.L.; Riegel, A.M. Unexplained lymphadenopathy: Evaluation and differential diagnosis. Am. Fam. Physician 2016, 94, 896–903. [Google Scholar] [PubMed]
- Long, M.; Reddy, D.N.; Akiki, S.; Barrowman, N.J.; Zemek, R. Paediatric acute lymphadenitis: Emergency department management and clinical course. Paediatr. Child. Health 2019, 25, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Zangwill, K.M. Cat Scratch Disease and Bartonellaceae: The known, the unknown and the curious. Pediatr. Infect. Dis. J. 2021, 40 (Suppl. 5), 11–15. [Google Scholar] [CrossRef] [PubMed]
- Bilal, J.A. Prevalence and clinical characteristics of primary Epstein–Barr Virus infection among children presented with cervical lymphadenopathy. J. Clin. Diagn. Res. 2015, 9, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Dunmire, S.K.; Hogquist, K.A.; Balfour, H.H. Infectious mononucleosis. Curr. Top. Microbiol. Immunol. 2015, 390, 211–240. [Google Scholar] [CrossRef]
- Bain, B.J. Diagnosis from the blood smear. N. Engl. J. Med. 2005, 353, 498–507. [Google Scholar] [CrossRef]
- Şen, H.S.; Ocak, S.; Yılmazbaş, P. Children with cervical lymphadenopathy: Reactive or not? Turk. J. Pediatr. 2021, 63, 363–371. [Google Scholar] [CrossRef]
| Qualitative Characteristic | Frequency (N) | Percentage of Patients |
|---|---|---|
| Fever | 88 | 63.3 |
| Recent contact with a cat | 48 | 34.5 |
| Residence in a rural area | 76 | 54.7 |
| Generalized lymphadenopathy | 9 | 6.5 |
| Bilateral lymphadenopathy | 31 | 22.3 |
| Inflammation (redness and/or fluctuation) | 31 | 22,3 |
| Lymph node tenderness | 105 | 75.5 |
| Pharyngitis | 60 | 43.2 |
| Increased blood liver enzyme levels 1 | 24 | 17.3 |
| Atypical lymphocytes (>10% of differential WBC count) | 25 | 18.0 |
| US 2, multiple enlarged lymph nodes | 95 | 84.1 |
| US 2, abscess formation | 27 | 23.9 |
| Quantitative characteristic | Median | Interquartile range |
| Age (months) | 50 | 53 |
| Duration of lymphadenopathy (days) | 4 | 5 |
| Size of lymph node (clinically-cm) | 3 | 3 |
| WBC count (×109/L) | 13.4 | 9.1 |
| Neutrophil count (×109/L) | 7.1 | 7.0 |
| CRP (mg/L) | 20 | 43 |
| LDH (μkat/L) | 4,8 | 1.8 |
| Size of lymph node (US 2-cm) | 2.5 | 1.0 |
| Etiology | Frequency (N) | Percentage of Patients |
|---|---|---|
| Bacterial lymphadenitis (Stapyhlococcus aureus or GAS) | 66 | 47.5 |
| Epstein-Barr virus | 29 | 20.9 |
| Cat scratch disease | 29 | 20.9 |
| Atypical mycobacteria | 4 | 2.9 |
| Lymphoma | 3 | 2.2 |
| Kawasaki disease | 2 | 1.4 |
| Cytomegalovirus | 2 | 1.4 |
| Other specified viral infections | 2 | 1.4 |
| Toxoplasmosis | 1 | 0.7 |
| Tularemia | 1 | 0.7 |
| Qualitative Characteristic [n (%)] 1 | CSD (n = 29) | IM (n = 31) | Bacterial (n = 66) | p Value 2 | Odds Ratio (95% Confidence Interval) 3 | Positive Predictive Value (%) 4 | Negative Predictive Value (%) 4 | |
|---|---|---|---|---|---|---|---|---|
| Female sex | 15 (51.7) | 11 (35.5) | 34 (51.5) | CI = 0.297 BC = 1.000 BI = 0.191 | CI = 1.40 (0.83–2.36) BC = 1.00 (0.76–1.30) BI = 1.23 (0.94–1.61) | 56.7 | 51.5 | |
| Fever | 9 (31.0) | 23 (74.2) | 48 (72.7) | CI = 0.002 BC < 0.001 BI = 1.000 | CI = 0.39 (0.22–0.72) BC = 1.78 (1.25–2.53) BI = 0.98 (0.72–1.32) | 60.0 | 60.9 | |
| Recent contact with a cat | 18 (62.1) | 5 (16.1) | 20 (30.3) | CI < 0.001 BC = 0.006 BI = 0.213 | CI = 2.63 (1.53–4.52) BC = 0.65 (0.47–0.91) BI = 1.25 (0.96–1.63) | 46.5 | 44.5 | |
| Residence in a rural area | 18 (62.1) | 21 (67.8) | 30 (45.5) | CI = 0.788 BC = 0.182 BI = 0.051 | CI = 0.88 (0.52–1.51) BC = 0.62 (0.33–1.27) BI = 0.53 (0.28–1.00) | 63.2 | 56.5 | |
| Generalized lymphadenopathy | 1 (3.4) | 8 (25.8) | 0 (0) | CI = 0.029 BC = 0.305 BI < 0.001 | CI = 0.53 (0.37–0.77) | 43.5 | ||
| Inflammation (redness and/or fluctuation) | 7 (24.1) | 0 (0) | 21 (31.8) | CI = 0.004 BC = 0.626 BI < 0.001 | CI = 2.41 (1.75–3.32) BC = 1.12 (0.85–1.47) BI = 1.67 (1.40–2.04) | 75.0 | 54.1 | |
| Lymph node tenderness | 23 (79.3) | 17 (54.8) | 58 (87.8) | CI = 0.058 BC = 0.348 BI = 0.001 | CI = 1.92 (0.93–3.94) BC = 1.25 (0.78–2.01) BI = 2.13 (1.21–3.75) | 59.2 | 71.4 | |
| Pharyngitis | 4 (13.8) | 22 (71.0) | 27 (40.9) | CI < 0.001 BC = 0.009 BI = 0.006 | CI = 0.21 (0.08–0.53) BC = 1.43 (1.13–1.82) BI = 0.68 (0.51–0.90) | 50.9 | 46.6 | |
| Increased blood liver enzyme levels 5 | 1 (3.4) | 20 (64.5) | 3 (4.5) | CI < 0.001 BC = 1.000 BI < 0.001 | CI = 0.07 (0.01–0.45) BC = 1.08 (0.60–1.94) BI = 0.15 (0.05–0.44) | 12.5 | 38.2 | |
| Atypical lymphocytes (>10% of differential WBC count) | 0 (0) | 25 (75.8) | 0 (0) | CI < 0.001 BI < 0.001 | 34.7 | |||
| Quantitative characteristic [median (IQR)] | ||||||||
| Age (months) | 78 (90) | 45 (61) | 45 (35) | CI = 0.089 BC = 0.010 BI = 0.358 | ||||
| Duration of lymphadenopathy (days) | 5 (13) | 3 (5) | 3 (4) | CI = 0.197 BC = 0.011 BI = 0.139 | ||||
| Size of lymph node (clinically-cm) | 3 (3.1) | 3 (2) | 4 (2) | CI = 0.945 BC = 0.043 BI = 0.019 | ||||
| WBC count (×109/L) | 9.9 (4.9) | 15.9 (9.8) | 15.1 (9.3) | CI = 0.002 BC < 0.001 BI = 0.634 | ||||
| Neutrophil count (×109/L) | 5.6 (4.5) | 4.0 (3.7) | 9.7 (7.4) | CI = 0.030 BC < 0.001 BI < 0.001 | ||||
| CRP (mg/L) | 9 (21) | 14 (34) | 34 (48) | CI = 0.124 BC < 0.001 BI = 0.002 | ||||
| LDH (μkat/L) | 4.1 (1.6) | 7.2 (4.3) | 4.4 (1.0) | CI < 0.001 BC = 1.000 BI < 0.001 | ||||
| Qualitative Characteristic [n (%)] 1 | CSD (n = 28) | IM (n = 13) | Bacterial (n = 60) | p Value 2 | Odds Ratio (95% Confidence Interval) 3 | Positive Predictive Value (%) 4 | Negative Predictive Value (%) 4 |
|---|---|---|---|---|---|---|---|
| Multiple enlarged lymph nodes | 22 (78.6) | 13 (100) | 50 (83.3) | CI = 0.152 BC = 0.569 BI = 0.192 | CI = 0.77 (0.51–1.29) BC = 1.23 (0.60–2.53) BI = 0.86 (0.57–1.39) | 58.8 | 37.5 |
| Abscess formation | 4 (14.3) | 1 (7.7) | 19 (31.6) | CI = 1.000 BC = 0.118 BI = 0.097 | CI = 1.20 (0.73–1.97) BC = 1.31 (1.01–1.71) BI = 1.23 (0.99–1.47) | 79.8 | 46.8 |
| Quantitative characteristic [median (IQR)] | |||||||
| Size of lymph node (cm) 5 | 2.7 (1.1) | 2.5 (0.8) | 2.3 (0.7) | CI = 0.441 BC = 0.027 BI = 0.512 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berce, V.; Rataj, N.; Dorič, M.; Zorko, A.; Kolarič, T. Association between the Clinical, Laboratory and Ultrasound Characteristics and the Etiology of Peripheral Lymphadenopathy in Children. Children 2023, 10, 1589. https://doi.org/10.3390/children10101589
Berce V, Rataj N, Dorič M, Zorko A, Kolarič T. Association between the Clinical, Laboratory and Ultrasound Characteristics and the Etiology of Peripheral Lymphadenopathy in Children. Children. 2023; 10(10):1589. https://doi.org/10.3390/children10101589
Chicago/Turabian StyleBerce, Vojko, Nina Rataj, Maja Dorič, Aleksandra Zorko, and Tjaša Kolarič. 2023. "Association between the Clinical, Laboratory and Ultrasound Characteristics and the Etiology of Peripheral Lymphadenopathy in Children" Children 10, no. 10: 1589. https://doi.org/10.3390/children10101589
APA StyleBerce, V., Rataj, N., Dorič, M., Zorko, A., & Kolarič, T. (2023). Association between the Clinical, Laboratory and Ultrasound Characteristics and the Etiology of Peripheral Lymphadenopathy in Children. Children, 10(10), 1589. https://doi.org/10.3390/children10101589





