Relation of Flatfoot Severity with Flexibility and Isometric Strength of the Foot and Trunk Extensors in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
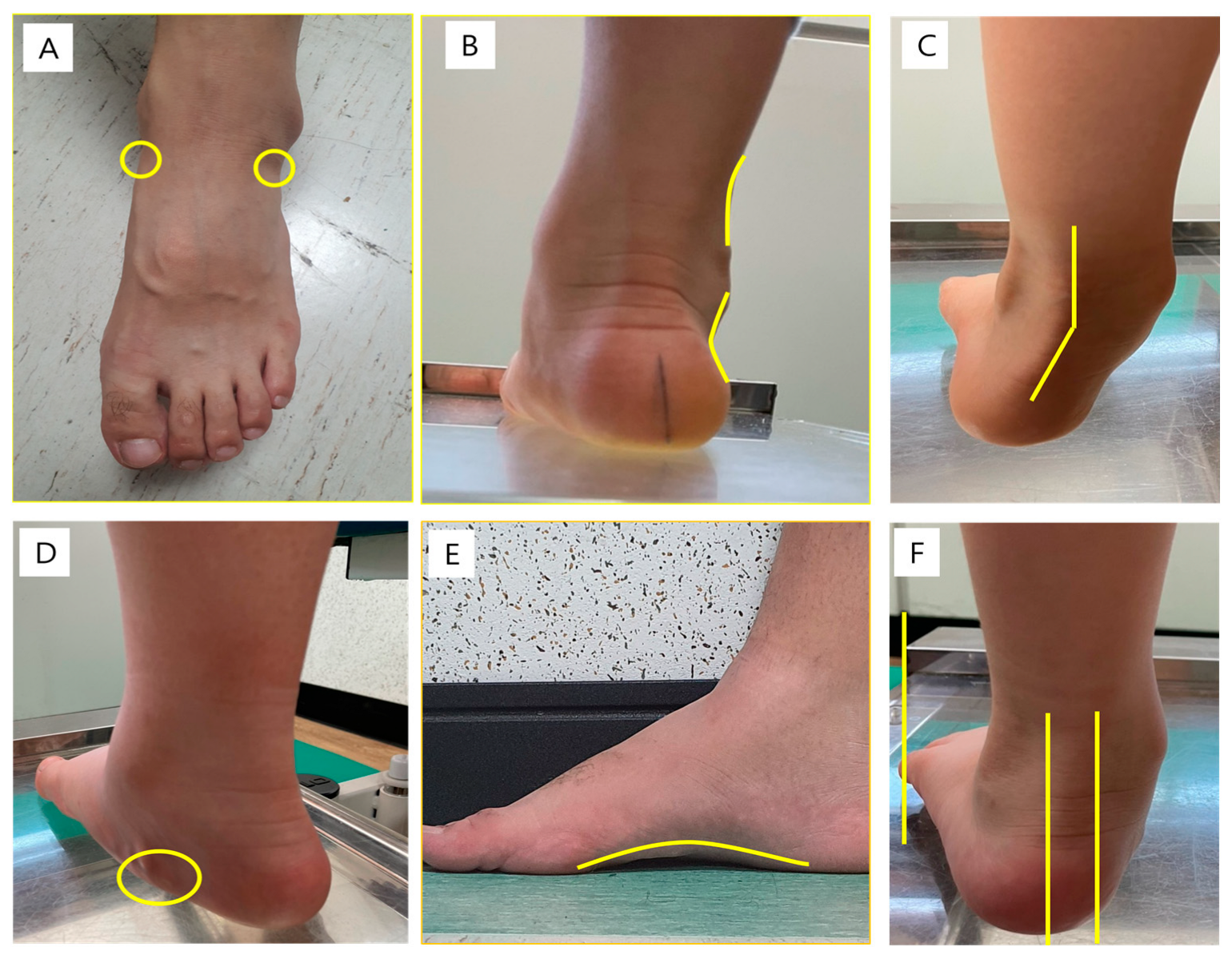
2.2. Physical Examination
2.3. Radiographic Assessment of the Foot
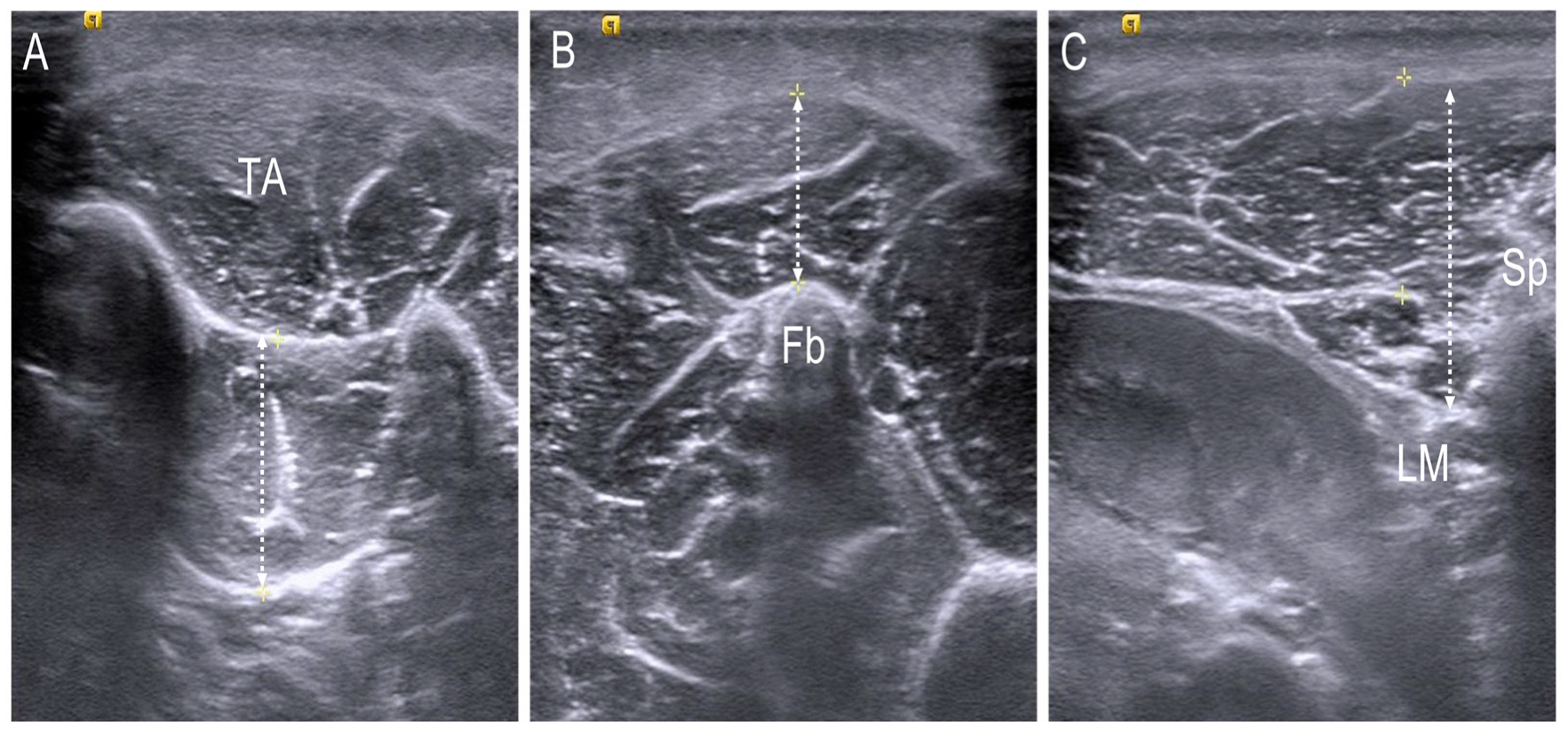
2.4. Thickness Assessment of the Foot and Trunk Muscle Using Ultrasonography
2.5. Isometric Strength Assessment of the Ankle and Trunk Muscle
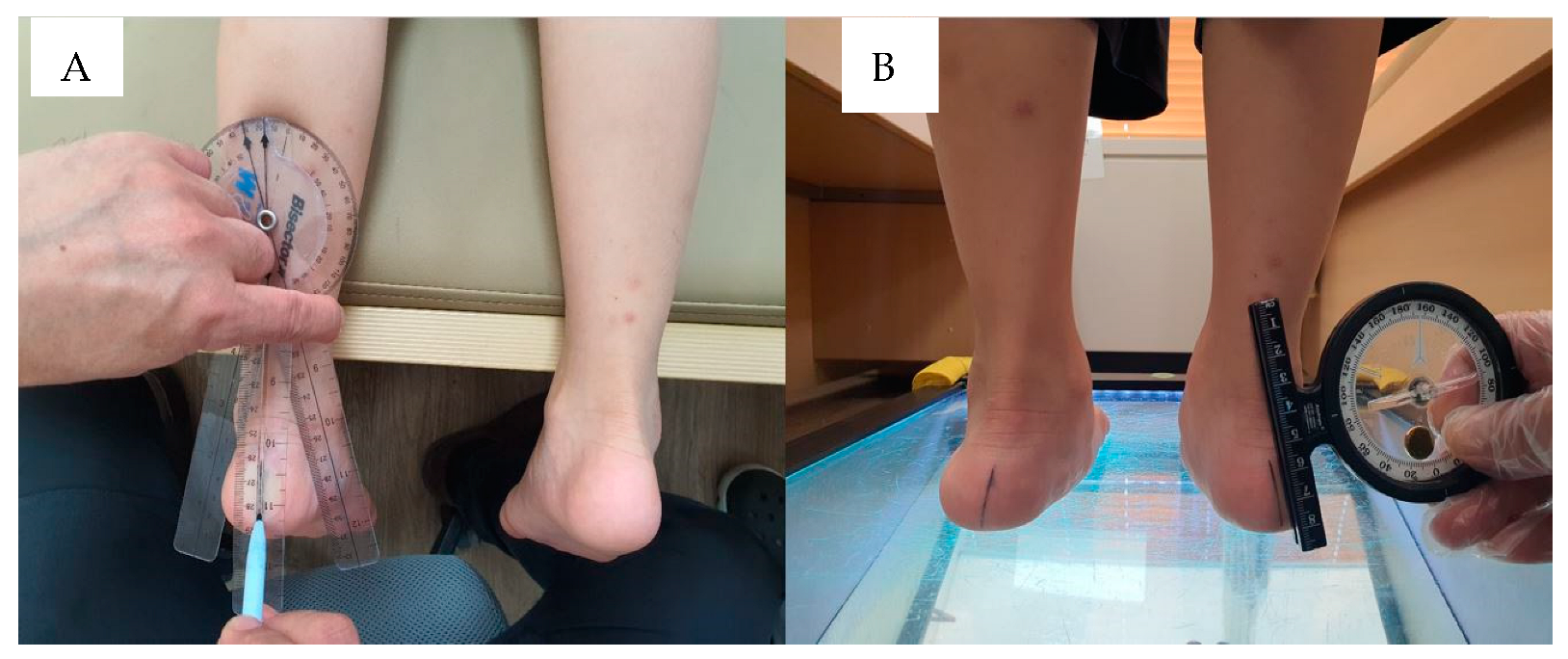
2.6. Flexibility of Joints
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angin, S.; Angin, S.; Crofts, G.; Mickle, K.J.; Nester, C.J. Ultrasound evaluation of foot muscles and plantar fascia in pes planus. Gait Posture 2014, 40, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lim, K.B.; Yoo, J.; Yoon, S.W.; Yun, H.J.; Jeong, T.H. Effect of custom-molded foot orthoses on foot pain and balance in children with symptomatic flexible flat feet. Ann. Rehabil. Med. 2015, 39, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Aenumulapalli, A.; Kulkarni, M.M.; Gandotra, A.R. Prevalence of flexible flat foot in adults: A cross-sectional study. J. Clin. Diagn. Res. 2017, 11, AC17–AC20. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.; Kotz, R.; Ledl, T.; Hauser, G.; Sluga, M. Prevalence of flat foot in preschool-aged children. Pediatrics 2006, 118, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Demneh, E.; Melvin, J.M.A.; Mickle, K. Prevalence of pathological flatfoot in school-age children. Foot 2018, 37, 38–44. [Google Scholar] [CrossRef]
- Yagerman, S.E.; Cross, M.B.; Positano, R.; Doyle, S.M. Evaluation and treatment of symptomatic pes planus. Curr. Opin. Pediatr. 2011, 23, 60–67. [Google Scholar] [CrossRef]
- Carr, J.B.; Yang, S.; Lather, L.A. Pediatric pes planus: A state-of-the-art review. Pediatrics 2016, 137, e20151230. [Google Scholar] [CrossRef]
- Chen, K.C.; Yeh, C.J.; Tung, L.C.; Yang, J.F.; Yang, S.F.; Wang, C.H. Relevant factors influencing flatfoot in preschool-aged children. Eur. J. Pediatr. 2011, 170, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Van Boerum, D.H.; Sangeorzan, B.J. Biomechanics and pathophysiology of flat foot. Foot Ankle Clin. 2003, 8, 419–430. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lou, S.Z.; Huang, C.Y.; Su, F.C. Effects of foot orthoses on gait patterns of flat feet patients. Clin. Biomech. 2010, 25, 265–270. [Google Scholar] [CrossRef]
- Cho, D.J.; Ahn, S.Y.; Bok, S.K. Effect of foot orthoses in children with symptomatic flexible flatfoot based on ultrasonography of the ankle invertor and evertor muscles. Ann. Rehabil. Med. 2021, 45, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; McKay, H.A.; Macdonald, H.; Nettlefold, L.; Baxter-Jones, A.D.; Cameron, N.; Brasher, P.M. Enhancing a Somatic Maturity Prediction Model. Med. Sci. Sports Exerc. 2015, 47, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Root, M.I. Biomechanical examination of the foot. J. Am. Podiatry Assoc. 1973, 63, 28–29. [Google Scholar] [PubMed]
- Shin, Y.; Ahn, S.Y.; Bok, S.K. Relationships between relative ankle muscle ratios, severity of symptoms, and radiologic parameters in adolescent patients with symptomatic flexible flat feet. Ann. Rehabil. Med. 2021, 45, 123–130. [Google Scholar] [CrossRef]
- Youn, K.J.; Ahn, S.Y.; Kim, B.O.; Park, I.S.; Bok, S.K. Long-term effect of rigid foot orthosis in children older than six years with flexible flat foot. Ann. Rehabil. Med. 2019, 43, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, K.B.; Jeong, J.O.; Kwon, N.Y.; Jeong, S.M. Correlation of foot posture index with plantar pressure and radiographic measurements in pediatric flatfoot. Ann. Rehabil. Med. 2015, 39, 10–17. [Google Scholar] [CrossRef]
- Gijon-Nogueron, G.; Marchena-Rodriguez, A.; Montes-Alguacil, J.; Evans, A.M. Evaluation of the paediatric foot using footprints and foot posture index: A cross-sectional study. J. Paediatr. Child Health 2020, 56, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.J.; Vanore, J.V.; Thomas, J.L.; Kravitz, S.R.; Mendelson, S.A.; Mendicino, R.W.; Silvani, S.H.; Gassen, S.C. Diagnosis and treatment of pediatric flatfoot. J. Foot Ankle Surg. 2004, 43, 341–373. [Google Scholar] [CrossRef]
- Sinha, S.; Song, H.R.; Kim, H.J.; Park, M.S.; Yoon, Y.C.; Song, S.H. Medial arch orthosis for paediatric flatfoot. J. Orthop. Surg. 2013, 21, 37–43. [Google Scholar] [CrossRef]
- Murley, G.S.; Tan, J.M.; Edwards, R.M.; De Luca, J.; Munteanu, S.E.; Cook, J.L. Foot posture is associated with morphometry of the peroneus longus muscle, tibialis anterior tendon, and Achilles tendon. Scand. J. Med. Sci. Sports 2014, 24, 535–541. [Google Scholar] [CrossRef]
- Stokes, M.; Rankin, G.; Newham, D.J. Ultrasound imaging of lumbar multifidus muscle: Normal reference ranges for measurements and practical guidance on the technique. Man. Ther. 2005, 10, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Shigeshima, K.; Yamasaki, H.; Katayama, K. Reliability, minimal detectable change and measurement errors in knee extension muscle strength measurement using a hand-held dynamometer in young children. J. Phys. Ther. Sci. 2022, 34, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Escobar, R.G.; Munoz, K.T.; Dominguez, A.; Banados, P.; Bravo, M.J. Maximal isometric muscle strength values obtained by hand-held dynamometry in children between 6 and 15 years of age. Muscle Nerve 2017, 55, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Beenakker, E.A.; van der Hoeven, J.H.; Fock, J.M.; Maurits, N.M. Reference values of maximum isometric muscle force obtained in 270 children aged 4–16 years by hand-held dynamometry. Neuromuscul. Disord. 2001, 11, 441–446. [Google Scholar] [CrossRef]
- Beighton, P.; Solomon, L.; Soskolne, C.L. Articular mobility in an African population. Ann. Rheum. Dis. 1973, 32, 413–418. [Google Scholar] [CrossRef]
- Vittore, D.; Patella, V.; Petrera, M.; Caizzi, G.; Ranieri, M.; Putignano, P.; Spinarelli, A. Extensor deficiency: First cause of childhood flexible flat foot. Orthopedics 2009, 32, 28. [Google Scholar]
- Benedetti, M.G.; Ceccarelli, F.; Berti, L.; Luciani, D.; Catani, F.; Boschi, M.; Giannini, S. Diagnosis of flexible flatfoot in children: A systematic clinical approach. Orthopedics 2011, 34, 94. [Google Scholar] [CrossRef] [PubMed]
- Halabchi, F.; Mazaheri, R.; Mirshahi, M.; Abbasian, L. Pediatric flexible flatfoot; clinical aspects and algorithmic approach. Iran. J. Pediatr. 2013, 23, 247–260. [Google Scholar] [PubMed]
- Jerosch, J.; Schunck, J.; Abdel-Aziz, H. The stop screw technique—A simple and reliable method in treating flexible flatfoot in children. Foot Ankle Surg. 2009, 15, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raoof, N. Influence of second-degree flatfoot on spinal and pelvic mechanics in young females. Int. J. Ther. Rehabil. 2013, 20, 428–434. [Google Scholar] [CrossRef]
- Resende, R.A.; Deluzio, K.J.; Kirkwood, R.N.; Hassan, E.A.; Fonseca, S.T. Increased unilateral foot pronation affects lower limbs and pelvic biomechanics during walking. Gait Posture 2015, 41, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Barwick, A.; Smith, J.; Chuter, V. The relationship between foot motion and lumbopelvic-hip function: A review of the literature. Foot 2012, 22, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Elataar, F.F.; Abdelmajeed, S.F.; Abdellatif, N.M.N.; Mohammed, M.M. Core muscles’ endurance in flexible flatfeet: A cross-sectional study. J. Musculoskelet. Neuronal Interact. 2020, 20, 404–410. [Google Scholar] [PubMed]
- Lin, C.J.; Lai, K.A.; Kuan, T.S.; Chou, Y.L. Correlating factors and clinical significance of flexible flatfoot in preschool children. J. Pediatr. Orthop. 2001, 21, 378–382. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chih, Y.-C.; Shih, C.-L.; Chen, S.-J.; Shen, P.-C.; Tien, Y.-C. Joint hypermobility and preschool-age flexible flatfoot. Medicine 2022, 101, e29608. [Google Scholar] [CrossRef]
- Lee, J.H.; Sung, I.Y.; Yoo, J.Y. Clinical or radiologic measurements and 3-D gait analysis in children with pes planus. Pediatr. Int. 2009, 51, 201–205. [Google Scholar] [CrossRef]


| −2 | −1 | 0 | 1 | 2 | |
|---|---|---|---|---|---|
| Talar head palpation | Talar head palpable on the lateral side/but not on the medial side | Talar head palpable on the lateral side/ slightly palpable on the medial side | Talar head equally palpable on the lateral and medial side | Talar head slightly palpable on the lateral side/palpable on the medial side | Talar head not palpable on the lateral side/but palpable on the medial side |
| Supra- and infra-lateral malleolar curvature | Curve below the malleolus either straight or convex | Curve below the malleolus concave, but flatter/more than the curve above the malleolus | Both infra- and supra-malleolar curves roughly equal | Curve below the malleolus more concave than the curve above the malleolus | Curve below the malleolus markedly more concave than the curve above the malleolus |
| Calcaneal frontal plane position | More than an estimated 5° inverted (varus) | Between vertical and an estimated 5° inverted (varus) | Vertical | Between vertical and an estimated 5° everted (valgus) | More than an estimated 5° everted (valgus) |
| Prominence in the region of the talonavicular joint (TNJ) | Area of the TNJ markedly concave | Area of the TNJ slightly, but definitely, concave | Area of the TNJ flat | Area of the TNJ bulging slightly | Area of the TNJ bulging markedly |
| Congruence of the medial longitudinal arch | Arch high and acutely angled towards the posterior end of the medial arch | Arch moderately high and slightly acute posteriorly | Arch height normal and concentrically curved | Arch lowered with some flattening in the central position | Arch very low with severe flattening in the central position—arch making ground contact |
| Abduction/adduction of the forefoot on the rare foot | No lateral toes visible; medial toes clearly visible | Medial toes clearly more visible than the lateral | Medial and lateral toes equally visible | Lateral toes clearly more visible than medial | No medial toes visible; lateral toes clearly visible |
| Characteristics | Total (n = 16) | Male (n = 12) | Female (n = 4) | |
|---|---|---|---|---|
| Age (year) | Median Interquartile range Minimum Maximum | 6 5–7 4 8 | 6 5–7 4 8 | 5.5 4.75–6 4 6 |
| Height (cm) | Median Interquartile range Minimum Maximum | 119.4 106.1–123.8 100.3 135.5 | 119.4 106.1–123.8 100.3 135.5 | 120.35 115.25–122.45 104 124.7 |
| Body weight (kg) | Median Interquartile range Minimum Maximum | 21.6 16.8–25.9 15.8 43.4 | 21 16.5–23.3 15.8 43.4 | 25.75 23.3–26.5 16.7 28 |
| BMI | Median Interquartile range Minimum Maximum | 15.46 14.9–17.2 14 23.63 | 15.01 14.4–16.0 14 23.63 | 17.78 17–18 16.7 18 |
| Maturity offset | Mean ± SD | −5.28 ± 0.57 | −5.53 ± 0.61 | −5.04 ± 0.56 |
| Characteristics | Total (n = 16) | Male (n = 12) Mean ± SD | Female (n = 4) |
|---|---|---|---|
| RCSPA (°) | −7.94 ± 2.37 | −8.08 ± 2.22 | −7.5 ± 2.74 |
| FPI score (points) | 9.45 ± 1.44 | 9.78 ± 1.31 | 8.0 ± 1.0 |
| Beighton’s score (points) | 8.43 ± 0.98 | 8.5 ± 0.81 | 8.25 ± 1.30 |
| Muscle thickness (mm) | |||
| Tibialis posterior | 17.08 ± 3.07 | 17.20 ± 3.35 | 16.73 ± 2.05 |
| Peroneus longus | 11.32 ± 2.74 | 11.33 ± 2.92 | 11.30 ± 2.11 |
| L1 multifidus | 15.68 ± 2.25 | 16.04 ± 2.22 | 14.61 ± 2.01 |
| Muscle power (N) | |||
| Ankle inverter | 12.08 ± 4.62 | 12.01 ± 4.89 | 12.28 ± 3.64 |
| Ankle evertor | 10.52 ± 3.20 | 10.82 ± 3.35 | 9.63 ± 2.51 |
| L1 multifidus | 10.90 ± 2.70 | 11.24 ± 2.88 | 9.90 ± 1.68 |
| Radiographic parameter (°) | |||
| CPA | 11.00 ± 2.37 | 11.13 ± 2.53 | 10.64 ± 1.73 |
| TMA | 22.81 ± 5.31 | 22.37 ± 5.28 | 24.15 ± 5.15 |
| TCA | 51.74 ± 4.09 | 51.38 ± 3.84 | 52.83 ± 4.60 |
| Muscle Thickness/Muscle Power | Correlation | p-Value |
|---|---|---|
| Tibialis posterior/ankle inverter | 0.679 ** | 0.000 |
| Peroneus longus/ankle evertor | 0.151 | 0.418 |
| L1 multifidus/lumbar | 0.431 * | 0.014 |
| Muscle Thickness/Muscle Power | Correlation | p-Value |
|---|---|---|
| Tibialis posterior/ankle inverter | 0.653 ** | 0.001 |
| Peroneus longus/ankle evertor | 0.080 | 0.718 |
| L1 multifidus/lumbar | 0.413 * | 0.045 |
| FPI | RCSPA | |||
|---|---|---|---|---|
| Correlation | p-Value | Correlation | p-Value | |
| Muscle Thickness | ||||
| TP | −0.558 ** | 0.009 | 0.258 | 0.162 |
| PL | −0.220 | 0.338 | −0.118 | 0.526 |
| L1 multifidus | −0.527 * | 0.012 | −0.177 | 0.331 |
| Muscle power | ||||
| Ankle inverter | −0.580 ** | 0.005 | 0.010 | 0.956 |
| Ankle evertor | −0.257 | 0.249 | −0.044 | 0.809 |
| Lumbar | −0.436 * | 0.043 | 0.245 | 0.176 |
| Radiologic parameter | ||||
| CPA | −0.290 | 0.191 | 0.165 | 0.368 |
| TMA | −0.214 | 0.339 | 0.017 | 0.925 |
| TCA | −0.078 | 0.730 | 0.489 ** | 0.005 |
| FPI | RCSPA | |||
|---|---|---|---|---|
| Correlation | p-Value | Correlation | p-Value | |
| Muscle Thickness | ||||
| TP | −0.493 * | 0.044 | 0.375 | 0.078 |
| PL | −0.127 | 0.628 | 0.018 | 0.935 |
| L1 multifidus | −0.507 * | 0.032 | −0.020 | 0.927 |
| Muscle power | ||||
| Ankle inverter | −0.603 ** | 0.008 | 0.124 | 0.565 |
| Ankle everter | −0.447 | 0.063 | 0.043 | 0.841 |
| Lumbar | −0.632 ** | 0.005 | 0.330 | 0.115 |
| Radiologic parameter | ||||
| CPA | −0.372 | 0.129 | 0.296 | 0.160 |
| TMA | −0.163 | 0.517 | −0.044 | 0.838 |
| TCA | −0.098 | 0.699 | 0.544 ** | 0.006 |
| Beighton Score (Flexibility) | ||
|---|---|---|
| Correlation | p-Value | |
| RCSPA | 0.136 | 0.491 |
| FPI | 0.378 | 0.122 |
| Radiologic parameter | ||
| CPA | −0.023 | 0.907 |
| TMA | 0.308 | 0.110 |
| TCA | 0.054 | 0.783 |
| Muscle thickness | ||
| TP | −0.071 | 0.720 |
| PL | −0.019 | 0.925 |
| L1 multifidus | −0.372 | 0.051 |
| Muscle power | ||
| Ankle inverter | 0.008 | 0.967 |
| Ankle everter | 0.242 | 0.215 |
| Lumbar | 0.077 | 0.698 |
| Beighton Score (Flexibility) | ||
|---|---|---|
| Correlation | p-Value | |
| RCSPA | 0.241 | 0.307 |
| FPI | 0.000 | 1.000 |
| Radiologic parameter | ||
| CPA | −0.252 | 0.284 |
| TMA | 0.422 | 0.063 |
| TCA | 0.025 | 0.916 |
| Muscle thickness | ||
| TP | 0.203 | 0.390 |
| PL | −0.226 | 0.339 |
| L1 multifidus | −0.387 | 0.089 |
| Muscle power | ||
| Ankle inverter | 0.227 | 0.335 |
| Ankle evertor | 0.411 | 0.072 |
| Lumbar | 0.129 | 0.587 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.H.; Cha, S.; Choi, J.E.; Jeon, M.; Choi, J.Y.; Yang, S.-S. Relation of Flatfoot Severity with Flexibility and Isometric Strength of the Foot and Trunk Extensors in Children. Children 2023, 10, 19. https://doi.org/10.3390/children10010019
Kim MH, Cha S, Choi JE, Jeon M, Choi JY, Yang S-S. Relation of Flatfoot Severity with Flexibility and Isometric Strength of the Foot and Trunk Extensors in Children. Children. 2023; 10(1):19. https://doi.org/10.3390/children10010019
Chicago/Turabian StyleKim, Min Hwan, Sangha Cha, Jae Eun Choi, Minsoo Jeon, Ja Young Choi, and Shin-Seung Yang. 2023. "Relation of Flatfoot Severity with Flexibility and Isometric Strength of the Foot and Trunk Extensors in Children" Children 10, no. 1: 19. https://doi.org/10.3390/children10010019
APA StyleKim, M. H., Cha, S., Choi, J. E., Jeon, M., Choi, J. Y., & Yang, S.-S. (2023). Relation of Flatfoot Severity with Flexibility and Isometric Strength of the Foot and Trunk Extensors in Children. Children, 10(1), 19. https://doi.org/10.3390/children10010019





