Exploratory Longitudinal Analysis of the Circulating CHIT1 Activity in Pediatric Patients with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Variables
2.2. Chitotriosidase Circulating Activity
2.3. Genetic Analysis of CHIT1 Gene
2.4. Statistical Analysis
3. Results
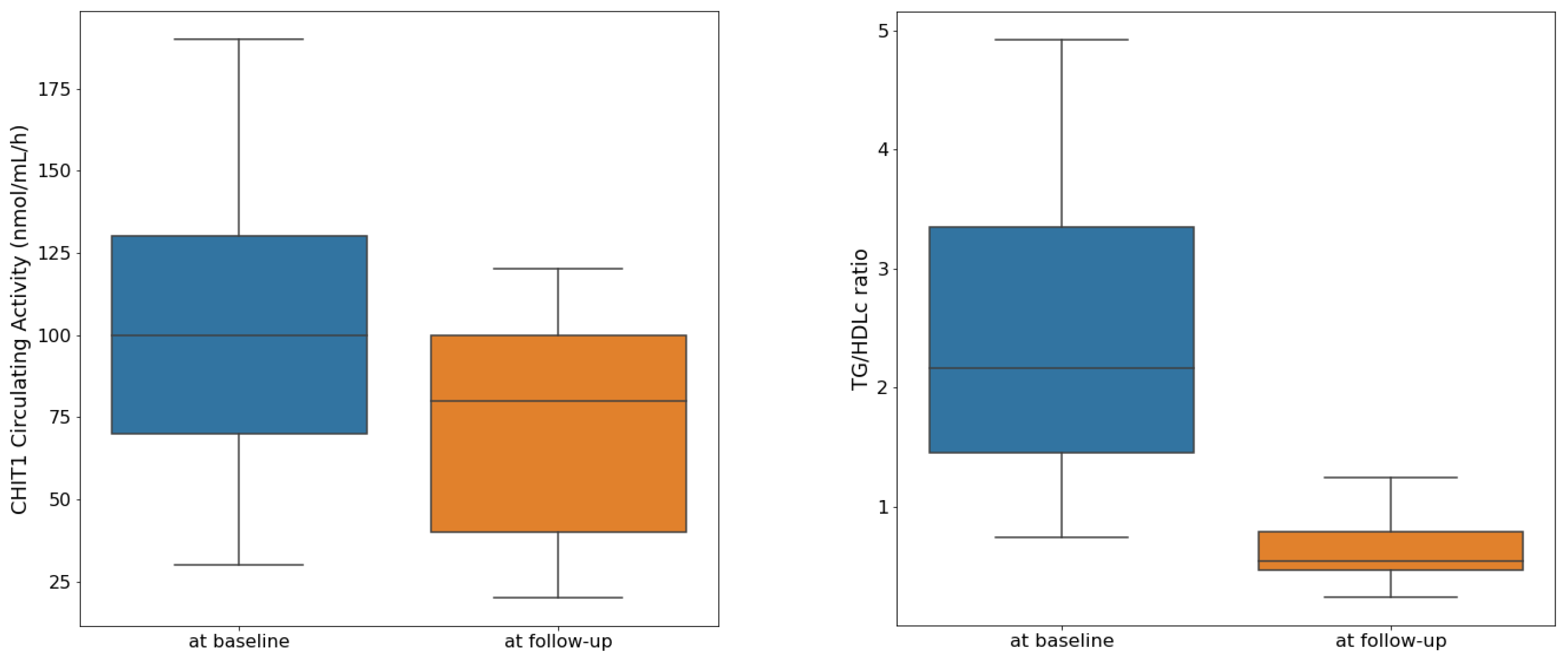
3.1. Description of the Study Sample and Longitudinal Analysis
3.2. Cross-Sectional Analysis at the Baseline
3.3. Cross-Sectional Analysis at the Follow-Up
3.4. Influence of dup24 in the CHIT1 Gene upon the Change of CHIT Circulating Activity
4. Discussions
4.1. CHIT1 Circulating Activity and the Insulin Resistance Expressed by TG/HDLc
4.2. Relationship between the Clinical Indicators of Adiposity and Insulin Resistance
4.3. The Influence of Puberty on Insulin Resistance
4.4. Limitations and Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P.J.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Twig, G. The Impact of Childhood and Adolescent Obesity on Cardiovascular Risk in Adulthood: A Systematic Review. Curr. Diab. Rep. 2018, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Horesh, A.; Tsur, A.M.; Bardugo, A.; Twig, G. Adolescent and Childhood Obesity and Excess Morbidity and Mortality in Young Adulthood—A Systematic Review. Curr. Obes. Rep. 2021, 10, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Armocida, B.; Monasta, L.; Sawyer, S.; Bustreo, F.; Segafredo, G.; Castelpietra, G.; Ronfani, L.; Pasovic, M.; Hay, S.; GBD 2019 Europe NCDs in Adolescents Collaborators; et al. Burden of non-communicable diseases among adolescents aged 10–24 years in the EU, 1990–2019: A systematic analysis of the Global Burden of Diseases Study 2019. Lancet Child Adolesc. Health 2022, 6, 367–383. [Google Scholar] [CrossRef]
- Harman-Boehm, I.; Blüher, M.; Redel, H.; Sion-Vardy, N.; Ovadia, S.; Avinoach, E.; Shai, I.; Klöting, N.; Stumvoll, M.; Bashan, N.; et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: Effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 2007, 92, 2240–2247. [Google Scholar] [CrossRef]
- Cotillard, A.; Poitou, C.; Torcivia, A.; Bouillot, J.L.; Dietrich, A.; Klöting, N.; Grégoire, C.; Lolmede, K.; Blüher, M.; Clément, K. Adipocyte Size Threshold Matters: Link with Risk of Type 2 Diabetes and Improved Insulin Resistance After Gastric Bypass. J. Clin. Endocrinol. Metab. 2014, 99, e1466–e1470. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Boden, G.; Duan, X.; Homko, C.; Molina, E.J.; Song, W.; Perez, O.; Cheung, P.; Merali, S. Increase in Endoplasmic Reticulum Stress–Related Proteins and Genes in Adipose Tissue of Obese, Insulin-Resistant Individuals. Diabetes 2008, 57, 2438–2444. [Google Scholar] [CrossRef]
- Michailidou, Z. Fundamental roles for hypoxia signalling in adipose tissue metabolism and inflammation in obesity. Curr. Opin. Physiol. 2019, 12, 39–43. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Inouye, K.E.; Shi, H.; Howard, J.K.; Daly, C.H.; Lord, G.M.; Rollins, B.J.; Flier, J.S. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 2007, 56, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, M.; van Roomen, C.P.A.A.; Renkema, G.H.; Bussink, A.P.; Andrews, L.; Blommaart, E.F.C.; Sugar, A.; Verhoeven, A.J.; Boot, R.G.; Aerts, J.M.F.G. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int. Immunol. 2005, 17, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Malaguarnera, G.; De Gregorio, C.; Drago, F.; Malaguarnera, L. Evaluation of CHI3L-1 and CHIT-1 expression in differentiated and polarized macrophages. Inflammation 2013, 36, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, M.; Voorn-Brouwer, T.; Scheij, S.S.; Verhoeven, A.J.; Boot, R.G.; Aerts, J.M.F.G. Curdlan-mediated regulation of human phagocyte-specific chitotriosidase. FEBS Lett. 2010, 584, 3165–3169. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Malaguarnera, G.; De Gregorio, C.; D’Amico, F.; Mazzarino, M.C.; Malaguarnera, L. Modulation of Chitotriosidase During Macrophage Differentiation. Cell Biochem. Biophys. 2013, 66, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Boot, R.G.; Renkema, G.H.; Verhoek, M.; Strijland, A.; Bliek, J.; de Meulemeester, T.M.; Mannens, M.M.; Aerts, J.M. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J. Biol. Chem. 1998, 273, 25680–25685. [Google Scholar] [CrossRef]
- Lee, P.; Waalen, J.; Crain, K.; Smargon, A.; Beutler, E. Human Chitotriosidase Polymorphisms G354R and A442V Associated with Reduced Enzyme Activity. Blood Cells Mol. Dis. 2007, 39, 353–360. [Google Scholar] [CrossRef]
- Huber, J.; Kiefer, F.W.; Zeyda, M.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; Zlabinger, G.J.; Stulning, T.M. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J. Clin. Endocrinol. Metab. 2008, 93, 3215–3221. [Google Scholar] [CrossRef]
- Correale, J.; Fiol, M. Chitinase effects on immune cell response in neuromyelitis optica and multiple sclerosis. Mult. Scler. 2011, 17, 521–531. [Google Scholar] [CrossRef]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef]
- Li, M.; Shu, W.; Zunong, J.; Amaerjiang, N.; Xiao, H.; Li, D.; Vermund, S.H.; Hu, Y. Predictors of non-alcoholic fatty liver disease in children. Pediatr. Res. 2022, 92, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Istiqomah, E.; Gurnida, D.A.; Hilmanto, D.; Hakim, D.D.L.; Fauziah, P.N. Waist circumference and waist-hip ratio as screening tools for hypertension in children aged 6–11 years. Paediatr. Indones. 2019, 59, 265–270. [Google Scholar] [CrossRef]
- Yamanaka, A.B.; Davis, J.D.; Wilkens, L.R.; Hurwitz, E.L.; Fialkowski, M.K.; Deenik, J.; Guerrero, R.T.L.; Novotny, R. Determination of Child Waist Circumference Cut Points for Metabolic Risk Based on Acanthosis Nigricans, the Children’s Healthy Living Program. Prev. Chronic Dis. 2021, 18, e64. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Bacha, F.; Gungor, N.; Arslanian, S.A. Waist circumference is an independent predictor of insulin resistance in black and white youths. J. Pediatr. 2006, 148, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2001, 109, 135–148. [Google Scholar] [CrossRef]
- Faienza, M.F.; Urbano, F.; Lassandro, G.; Valente, F.; D’Amato, G.; Portincasa, P.; Giordano, P. The Cardiovascular Disease (CVD) Risk Continuum from Prenatal Life to Adulthood: A Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 8282. [Google Scholar] [CrossRef]
- Owen, C.G.; Whincup, P.H.; Orfei, L.; Chou, Q.A.; Rudnicka, A.R.; Wathern, A.K.; Kaye, S.J.; Eriksson, J.G.; Osmond, C.; Cook, D.G. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int. J. Obes. 2009, 33, 866–877. [Google Scholar] [CrossRef]
- Bjørge, T.; Engeland, A.; Tverdal, A.; Smith, G.D. Body mass index in adolescence in relation to cause-specific mortality: A follow-up of 230,000 Norwegian adolescents. Am. J. Epidemiol. 2008, 168, 30–37. [Google Scholar] [CrossRef]
- Caprio, S.; Santoro, N.; Weiss, R. Childhood obesity and the associated rise in cardiometabolic complications. Nat. Metab. 2020, 2, 223–232. [Google Scholar] [CrossRef]
- Perreault, M.; Marette, A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat. Med. 2001, 7, 1138–1143. [Google Scholar] [CrossRef]
- Samad, F.; Yamamoto, K.; Pandey, M.; Loskutoff, D.J. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol. Med. Camb. Mass 1997, 3, 37–48. [Google Scholar] [PubMed]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Yudkin, J.S.; Stehouwer, C.D.A.; Schalkwijk, C.G.; Pratley, R.E.; Tataranni, P.A. Humoral markers of inflammation and endothelial dysfunction in relation to adiposity and in vivo insulin action in Pima Indians. Atherosclerosis 2002, 161, 233–242. [Google Scholar] [CrossRef]
- Weiss, R.; Dufour, S.; Taksali, S.E.; Tamborlane, W.V.; Petersen, K.F.; Bonadonna, R.C.; Boselli, L.; Narbetta, G.; Allen, K.; Rife, F.; et al. Prediabetes in obese youth: A syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 2003, 362, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Cali, A.M.G.; De Oliveira, A.M.; Kim, H.; Chen, S.; Reyes-Mugica, M.; Escalera, S.; Dziura, J.; Taksali, S.E.; Kursawe, R.; Shaw, M.; et al. Glucose dysregulation and hepatic steatosis in obese adolescents: Is there a link? Hepatology 2009, 49, 1896–1903. [Google Scholar] [CrossRef]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Kursawe, R.; Caprio, S.; Giannini, C.; Narayan, D.; Lin, A.; D’Adamo, E.; Shaw, M.; Pierpont, B.; Cushman, S.W.; Shulman, G.I. Decreased Transcription of ChREBP-α/β Isoforms in Abdominal Subcutaneous Adipose Tissue of Obese Adolescents with Prediabetes or Early Type 2 Diabetes. Diabetes 2013, 62, 837–844. [Google Scholar] [CrossRef]
- Taksali, S.E.; Caprio, S.; Dziura, J.; Dufour, S.; Calí, A.M.G.; Goodman, T.R.; Papademetris, X.; Burgert, T.S.; Pierpontr, B.M.; Savoye, M.; et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: A determinant of an adverse metabolic phenotype. Diabetes 2008, 57, 367–371. [Google Scholar] [CrossRef]
- Cao, C.; Koh, H.C.E.; Van Vliet, S.; Patterson, B.W.; Reeds, D.N.; Laforest, R.; Gropler, R.J.; Mittendorfer, B. Increased plasma fatty acid clearance, not fatty acid concentration, is associated with muscle insulin resistance in people with obesity. Metabolism 2022, 132, 155216. [Google Scholar] [CrossRef]
- Caprio, S.; Pierpont, B.; Kursawe, R. The “adipose tissue expandability” hypothesis: A potential mechanism for insulin resistance in obese youth. Horm. Mol. Biol. Clin. Investig. 2018, 33, 20180005. [Google Scholar] [CrossRef]
- Roden, M.; Price, T.B.; Perseghin, G.; Petersen, K.F.; Rothman, D.L.; Cline, G.W.; Shulman, G.I. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Investig. 1996, 97, 2859–2865. [Google Scholar] [CrossRef] [PubMed]
- Tagi, V.M.; Giannini, C.; Chiarelli, F. Insulin Resistance in Children. Front. Endocrinol. 2019, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Chaychenko, T.; Argente, J.; Spiliotis, B.E.; Wabitsch, M.; Marcus, C. Difference in Insulin Resistance Assessment between European Union and Non-European Union Obesity Treatment Centers (ESPE Obesity Working Group Insulin Resistance Project). Horm. Res. Paediatr. 2020, 93, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Iwani, N.A.K.Z.; Jalaludin, M.Y.; Zin, R.M.W.M.; Fuziah, M.Z.; Hong, J.Y.H.; Abqariyah, Y.; Mokhtar, A.H.; Nazaimoon, W.M.W. Triglyceride to HDL-C Ratio is Associated with Insulin Resistance in Overweight and Obese Children. Sci. Rep. 2017, 7, 40055. [Google Scholar] [CrossRef]
- Giannini, C.; Santoro, N.; Caprio, S.; Kim, G.; Lartaud, D.; Shaw, M.; Pierpont, B.; Weiss, R. The triglyceride-to-HDL cholesterol ratio: Association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care 2011, 34, 1869–1874. [Google Scholar] [CrossRef]
- Țaranu, I.; Iancu, M.; Lazea, C.; Alkhzouz, C.; Răcătăianu, N.; Cătană, C.S.; Mirea, A.M.; Miclea, D.; Bolboacă, S.D. Evaluation of Circulating Chitotriosidase Activity in Children with Obesity. J. Clin. Med. 2022, 11, 3634. [Google Scholar] [CrossRef]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Hollak, C.E.; van Weely, S.; van Oers, M.H.; Aerts, J.M. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J. Clin. Investig. 1994, 93, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Püschel, G.P.; Klauder, J.; Henkel, J. Macrophages, Low-Grade Inflammation, Insulin Resistance and Hyperinsulinemia: A Mutual Ambiguous Relationship in the Development of Metabolic Diseases. J. Clin. Med. 2022, 11, 4358. [Google Scholar] [CrossRef] [PubMed]
- Singer, K.; Lumeng, C.N. The initiation of metabolic inflammation in childhood obesity. J. Clin. Investig. 2017, 127, 65–73. [Google Scholar] [CrossRef]
- Kursawe, R.; Dixit, V.D.; Scherer, P.E.; Santoro, N.; Narayan, D.; Gordillo, R.; Giannini, C.; Lopez, X.; Pierpont, B.; Nouws, J.; et al. A Role of the Inflammasome in the Low Storage Capacity of the Abdominal Subcutaneous Adipose Tissue in Obese Adolescents. Diabetes 2016, 65, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Li, P.; Huh, J.Y.; Hwang, I.J.; Lu, M.; Kim, J.I.; Ham, M.; Talukdar, S.; Chen, A.; Lu, W.J.; et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 2011, 60, 2474–2483. [Google Scholar] [CrossRef]
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Lumeng, C.N.; DelProposto, J.B.; Westcott, D.J.; Saltiel, A.R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008, 57, 3239–3246. [Google Scholar] [CrossRef] [PubMed]
- Jaitin, D.A.; Adlung, L.; Thaiss, C.A.; Weiner, A.; Li, B.; Descamps, H.; Lundgren, P.; Bleriot, C.; Liu, Z.; Deczkowska, A.; et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 2019, 178, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Lim, H.W.; Kim, Y.H.; Ho, W.Y.; Foong, Y.H.; Nelson, V.L.; Nguyen, H.C.B.; Chegireddy, K.; Kim, J.; Habertheuer, A.; et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc. Natl. Acad. Sci. USA 2018, 115, e5096–e5105. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Létuvé, S.; Kozhich, A.; Humbles, A.; Brewah, Y.; Dombret, M.C.; Grandsaigne, M.; Adle, H.; Kolbeck, R.; Aubier, M.; Coyle, A.J.; et al. Lung Chitinolytic Activity and Chitotriosidase Are Elevated in Chronic Obstructive Pulmonary Disease and Contribute to Lung Inflammation. Am. J. Pathol. 2010, 176, 638–649. [Google Scholar] [CrossRef]
- Di Rosa, M.; Mangano, K.; De Gregorio, C.; Nicoletti, F.; Malaguarnera, L. Association of chitotriosidase genotype with the development of non-alcoholic fatty liver disease. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2013, 43, 267–275. [Google Scholar] [CrossRef]
- Kabaroğlu, C.; Onur, E.; Barutçuoğlu, B.; Özhan, B.; Erdinç, S.; Var, A.; Bayindir, O.; Ersoy, B. Inflammatory marker levels in obese adolescents with glucose intolerance: Increased chitotriosidase activity. Clin. Biochem. 2012, 45, 281–284. [Google Scholar] [CrossRef]
- Yap, J.; McCurdy, S.; Alcala, M.; Irei, J.; Garo, J.; Regan, W.; Lee, B.H.; Kitamoto, S.; Boisvert, W.A. Expression of Chitotriosidase in Macrophages Modulates Atherosclerotic Plaque Formation in Hyperlipidemic Mice. Front. Physiol. 2020, 11, 714. [Google Scholar] [CrossRef]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef]
- Yu, Y.H.; Ginsberg, H.N. Adipocyte Signaling and Lipid Homeostasis. Sequelae of Insulin-Resistant Adipose Tissue. Circ. Res. 2005, 96, 1042–1052. [Google Scholar] [CrossRef]
- Feingold, K.R.; Anawalt, B.; Boyce, A.; Chrousos, G.; de Herder, W.W.; Dhatariya, K.; Dungan, K.; Hershman, J.M.; Hofland, J.; Kalra, S.; et al. (Eds.) Obesity and Dyslipidemia; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Quijada, Z.; Paoli, M.; Zerpa, Y.; Camacho, N.; Cichetti, R.; Villarroel, V.; Arata-Bellabarba, G.; Lanes, R. The triglyceride/HDL-cholesterol ratio as a marker of cardiovascular risk in obese children; association with traditional and emergent risk factors. Pediatr. Diabetes 2008, 9, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Moio, N.; Scilla, C.; Cavuto, L.; Sibilio, G.; Sanguigno, E.; Forziato, C.; Saitta, F.; Iardino, M.R.; Di Carluccio, C.; et al. Usefulness of the High Triglyceride-to-HDL Cholesterol Ratio to Identify Cardiometabolic Risk Factors and Preclinical Signs of Organ Damage in Outpatient Children. Diabetes Care 2012, 35, 158–162. [Google Scholar] [CrossRef] [PubMed]
- De Giorgis, T.; Marcovecchio, M.L.; Di Giovanni, I.; Giannini, C.; Chiavaroli, V.; Chiarelli, F.; Mohn, A. Triglycerides-to-HDL ratio as a new marker of endothelial dysfunction in obese prepubertal children. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 2013, 170, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Kim, J.H. Validation of surrogate markers for metabolic syndrome and cardiometabolic risk factor clustering in children and adolescents: A nationwide population-based study. PLoS ONE 2017, 12, e0186050. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Valerio, G.; Grugni, G.; Licenziati, M.R.; Maffeis, C.; Manco, M.; Miraglia del Giudice, E.; Pacifico, L.; Pellegrin, M.C.; Tomat, M.; et al. Comparison of non-HDL-cholesterol versus triglycerides-to-HDL-cholesterol ratio in relation to cardiometabolic risk factors and preclinical organ damage in overweight/obese children: The CARITALY study. Nutr. Metab. Cardiovasc. Dis. NMCD 2015, 25, 489–494. [Google Scholar] [CrossRef] [PubMed]
- De Ferranti, S.D.; Gauvreau, K.; Ludwig, D.S.; Neufeld, E.J.; Newburger, J.W.; Rifai, N. Prevalence of the metabolic syndrome in American adolescents: Findings from the Third National Health and Nutrition Examination Survey. Circulation 2004, 110, 2494–2497. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Weitzman, M.; Auinger, P.; Nguyen, M.; Dietz, W.H. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch. Pediatr. Adolesc. Med. 2003, 157, 821–827. [Google Scholar] [CrossRef]
- Viner, R.; Segal, T.; Lichtarowicz-Kryn, E.; Hindmarsh, P. Prevalence of the insulin resistance syndrome in obesity. Arch. Dis. Child. 2005, 90, 10–14. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Ahrens, W.; Moreno, L.A.; Mårild, S.; Molnár, D.; Siani, A.; De Henauw, S.; Böhmann, J.; Günther, K.; Hadjigeorgiou, C.; Iacoviello, L.; et al. Metabolic syndrome in young children: Definitions and results of the IDEFICS study. Int. J. Obes. 2014, 38, S4–S14. [Google Scholar] [CrossRef]
- Suliga, E. Visceral adipose tissue in children and adolescents: A review. Nutr. Res. Rev. 2009, 22, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Park, H.S. Association of abdominal fat distribution and cardiometabolic risk factors among obese Korean adolescents. Diabetes Metab. 2008, 34, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Polat, T.B.; Urganci, N.; Caliskan, K.C.; Akyildiz, B. Correlation of abdominal fat accumulation and stiffness of the abdominal aorta in obese children. JPEM 2008, 21, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Abraham, T.M.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Fox, C.S. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation 2015, 132, 1639–1647. [Google Scholar] [CrossRef]
- Cancello, R.; Tordjman, J.; Poitou, C.; Guilhem, G.; Bouillot, J.L.; Hugol, D.; Coussieu, C.; Basdevant, A.; Bar Hen, A.; Bedossa, P.; et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 2006, 55, 1554–1561. [Google Scholar] [CrossRef]
- Barlow, S.E.; Dietz, W.H. Obesity evaluation and treatment: Expert Committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics 1998, 102, e29. [Google Scholar] [CrossRef]
- Hardy, O.T.; Perugini, R.A.; Nicoloro, S.M.; Gallagher-Dorval, K.; Puri, V.; Straubhaar, J.; Czech, M.P. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2011, 7, 60–77. [Google Scholar] [CrossRef]
- Sinaiko, A.R.; Steinberger, J.; Moran, A.; Hong, C.P.; Prineas, R.J.; Jacobs, D.R. Influence of insulin resistance and body mass index at age 13 on systolic blood pressure, triglycerides, and high-density lipoprotein cholesterol at age 19. Hypertension 2006, 48, 730–736. [Google Scholar] [CrossRef]
- Wiebe, N.; Ye, F.; Crumley, E.T.; Bello, A.; Stenvinkel, P.; Tonelli, M. Temporal Associations among Body Mass Index, Fasting Insulin, and Systemic Inflammation: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e211263. [Google Scholar] [CrossRef]
- Dattani, M.T.; Brook, C.G.D. Brook’s Clinical Pediatric Endocrinology, 7th ed.; Wiley-Blackwell: Oxford, UK, 2020; pp. 701–726. [Google Scholar]
- Hannon, T.S.; Janosky, J.; Arslanian, S.A. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr. Res. 2006, 60, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Marwitz, S.E.; Gaines, M.V.; Brady, S.M.; Mi, S.J.; Broadney, M.M.; Yanovski, S.Z.; Hubbard, V.S.; Yanovski, J.A. Cross-Sectional and Longitudinal Examination of Insulin Sensitivity and Secretion across Puberty among Non-Hispanic Black and White Children. Endocrinol. Metab. 2020, 35, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, F.A. Magnetic resonance imaging of abdominal masses in the pediatric patient. Semin. Ultrasound CT MR 2005, 26, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.R.; Peters, D.M.; Sharpe, P.; Bell, M. Assessment of abdominal fat development in young adolescents using magnetic resonance imaging. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1653–1659. [Google Scholar] [CrossRef]
- Dong, Y.; Bai, L.; Cai, R.; Zhou, J.; Ding, W. Children’s Lipid Accumulation Product Combining Visceral Adiposity Index is a Novel Indicator for Predicting Unhealthy Metabolic Phenotype Among Chinese Children and Adolescents. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 23, 4579–4587. [Google Scholar] [CrossRef]

| Characteristic | At Baseline | At Follow-Up | p-Value |
|---|---|---|---|
| Age, years | 11.36 ± 3.61 | 11.95 ± 3.6 | <0.001 |
| 11.42 [8.83 to 13.33] | 12.08 [9.67 to 14.17] | ||
| {5.08 to 18.42} | {5.5 to 18.92} | ||
| Tanner staging | 0.264 * | ||
| prepubertal | 14 (48.28) | 8 (30.77) | |
| pubertal | 15 (51.72) | 18 (69.23) | |
| BMI-for-age z score | 3.01 ± 1.09 | 2.9 ± 0.94 | 0.127 |
| 2.57 [2.25 to 3.78] | 2.67 [2.34 to 3.32] | ||
| {1.82 to 6.32} | {1.86 to 5.86} | ||
| Waist circumference, cm | 91.79 ± 16.78 | 91.11 ± 15.16 | 0.331 |
| 91 [80 to 98] | 90 [80 to 97.5] | ||
| {64 to 136} | {63 to 130} | ||
| Waist-to-hip ratio | 0.96 ± 0.06 | 0.95 ± 0.05 | 0.327 |
| 0.95 [0.91 to 1.01] | 0.95 [0.90 to 0.98] | ||
| {0.83 to 1.05} | {0.87 to 1.06} | ||
| Waist-to-height ratio | 0.61 ± 0.06 | 0.6 ± 0.06 | 0.145 |
| 0.61 [0.57 to 0.65] | 0.59 [0.55 to 0.64] | ||
| {0.48 to 0.8} | {0.49 to 0.72} |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țaranu, I.; Răcătăianu, N.; Drugan, C.; Cătană, C.-S.; Mirea, A.-M.; Miclea, D.; Bolboacă, S.D. Exploratory Longitudinal Analysis of the Circulating CHIT1 Activity in Pediatric Patients with Obesity. Children 2023, 10, 124. https://doi.org/10.3390/children10010124
Țaranu I, Răcătăianu N, Drugan C, Cătană C-S, Mirea A-M, Miclea D, Bolboacă SD. Exploratory Longitudinal Analysis of the Circulating CHIT1 Activity in Pediatric Patients with Obesity. Children. 2023; 10(1):124. https://doi.org/10.3390/children10010124
Chicago/Turabian StyleȚaranu, Ioana, Nicoleta Răcătăianu, Cristina Drugan, Cristina-Sorina Cătană, Andreea-Manuela Mirea, Diana Miclea, and Sorana D. Bolboacă. 2023. "Exploratory Longitudinal Analysis of the Circulating CHIT1 Activity in Pediatric Patients with Obesity" Children 10, no. 1: 124. https://doi.org/10.3390/children10010124
APA StyleȚaranu, I., Răcătăianu, N., Drugan, C., Cătană, C.-S., Mirea, A.-M., Miclea, D., & Bolboacă, S. D. (2023). Exploratory Longitudinal Analysis of the Circulating CHIT1 Activity in Pediatric Patients with Obesity. Children, 10(1), 124. https://doi.org/10.3390/children10010124








