Modern Wound Dressings: Hydrogel Dressings
Abstract
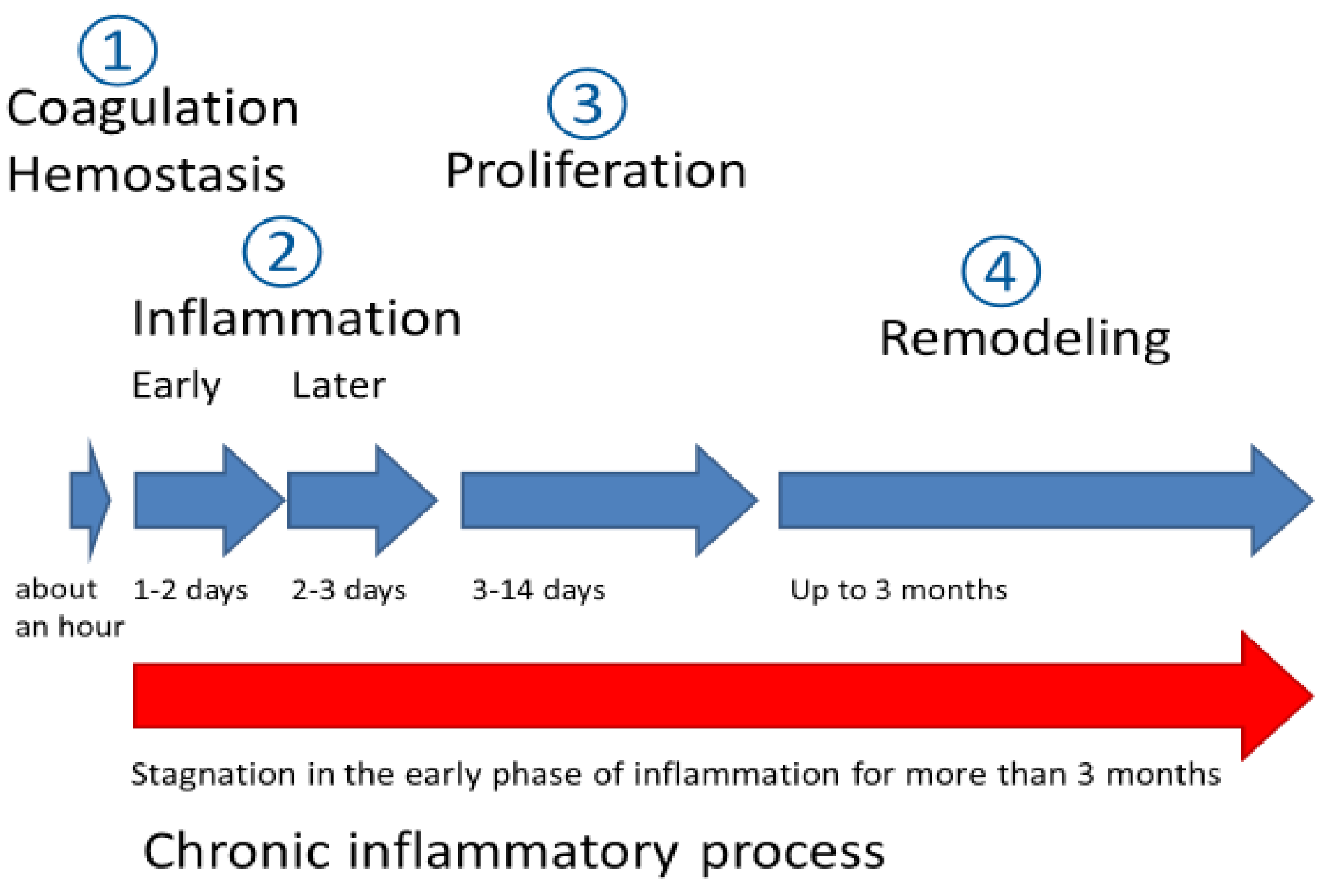
:1. Introduction
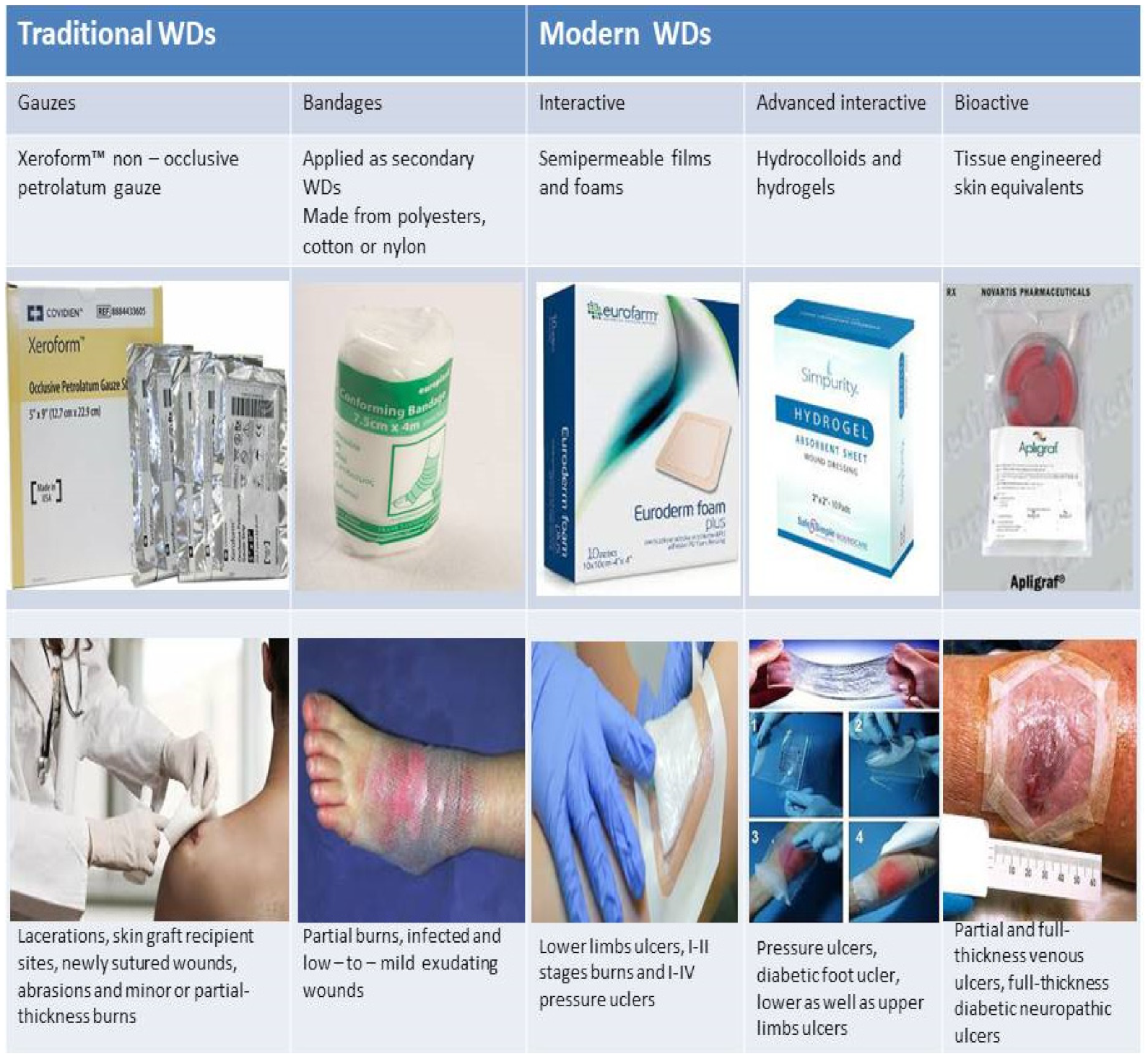
2. Types of Wound Dressings (WDs)
| Wound Type | Etiology | Properties | Applicable Dressing (Example) |
|---|---|---|---|
| Diabetic foot ulcer | Neuropathy and lower limb diseases | Insufficient oxygen and blood supply to the wound bed; healing stagnates in the inflammation stage Weak, moderate, or profuse exudation | Semipermeable non-adhesive and adhesive seals presented by foams and hydrocolloids Examples: UrgoStart contact dressing Allevyn, Biatain and Tegaderm dressings |
| Pressure ulcers | Local ischemia and tissue injury | Local injury of skin or subcutaneous fat Low–to–moderate exudation | Semipermeable non-adhesive foam dressings and hydrocolloids. Examples: Mepilex Ag, DuoDerm, SignalTM, and DuoDerm ExtraThin |
| Burns | Thermal, chemical or radiation skin injuries | Propensity to secondary infection, wounds with profuse exudation potentially extending to dermal layers, subcutaneous fat, muscles and bone tissue | Occlusive dressings with high absorptive capacities Examples: alginate, chitosane, collagen, hyaluronic acid hydrogels or fibrous dressings able to form a gel under a contact with wound surface (carboxymethylcelluloseHydrofiber, Algisite M, HemCon Bandage Pro, Hydrofiber) |
| Chronic venous ulcers | Lower limb vascular diseases | Blood supply disturbance; pronounced formation of necrotic tissue, abundant exudation on ulcer surface, accompanied by multiple infections | Semipermeable foam dressings Examples: Mepilex, Allevyn, Contreet Ag, Coloplast |
| Radiation dermatitis | Local radiation induced skin injury | Impairment of wound healing in proliferation stage and consequent alteration of granulation tissue | Film barrier dressings in form of gauze or spay Examples: 3MTM Cavilon, SECURA, Medi Derma S |
3. Discussion
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef]
- Maksimova, N.V.; Lyundup, A.V.; Lubimov, R.O.; Mel’nichenko, G.A.; Nikolenko, V.N. Pathophysiological aspects of wound healing in normal and diabetic foot. Ann. Russ. Acad. Med. Sci. 2014, 69, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomennikov, A.V.; Tyukavin, A.I.; Arseniev, N.A. The effect of platelet degranulation on local inflammatory process formation. J. Med. Biol. Stud. 2019, 7, 280–289. [Google Scholar] [CrossRef]
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Moreno Rodriguez, R.; Markwald, R.R.; Misra, S. Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis. Int. J. Cell Biol. 2015, 2015, 834893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avishai, E.; Yeghiazaryan, K.; Golubnitschaja, O. Impaired wound healing: Facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017, 8, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Savina, I.N.; Zoughaib, M.; Yergeshov, A.A. Design and Assessment of Biodegradable Macroporous Cryogels as Advanced Tissue Engineering and Drug Carrying Materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef]
- Sun, C.-K.; Ke, C.-J.; Lin, Y.-W.; Lin, F.-H.; Tsai, T.-H.; Sun, J.-S. Transglutaminase Cross-Linked Gelatin-Alginate-Antibacterial Hydrogel as the Drug Delivery-Coatings for Implant-Related Infections. Polymers 2021, 13, 414. [Google Scholar] [CrossRef]
- Sabir, F.; Katona, G.; Ismail, R.; Sipos, B.; Ambrus, R.; Csóka, I. Development and Characterization of n-Propyl Gallate Encapsulated Solid Lipid Nanoparticles-Loaded Hydrogel for Intranasal Delivery. Pharmaceuticals 2021, 14, 696. [Google Scholar] [CrossRef]
- Eskens, O.; Villani, G.; Amin, S. Rheological Investigation of Thermoresponsive Alginate-Methylcellulose Gels for Epidermal Growth Factor Formulation. Cosmetics 2021, 8, 3. [Google Scholar] [CrossRef]
- Doostmohammadi, M.; Ameri, A.; Mohammadinejad, R.; Dehghannoudeh, N.; Banat, I.M.; Ohadi, M.; Dehghannoudeh, G. Hydrogels For Peptide Hormones Delivery: Therapeutic And Tissue Engineering Applications. Drug design, development and therapy. Drug Des. Devel. Ther. 2019, 13, 3405–3418. [Google Scholar] [CrossRef] [Green Version]
- Barillo, D.J.; Barillo, A.R.; Korn, S.; Lam, K.; Attar, P.S. The antimicrobial spectrum of Xeroform®. Burns 2017, 43, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Pereira, C.V.; Mano, F.; Silva, E.; Castro, V.I.B.; Sá-Nogueira, I.; Reis, R.L.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Therapeutic Role of Deep Eutectic Solvents Based on Menthol and Saturated Fatty Acids on Wound Healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef] [PubMed]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings. Mini Rev. Med. Chem. 2018, 18, 414–427. [Google Scholar] [CrossRef]
- Przekora, A.A. Concise Review on Tissue Engineered Artificial Skin Grafts for Chronic Wound Treatment: Can We Reconstruct Functional Skin Tissue In Vitro. Cells. 2020, 9, 1622. [Google Scholar] [CrossRef]
- Abruzzo, A.; Cappadone, C.; Sallustio, V.; Picone, G.; Rossi, M.; Nicoletta, F.P.; Luppi, B.; Bigucci, F.; Cerchiara, T. Development of Spanish Broom and Flax Dressings with Glycyrrhetinic Acid-Loaded Films for Wound Healing. Charact. Eval. Biol. Prop. Pharm. 2021, 13, 1192. [Google Scholar] [CrossRef]
- Assis, A.C.L.d.; Moreira, L.M.C.d.C.; Rocha, B.P.; Pereira, M.R.B.; de Melo, D.F.; Moura, R.O.d.; Azevedo, E.P.d.; Oshiro-Junior, J.A.; Damasceno, B.P.G.d.L. N-acylhydrazone Derivative-Loaded Cellulose Acetate Films: Thermoanalytical, Spectroscopic, Mechanical and Morphological Characterization. Polymers 2021, 13, 2345. [Google Scholar] [CrossRef] [PubMed]
- Michelin, R.M.; Ahdoot, E.; Zakhary, B.L.; McDowell, M.; French, M. Choosing the Optimal Wound Dressing for Bathing After Total Knee Arthroplasty. J. Arthroplast. 2021, 36, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Lee, H.-C.; Chen, C.-L.; Kuo, M.-C.; Ramachandran, S.; Chen, R.-F.; Kuo, Y.-R. The Effects of Silver-Releasing Foam Dressings on Diabetic Foot Ulcer Healing. J. Clin. Med. 2021, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Vivcharenko, V.; Wojcik, M.; Palka, K.; Przekora, A. Highly Porous and Superabsorbent Biomaterial Made of Marine-Derived Polysaccharides and Ascorbic Acid as an Optimal Dressing for Exuding Wound Management. Materials 2021, 14, 1211. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westby, M.J.; Dumville, J.C.; Soares, M.O.; Stubbs, N.; Norman, G. Dressings and topical agents for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 6, CD006876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamińska, M.S.; Cybulska, A.M.; Skonieczna-Żydecka, K.; Augustyniuk, K.; Grochans, E.; Karakiewicz, B. Effectiveness of Hydrocolloid Dressings for Treating Pressure Ulcers in Adult Patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 7881. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.J.; Kim, J.H.; Moon, B.M.; Chao, J.R.; Yoon, J.; Ju, H.W.; Lee, J.M.; Park, H.J.; Kim, D.W.; Kim, S.J.; et al. Fabrication and characterization of hydrocolloid dressing with silk fibroin nanoparticles for wound healing. Tissue Eng. Regen. Med. 2016, 13, 218–226. [Google Scholar] [CrossRef]
- Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [Green Version]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Properties of Water Bound in Hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef]
- Wang, H.; Heilshorn, S.C. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 2015, 27, 3717–3736. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Rebers, L.; Reichsöllner, R.; Regett, S.; Tovar, G.; Borchers, K.; Baudis, S.; Southan, A. Differentiation of physical and chemical cross-linking in gelatin methacryloyl hydrogels. Sci. Rep. 2021, 11, 3256. [Google Scholar] [CrossRef] [PubMed]
- Morello, G.; Polini, A.; Scalera, F.; Rizzo, R.; Gigli, G.; Gervaso, F. Preparation and Characterization of Salt-Mediated Injectable Thermosensitive Chitosan/Pectin Hydrogels for Cell Embedding and Culturing. Polymers 2021, 13, 2674. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, S.; Martyn-St, J.; Adderley, M.U.J. Alginate dressings for venous leg ulcers. Cochrane Database Syst. Rev. 2015, 2015, CD010182. [Google Scholar] [CrossRef]
- Echalier, C.; Laurine, V.; Martinez, J.; Mehdi, A.; Gilles, S. Chemical cross linking methods for cell encapsulation in hydrogels. In Materials Today Communications; Elsevier: Amsterdam, The Netherlands, 2019; Volume 20, p. 100536. [Google Scholar] [CrossRef]
- Chang, N.S.; Lin, R.; Sze, C.I.; Aqeilan, R.I. Editorial: WW Domain Proteins in Signaling, Cancer Growth, Neural Diseases, and Metabolic Disorders. Front. Oncol. 2019, 9, 719. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, H.; Biswas, N.; Li, P.; Zhong, C.; Chan, T.C.; Nudleman, E.; Ferrara, N. Heparin-binding VEGFR1 variants as long-acting VEGF inhibitors for treatment of intraocular neovascular disorders. Proc. Natl. Acad. Sci. USA 2021, 118, e1921252118. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.; Wu, Y.; Zhang, M.; Liao, J. A Review on Hydrogels with Photothermal Effect in Wound Healing and Bone Tissue Engineering. Polymers 2021, 13, 2100. [Google Scholar] [CrossRef]
- Verhelst, S.; Bonger, K.M.; Willems, L.I. Bioorthogonal Reactions in Activity-Based Protein Profiling. Molecules 2020, 25, 5994. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Nešović, K.; Janković, A.; Kojić, V.; Vukašinović-Sekulić, M.; Perić-Grujić, A.; Rhee, K.Y.; Mišković-Stanković, V. Silver/poly(vinyl alcohol)/chitosan/graphene hydrogels—Synthesis, biological and physicochemical properties and silver release kinetics. Compos. Part B Eng. 2018, 154, 175–185. [Google Scholar] [CrossRef]
- Sun, A.; He, X.; Li, L.; Li, T.; Liu, Q.; Zhou, X.; Ji, X.; Li, W.; Qian, Z. An injectable photopolymerized hydrogel with antimicrobial and biocompatible properties for infected skin regeneration. NPG Asia Mater. 2020, 12, 25. [Google Scholar] [CrossRef]
- Abdollahi, Z.; Zare, E.N.; Salimi, F.; Goudarzi, I.; Tay, F.R.; Makvandi, P. Bioactive Carboxymethyl Starch-Based Hydrogels Decorated with CuO Nanoparticles: Antioxidant and Antimicrobial Properties and Accelerated Wound Healing In Vivo. Int. J. Mol. Sci. 2021, 22, 2531. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS. Appl. Mater. Interfaces 2018, 10, 30103–30114. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Eritja, R.; Díaz, D. Liposomes-in-chitosan hydrogels. Challenges and opportunities for biomedical applications. In Materials Science and Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019. [Google Scholar]
- Oryan, A.; Jalili, M.; Kamali, A.; Nikahval, B. The concurrent use of probiotic microorganism and collagen hydrogel/scaffold enhances burn wound healing: An in vivo evaluation. Burns 2018, 7, 1775–1786. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Wang, P.; Huang, S.; Hu, Z.; Yang, W.; Lan, Y.; Zhu, J.; Hancharou, A.; Guo, R.; Tang, B. In situ formed anti-inflammatory hydrogel loading plasmid DNA encoding VEGF for burn wound healing. Acta Biomater. 2019, 100, 191–201. [Google Scholar] [CrossRef]
- Miller, K.J.; Brown, D.A.; Ibrahim, M.M.; Ramchal, T.D.; Levinson, H. MicroRNAs in skin tissue engineering. Adv. Drug Deliv. Rev. 2015, 88, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Larouche, D.; Cantin-Warren, L.; Desgagné, M.; Guignard, R.; Martel, I.; Ayoub, A.; Lavoie, A.; Gauvin, R.; Auger, F.A.; Moulin, V.J.; et al. Improved Methods to Produce Tissue-Engineered Skin Substitutes Suitable for the Permanent Closure of Full-Thickness Skin Injuries. BioRes. Open Access 2016, 5, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guo, X. A review: Therapeutic potential of adipose-derived stem cells in cutaneous wound healing and regeneration. Stem Cell Res. Ther. 2018, 9, 302. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, L.; Liu, J.; Gong, N.; Chen, L. The effects of cytokines in adipose stem cell-conditioned medium on the migration and proliferation of skin fibroblasts in vitro. Biomed. Res. Int. 2013, 2013, 578479. [Google Scholar] [CrossRef] [Green Version]
- An, Y.; Li, C.; Xu, Q.; Sun, Y.; Wang, Z. Exosomes from Adipose-Derived Mesenchymal Stem Cells Can Promote Fibroblasts Proliferation, Migration, and Collagen Synthesis via Activating Wnt/β-Catenin Signaling Pathway during Wound Healing. 2021. Available online: https://assets.researchsquare.com/files/rs-520642/v1/d9e406e0-e2d0-4bde-a65f-72c4922bb94f.pdf?c=1621279716 (accessed on 30 August 2021).
- Ma, H.; Lam, P.K.; Siu, W.S.; Tong, C.S.W.; Lo, K.K.Y.; Koon, C.M.; Wu, X.X.; Li, X.; Cheng, W.; Shum, W.T.; et al. Adipose Tissue-Derived Mesenchymal Stem Cells (ADMSCs) and ADMSC-Derived Secretome Expedited Wound Healing in a Rodent Model—A Preliminary Study. Clin. Cosmet. Investig. Dermatol. 2021, 14, 753–764. [Google Scholar] [CrossRef]
- Lu, T.-Y.; Yu, K.-F.; Kuo, S.-H.; Cheng, N.-C.; Chuang, E.-Y.; Yu, J. Enzyme-Crosslinked Gelatin Hydrogel with Adipose-Derived Stem Cell Spheroid Facilitating Wound Repair in the Murine Burn Model. Polymers 2020, 12, 2997. [Google Scholar] [CrossRef]
- Yang, M.; He, S.; Su, Z.; Yang, Z.; Liang, X.; Wu, Y. Thermosensitive Injectable Chitosan/Collagen/β-Glycerophosphate Composite Hydrogels for Enhancing Wound Healing by Encapsulating Mesenchymal Stem Cell Spheroids. ACS. Omega 2020, 5, 21015–21023. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.W.; Azevedo Gomes, F.; Rode, M.P.; Marques da Silva, M.; Veleirinho, M.B.D.R.; Maraschin, M.; Hayashi, L.; Wosgrau Calloni, G.; Stimamiglio, M.A. The skin regeneration potential of a pro-angiogenic secretome from human skin-derived multipotent stromal cells. J. Tissue Eng. 2019, 10, 2041731419833391. [Google Scholar] [CrossRef] [Green Version]
- Smith, O.J.; Kanapathy, M.; Khajuria, A.; Prokopenko, M.; Hachach-Haram, N.; Mann, H.; Mosahebi, A. Systematic review of the efficacy of fat grafting and platelet-rich plasma for wound healing. Int. Wound J. 2018, 15, 519–526. [Google Scholar] [CrossRef]
- Semenič, D.; Cirman, T.; Rožman, P.; Smrke, D.M. Regeneration of chronic wounds with allogeneic platelet gel versus hydrogel treatment:a prospective study. Acta Clin. Croat. 2018, 57, 434–442. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, B.; D’Autilio, M.F.L.M.; Orlandi, F.; Pepe, G.; Garcovich, S.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Wound Healing: In Vitro and In Vivo Evaluation of a Bio-Functionalized Scaffold Based on Hyaluronic Acid and Platelet-Rich Plasma in Chronic Ulcers. J. Clin. Med. 2019, 8, 1486. [Google Scholar] [CrossRef] [Green Version]
- Notodihardjo, P.V.; Morimoto, N.; Kakudo, N.; Matsui, M.; Sakamoto, M.; Liem, P.H.; Suzuki, K.; Tabata, Y.; Kusumoto, K. Gelatin hydrogel impregnated with platelet-rich plasma releasate promotes angiogenesis and wound healing in murine model. J. Artif. Organs 2015, 18, 64–71. [Google Scholar] [CrossRef] [PubMed]
| Type of Physical Interaction | Exact Binding Mechanism | Example |
|---|---|---|
| Guest–host interaction | Interaction between 6,7,8-membered d-glucose (cyclodextrin) units, forming a cavity, with a guest molecule. This interaction is similar to hydrophobic and depends on the geometry of molecules | Cyclodextrin–adamantane |
| Dynamic protein–protein interactions | Complex interactions, the nature of which is determined by the affinity of the peptide to the protein, the number of repeating units, etc. | WW domain with proline enriched peptide [41] |
| Hydrophobic interactions “self-assambley” | Manifoldly repeating sequences that provide spiral-spiral interactions (so-called “self-assembly”). Self-assembly is based on a network entropy increase during aggregation of hydrophobic residues inward and exposure of hydrophilic residues in aqueous medium. | Collagen type I XaaYaaGly Gelatin Extracellular tissue matrix hydrogels [42] |
| Electrostatic interactions | Alginate-Ca2+ Heparin-heparin-binding domains of growth factors VEGF, bFGF [42]. |
| Reaction Type | Reagents Used for Hydrogel Precursors Functionalization | Synthetic or Natural Polymers Used | Embedded Cells | |
|---|---|---|---|---|
 | Chain growth radical photopolymerization | Acryloylchloride, methacryolyl chloride, methacrylic anhydride, 2-isocyanatoethyl methacrylate, glycidyl methacrylate | PEG, PLA-PEG-PLA, PVA, chondroitin sulfate, alginate, hyaluronic acid, collagen, chitosan, gelatin | Aorta smooth muscle cells, calvaria osteoblasts, articular chondrocytes, valvuar interstitial cells |
 | Thiol–ene photopolymerization | 5-norbornene-2-carbonic acid, cysteine derivatives, dithiothreitol, 3-mercaptopropionic acid | PEG, gelatin | Mesenchymal stem cells, motor neurons |
 | Michael’s addition | N-(2-aminoethyl) maleimide trifluoroacetate, 4-mercaptophenylpropionic acid, mercaptoisobutyrate, 2-dimethyl-3- (4-mercaptophenyl)-propionic acid | PEG, heparin | Pancreatic islets, myoblasts |
 | Azide-alkyne cyclocondensation | 2-(2-cyclooctin-1-yloxy) acetic acid, bicyclo [6.1.0] non-4-yn-9-ylmethanol or methyl N-succinimidylcarbonate, 11,12-didehydro-5,6-dihydrodibenzo [a, e] cyclooctene-5-ol, 11,12-didehydro-γ-oxodibenz [b, f] azo-cine-5 (6H) -butanoic acid, sodium azide, 4-azidobutanoic acid | PEG | 3T3 fibroblasts, mesenchymal stem cells, bone marrow stem cells |
 | Diels Alder reaction | Furfuryl methacrylate, furfurylamine, 3-(2-Furyl) propanoic acid, N- (2-aminoethyl) male-imide trifluoroacetate salt, 4-(4-N-maleimidophenyl) butyric acid hydrazide, N-maleoyl-β-alanine, N-methoxycarbonylmale-imide | PEG, dextran hyaluronate, poly (N, N-dimethylacrylamide-co-furfuryl methacrylate) | Chondrogenic cells |
 | The inverse electron demand Diels Alder reaction | 5-[4-(1,2,4,5-tetrazin-3-yl) benzylamino]-5-oxopentanoic acid, 5-norbornene 2-carboxylic acid | PEG, hyaluronate | Mesenchymal stem cell, prostate cancer cells |
 | Chemical ligation | 5-ethyl-3-mercaptopropionate, Boc-Cys (Trt)-OH | PEG | Insulinoma cells, induced pluripotent stem cells |
 | (Acyl)hydrazine formation | Oxalyl chloride / DMSO (Swern oxidation), sodium periodate, tri-Boc hydrazinoacetic acid, hydrazine monohydrate, adipic acid dihydrazide, carbohydrazide, oxalyl dihydrazide | PEG, poly(vinyl alcohol), hyaluronic acid, dexran, carboxymethylcellulose, poly(isopropylacrylamide), poly(aspartic acid), heparin, poly (l-glutamic acid), alginate | Adipose fibroblasts, myoblasts, neuroblastoma cells, vocal cord fibroblasts, neonatal cardiomyocytes |
 | Oxime formation | Sodium periodate, N-hydroxyphthalimide | PEG, hyaluronic acid, alginate | Mesenchymal stem cells, adipose tissue fibroblasts |
 | Disulfide bond formation | Dithiobis (propanoic or butyric) dihydrazide, N, N’-bis (acryloyl) cystamine (requires a reduction step), thioacetic acid (requires saponification), N-acetyl-l-cysteine or l-cysteine | Hyaluronic acid, chitosan, PEG, gellan gum, copoly(acrylamide) | - |
 | Sol-gel transition | 3-isocyanatopropyltriethoxysilane, 3-(glycidoxypropyl) triethoxysilane, 3-aminopropyltriethoxysilane | PEG, gelatin, chitosan, collagen, hydroxypropylmethyl cellulose, alginate | Articular chondrocytes, cardiomyocytes, chondrosarcoma cells, mesenchymal stem cells |
 | Imine formation | Sodium periodate (oxidative degradation of vicinal diols), 4-formylbenzoic acid, ethylenediamine | Alginate, gelatin, dextran, PEG, chitosan, starch, polyvinylamine, polyphosphasen | Hepatocytes, breast adenocarcinoma, articular chondrocytes, dermal fibroblasts |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. https://doi.org/10.3390/biomedicines9091235
Brumberg V, Astrelina T, Malivanova T, Samoilov A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines. 2021; 9(9):1235. https://doi.org/10.3390/biomedicines9091235
Chicago/Turabian StyleBrumberg, Valentin, Tatiana Astrelina, Tatiana Malivanova, and Alexander Samoilov. 2021. "Modern Wound Dressings: Hydrogel Dressings" Biomedicines 9, no. 9: 1235. https://doi.org/10.3390/biomedicines9091235
APA StyleBrumberg, V., Astrelina, T., Malivanova, T., & Samoilov, A. (2021). Modern Wound Dressings: Hydrogel Dressings. Biomedicines, 9(9), 1235. https://doi.org/10.3390/biomedicines9091235






