Topical Administration of Heat-Killed Enterococcus faecalis Strain KH2 Promotes Re-Epithelialization and Granulation Tissue Formation during Skin Wound-Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Wound Creation and Tissue Collection
2.3. Preparation of Heat-Killed E. faecalis KH2
2.4. Heat-Killed KH2 Treatment of Wounds
2.5. Measurement of the Wound Area
2.6. Histopathology and Immunohistochemistry
2.7. Measurement of Cytokine and Growth Factor Concentrations
2.8. Induction of Diabetes Mellitus
2.9. Statistical Analysis
3. Results
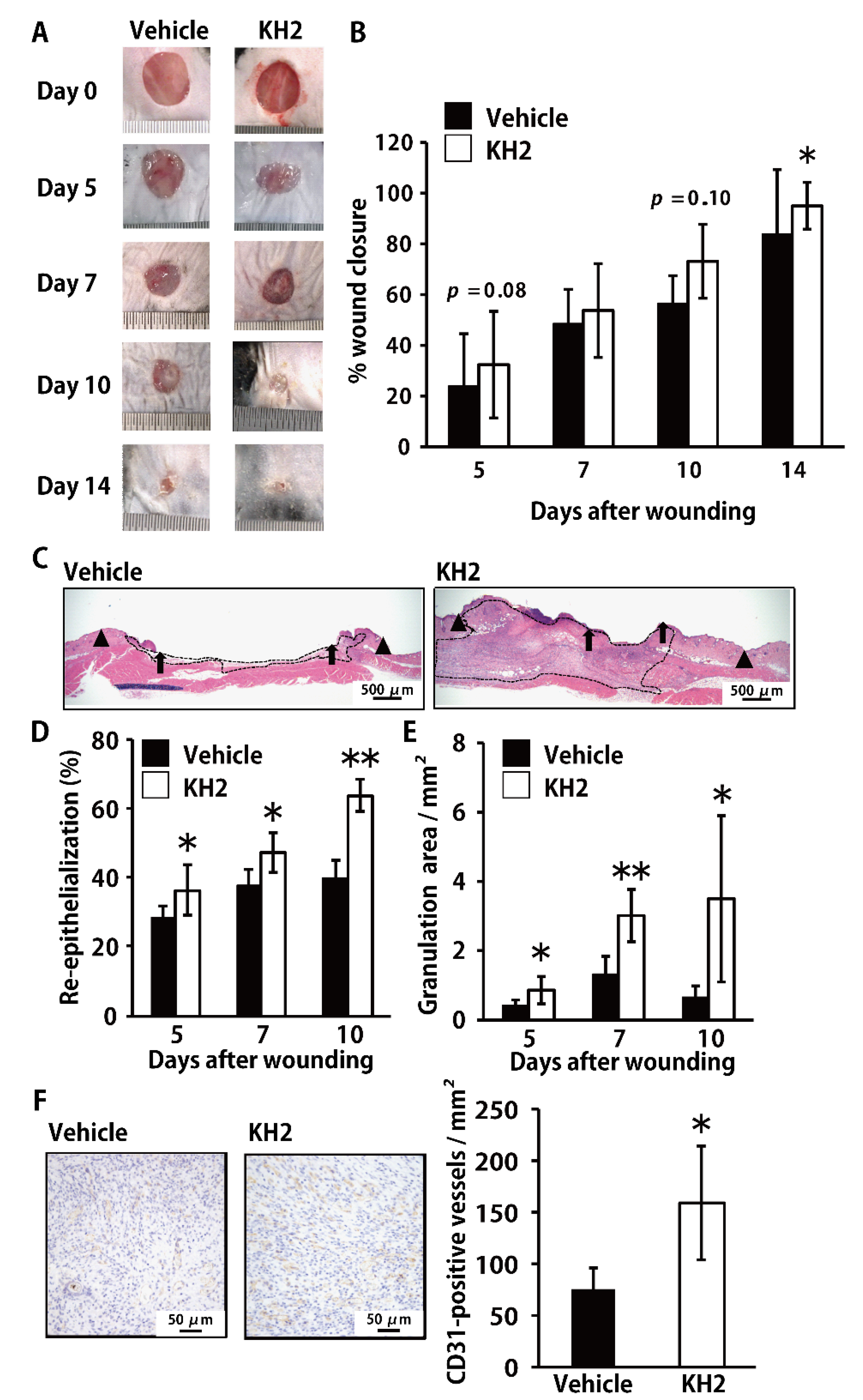
3.1. Accelerated Skin Wound-Healing by Treatment with Heat-Killed KH2
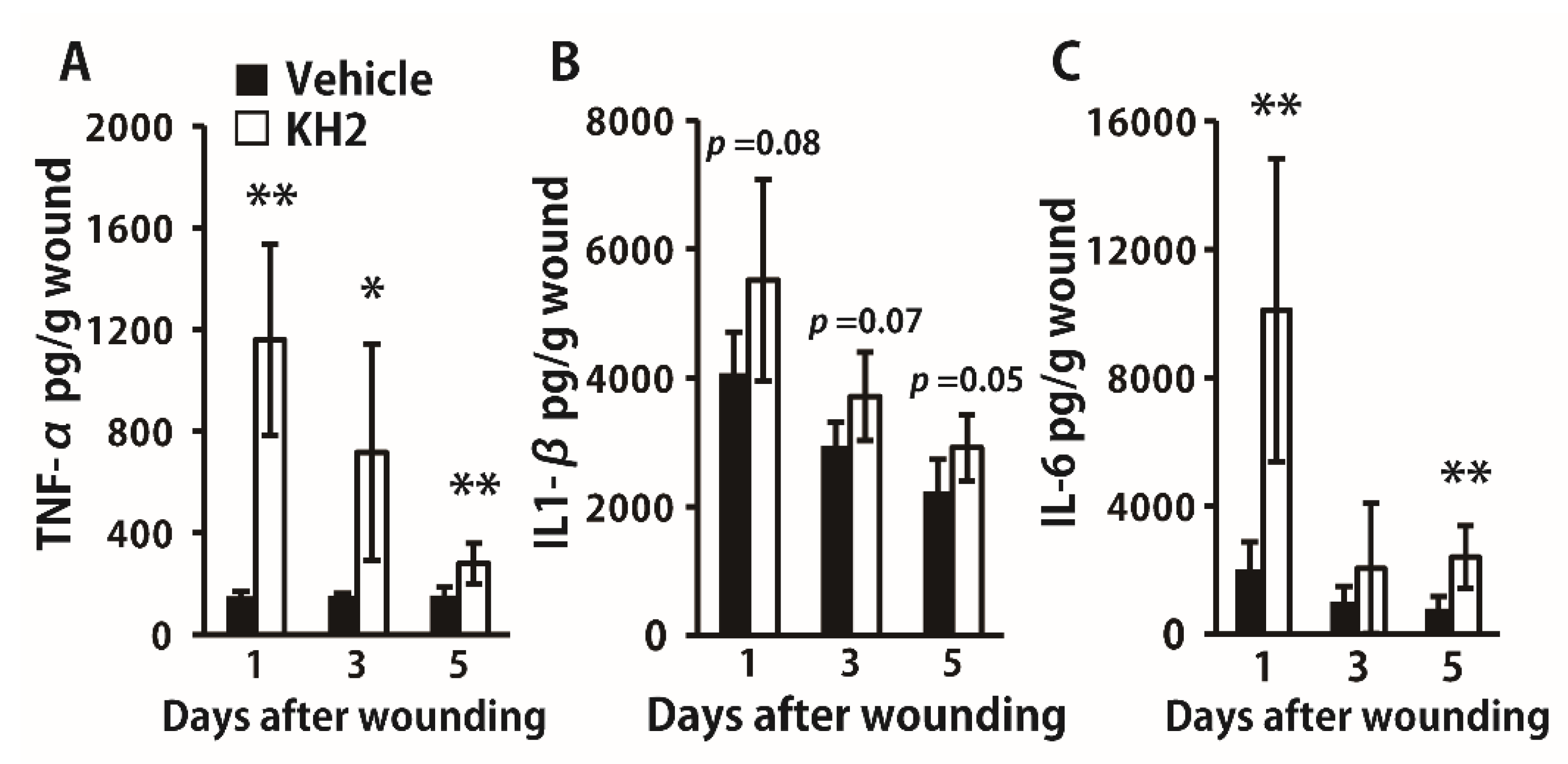
3.2. Enhanced Synthesis of Inflammatory Cytokines by Heat-Killed KH2 Treatment
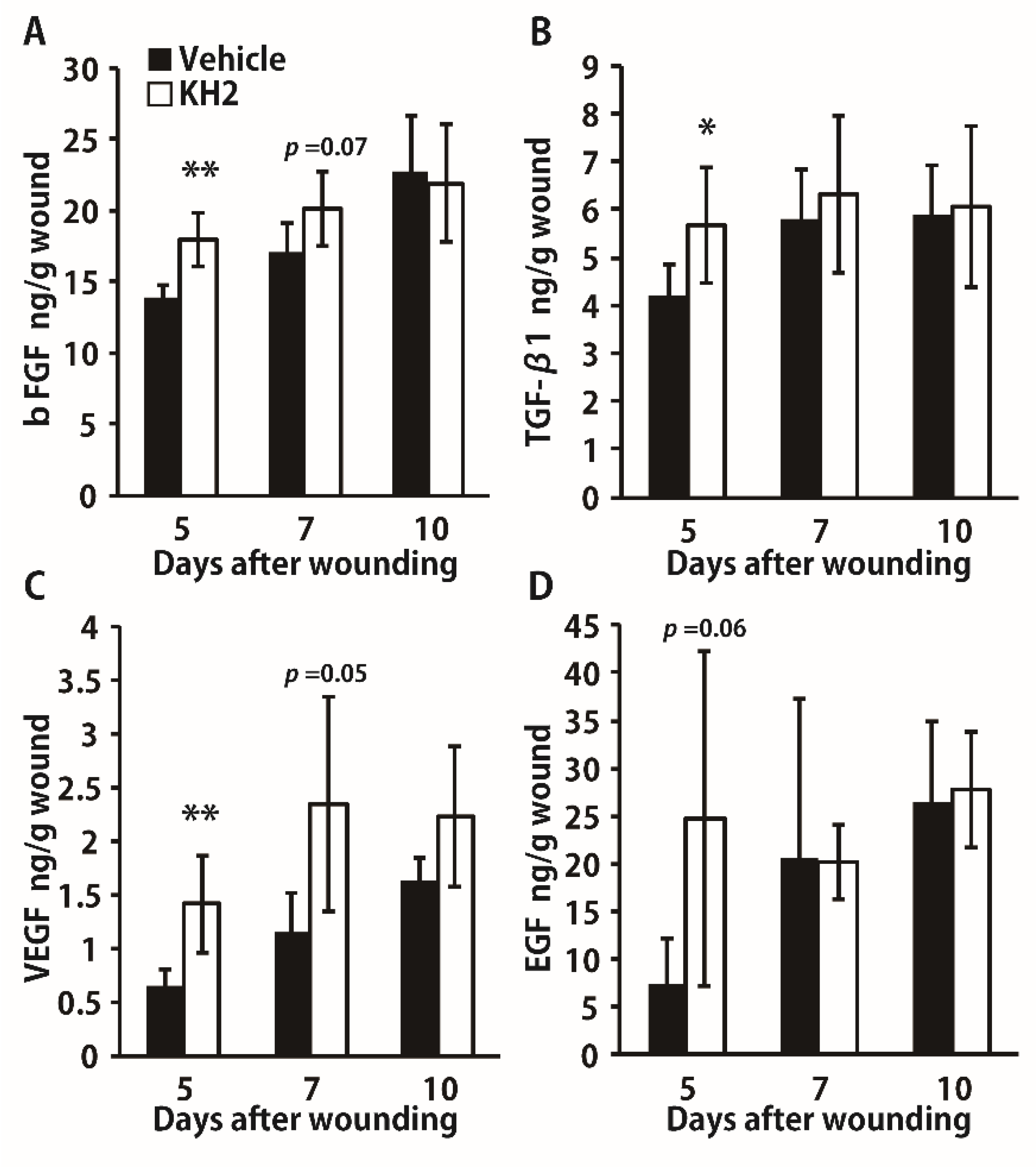
3.3. Enhanced Synthesis of Growth Factors by Heat-Killed KH2 Treatment
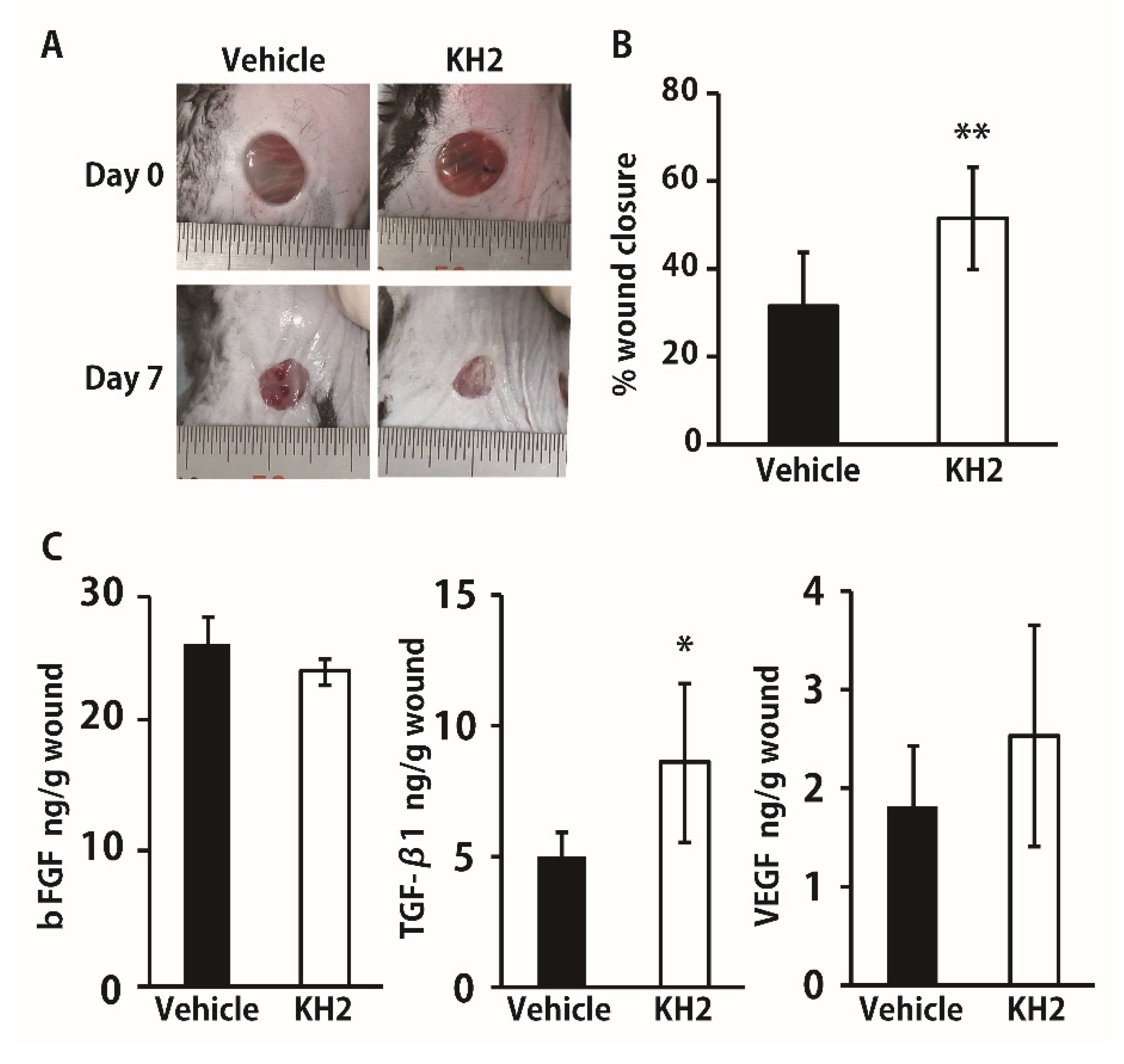
3.4. Improved Wound Closure and TGF-β1 Synthesis in STZ-Induced Diabetic Mice by Heat-Killed KH2 Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2018, 99, 665–706. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Kiya, K.; Kubo, T. Neurovascular interactions in skin wound healing. Neurochem. Int. 2019, 125, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Krisp, C.; Jacobsen, F.; McKay, M.J.; Molloy, M.P.; Steinstraesser, L.; Wolters, D.A. Proteome analysis reveals antiangiogenic environments in chronic wounds of diabetes mellitus type 2 patients. Proteomics 2013, 13, 2670–2681. [Google Scholar] [CrossRef] [PubMed]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Fink, L.N.; Zeuthen, L.H.; Ferlazzo, G.; Frøkiaer, H. Human antigen-presenting cells respond differently to gut-derived probiotic bacteria but mediate similar strain-dependent NK and T cell activation. FEMS Immunol. Med. Microbiol. 2007, 51, 535–546. [Google Scholar] [CrossRef] [Green Version]
- Jounai, K.; Sugimura, T.; Ohshio, K.; Fujiwara, D. Oral Administration of Lactococcus lactis Subsp. lactis JCM5805 Enhances Lung Immune Response Resulting in Protection from Murine Parainfluenza Virus Infection. PLoS ONE 2015, 10, e0119055. [Google Scholar] [CrossRef]
- Al Atya, A.K.; Drider-Hadiouche, K.; Ravallec, R.; Silvain, A.; Vachee, A.; Drider, D. Probiotic potential of Enterococcus faecalis strains isolated from meconium. Front. Microbiol. 2015, 6, 15. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef] [PubMed]
- Poutahidis, T.; Kearney, S.M.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Microbial Symbionts Accelerate Wound Healing via the Neuropeptide Hormone Oxytocin. PLoS ONE 2013, 8, e78898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argenta, A.; Satish, L.; Gallo, P.; Liu, F.; Kathju, S. Local Application of Probiotic Bacteria Prophylaxes against Sepsis and Death Resulting from Burn Wound Infection. PLoS ONE 2016, 11, e0165294. [Google Scholar] [CrossRef] [Green Version]
- Inoue, R.; Nagino, T.; Hoshino, G.; Ushida, K. Nucleic acids of Enterococcus faecalis strain EC-12 are potent Toll-like receptor 7 and 9 ligands inducing interleukin-12 production from murine splenocytes and murine macrophage cell line J774.1. FEMS Immunol. Med. Microbiol. 2010, 61, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukahara, T.; Nakamura, S.-I.; Romero-Pérez, G.A.; Ohwaki, M.; Yanagisawa, T.; Kan, T. Stimulation of murine cell-mediated immunity by dietary administration of a cell preparation of Enterococcus faecalis strain KH-2 and its possible activity against tumour development in mice. Biosci. Microbiota, Food Health 2018, 37, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Pinto, D.; Marzani, B.; Minervini, F.; Calasso, M.; Giuliani, G.; Gobbetti, M.; De Angelis, M. Plantaricin A synthesized by Lactobacillus plantarum induces in vitro proliferation and migration of human keratinocytes and increases the expression of TGF-β1, FGF7, VEGF-A and IL-8 genes. Peptides 2011, 32, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hayashi, K.; Takahashi, I.; Ohwaki, M.; Kan, T.; Kawahara, T. Physical properties of lactic acid bacteria influence the level of protection against influenza infection in mice. PLoS ONE 2021, 16, e0251784. [Google Scholar] [CrossRef]
- Tanno, H.; Kanno, E.; Sato, S.; Asao, Y.; Shimono, M.; Kurosaka, S.; Oikawa, Y.; Ishi, S.; Shoji, M.; Sato, K.; et al. Contribution of Invariant Natural Killer T Cells to the Clearance of Pseudomonas aeruginosa from Skin Wounds. Int. J. Mol. Sci. 2021, 22, 3931. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kanno, E.; Tanno, H.; Sasaki, A.; Kitai, Y.; Miura, T.; Takagi, N.; Shoji, M.; Kasamatsu, J.; Sato, K.; et al. Distinct roles for Dectin-1 and Dectin-2 in skin wound healing and neutrophilic inflammatory responses. J. Investig. Dermatol. 2020, 9, 15. [Google Scholar]
- Miura, T.; Kawakami, K.; Kanno, E.; Tanno, H.; Tada, H.; Sato, N.; Masaki, A.; Yokoyama, R.; Kawamura, K.; Kitai, Y.; et al. Dectin-2–Mediated Signaling Leads to Delayed Skin Wound Healing through Enhanced Neutrophilic Inflammatory Response and Neutrophil Extracellular Trap Formation. J. Investig. Dermatol. 2018, 139, 702–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, Y.; Kuninaka, Y.; Nosaka, M.; Furuta, M.; Kimura, A.; Taruya, A.; Yamamoto, H.; Shimada, E.; Akiyama, M.; Mukaida, N.; et al. CCL2-Mediated Reversal of Impaired Skin Wound Healing in Diabetic Mice by Normalization of Neovascularization and Collagen Accumulation. J. Investig. Dermatol. 2019, 139, 2517–2527.e5. [Google Scholar] [CrossRef]
- Gu, X.-Y.; Shen, S.-E.; Huang, C.-F.; Liu, Y.-N.; Chen, Y.-C.; Luo, L.; Zeng, Y.; Wang, A.-P. Effect of activated autologous monocytes/macrophages on wound healing in a rodent model of experimental diabetes. Diabetes Res. Clin. Pract. 2013, 102, 53–59. [Google Scholar] [CrossRef]
- Noguchi, F.; Nakajima, T.; Inui, S.; Reddy, J.K.; Itami, S. Alteration of Skin Wound Healing in Keratinocyte-Specific Mediator Complex Subunit 1 Null Mice. PLoS ONE 2014, 9, e102271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loyd, C.M.; Diaconu, I.; Fu, W.; Adams, G.N.; Brandt, E.; Knutsen, D.A.; Wolfram, J.A.; McCormick, T.; Ward, N.L. Transgenic overexpression of keratinocyte-specific VEGF and Ang1 in combination promotes wound healing under nondiabetic but not diabetic conditions. Int. J. Clin. Exp. Pathol. 2012, 5, 1–11. [Google Scholar] [PubMed]
- Chen, L.; Guo, S.; Ranzer, M.J.; DiPietro, L.A. Toll-Like Receptor 4 Has an Essential Role in Early Skin Wound Healing. J. Investig. Dermatol. 2013, 133, 258–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritsu, M.; Kanno, E.; Tanno, H.; Imai, Y.; Maruyama, R.; Tachi, M.; Kawakami, K.; Ishii, K. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Gallucci, R.M.; Simeonova, P.P.; Matheson, J.M.; Kommineni, C.; Guriel, J.L.; Sugawara, T.; Luster, M.I. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000, 14, 2525–2531. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.-Q.; Kondo, T.; Ishida, Y.; Takayasu, T.; Mukaida, N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J. Leukoc. Biol. 2003, 73, 713–721. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Sig. Transduct. Target Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.J.; Jo, S.G.; Kim, D.J.; Park, J.H. NLRP3 inflammasome mediates interleukin-1β production in immune cells in response to Acinetobacter baumannii and contributes to pulmonary inflammation in mice. Immunology 2017, 150, 495–505. [Google Scholar] [CrossRef]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Dionisi, L.; Pizzicannella, J.; Kothari, A.; De Fazio, D.; Garcovich, S. Impact of the Different Preparation Methods to Obtain Autologous Non-Activated Platelet-Rich Plasma (A-PRP) and Activated Platelet-Rich Plasma (AA-PRP) in Plastic Surgery: Wound Healing and Hair Regrowth Evaluation. Int. J. Mol. Sci. 2020, 21, 431. [Google Scholar] [CrossRef] [Green Version]
- Cervelli, V.; Gentile, P. Use of Cell Fat Mixed with Platelet Gel in Progressive Hemifacial Atrophy. Aesthetic Plast. Surg. 2008, 33, 22–27. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, B.; D’Autilio, M.F.; Orlandi, F.; Pepe, G.; Garcovich, S.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Wound healing: In vitro and in vivo evaluation of a bio-functionalized scaffold based on hyaluronic acid and platelet-rich plasma in chronic Ulcers. J. Clin. Med. 2019, 8, 1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelo, L.S.; Kurzrock, R. Vascular Endothelial Growth Factor and Its Relationship to Inflammatory Mediators: Figure 1. Clin. Cancer Res. 2007, 13, 2825–2830. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.; Stringer, S. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell Sci. 2001, 114, 853–865. [Google Scholar] [CrossRef]
- Luckett-Chastain, L.; Gallucci, R. Interleukin (IL)-6 modulates transforming growth factor-β expression in skin and dermal fibroblasts from IL-6-deficient mice. Br. J. Dermatol. 2009, 161, 237–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okizaki, S.-I.; Ito, Y.; Hosono, K.; Oba, K.; Ohkubo, H.; Amano, H.; Shichiri, M.; Majima, M. Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomed. Pharmacother. 2014, 70, 317–325. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Yu, Q.; Zhou, Z.; Wang, P. CpG oligonucleotides induce an immune response of odontoblasts through the TLR9, MyD88 and NF-κB pathways. Biochem. Biophys. Res. Commun. 2010, 399, 274–278. [Google Scholar] [CrossRef]
- Meng, W.; Yamazaki, T.; Nishida, Y.; Hanagata, N. Nuclease-resistant immunostimulatory phosphodiester CpG oligodeoxynucleotides as human Toll-like receptor 9 agonists. BMC Biotechnol. 2011, 11, 88. [Google Scholar] [CrossRef] [Green Version]
- Lebre, M.C.; van der Aar, A.M.; van Baarsen, L.; van Capel, T.M.; Schuitemaker, J.H.; Kapsenberg, M.L.; de Jong, E.C. Human keratinocytes express functional toll-like receptor 3, 4, 5, and 9. J. Investig. Dermatol. 2007, 127, 331–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Hori, K.; Ding, J.; Huang, Y.; Kwan, P.; Ladak, A.; Tredget, E.E. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J. Cell. Physiol. 2010, 226, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; Kim, Y.H.; Kwon, H.J.; Kim, D.S. The LRP1-independent mechanism of PAI-1-induced migration in CpG-ODN activated macrophages. Int. J. Biochem. Cell Biol. 2014, 49, 17–25. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Yu, H.; Wang, R.; Gou, Y.; Zhang, M.; Kang, C.; Liu, T.; Lan, Y.; Wang, X.-B.; et al. Membrane TLR9 Positive Neutrophil Mediated MPLA Protects Against Fatal Bacterial Sepsis. Theranostics 2019, 9, 6269–6283. [Google Scholar] [CrossRef]
- Reynolds, J.; Dong, C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Sato, T.; Yamamoto, M.; Shimosato, T.; Klinman, D.M. Accelerated wound healing mediated by activation of Toll-like receptor. Wound Repair Regen. 2010, 18, 586–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Xu, Z.; Zuo, J.; Ding, J. A C-type CpG ODN accelerates wound healing via regulating fibroblasts and immune response. J. Cell. Biochem. 2018, 120, 7868–7875. [Google Scholar] [CrossRef]
- Thakur, B.K.; Saha, P.; Banik, G.; Saha, D.R.; Grover, S.; Batish, V.K.; Das, S. Live and heat-killed probiotic Lactobacillus casei Lbs2 protects from experimental colitis through Toll-like receptor 2-dependent induction of T-regulatory response. Int. Immunopharmacol. 2016, 36, 39–50. [Google Scholar] [CrossRef]
- Hsieh, F.-C.; Lan, C.-C.E.; Huang, T.-Y.; Chen, K.-W.; Chai, C.-Y.; Chen, W.-T.; Fang, A.-H.; Chen, Y.-H.; Wu, C.-S. Heat-killed and live Lactobacillus reuteri GMNL-263 exhibit similar effects on improving metabolic functions in high-fat diet-induced obese rats. Food Funct. 2016, 7, 2374–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorjão, A.L.; De Oliveira, F.E.; Leão, M.V.P.; Carvalho, C.A.T.; Jorge, A.O.C.; Oliveira, L. Live and Heat-KilledLactobacillus rhamnosusATCC 7469 May Induce Modulatory Cytokines Profiles on Macrophages RAW 264.7. Sci. World J. 2015, 2015, 716749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanno, H.; Kanno, E.; Kurosaka, S.; Oikawa, Y.; Watanabe, T.; Sato, K.; Kasamatsu, J.; Miyasaka, T.; Ishi, S.; Shoji, M.; et al. Topical Administration of Heat-Killed Enterococcus faecalis Strain KH2 Promotes Re-Epithelialization and Granulation Tissue Formation during Skin Wound-Healing. Biomedicines 2021, 9, 1520. https://doi.org/10.3390/biomedicines9111520
Tanno H, Kanno E, Kurosaka S, Oikawa Y, Watanabe T, Sato K, Kasamatsu J, Miyasaka T, Ishi S, Shoji M, et al. Topical Administration of Heat-Killed Enterococcus faecalis Strain KH2 Promotes Re-Epithelialization and Granulation Tissue Formation during Skin Wound-Healing. Biomedicines. 2021; 9(11):1520. https://doi.org/10.3390/biomedicines9111520
Chicago/Turabian StyleTanno, Hiromasa, Emi Kanno, Shiho Kurosaka, Yukari Oikawa, Takumi Watanabe, Ko Sato, Jun Kasamatsu, Tomomitsu Miyasaka, Shinyo Ishi, Miki Shoji, and et al. 2021. "Topical Administration of Heat-Killed Enterococcus faecalis Strain KH2 Promotes Re-Epithelialization and Granulation Tissue Formation during Skin Wound-Healing" Biomedicines 9, no. 11: 1520. https://doi.org/10.3390/biomedicines9111520
APA StyleTanno, H., Kanno, E., Kurosaka, S., Oikawa, Y., Watanabe, T., Sato, K., Kasamatsu, J., Miyasaka, T., Ishi, S., Shoji, M., Takagi, N., Imai, Y., Ishii, K., Tachi, M., & Kawakami, K. (2021). Topical Administration of Heat-Killed Enterococcus faecalis Strain KH2 Promotes Re-Epithelialization and Granulation Tissue Formation during Skin Wound-Healing. Biomedicines, 9(11), 1520. https://doi.org/10.3390/biomedicines9111520





