Complementary Effect of Non-Persistent Silver Nano-Architectures and Chlorhexidine on Infected Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Synthesis
2.2.1. Silver Nanoarchitectures
Synthesis of Silver Clusters
Synthesis of Silver Nanoparticles Arrays
Synthesis of AgNAs
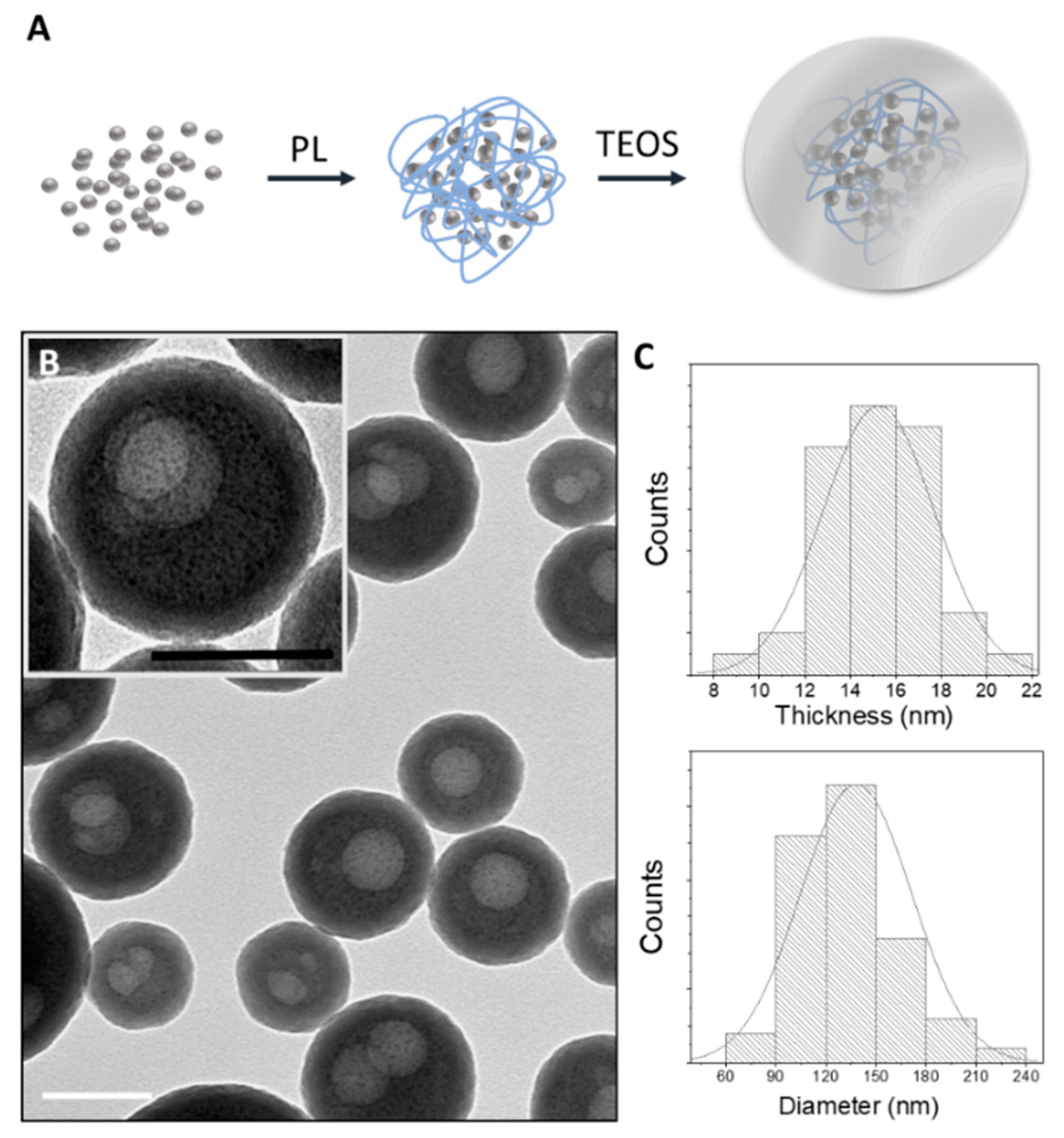
2.2.2. Nanoarchitecture Characterization
Transmission Electron Microscopy (TEM)
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Analyses
2.2.3. Antibacterial Activity Assessment
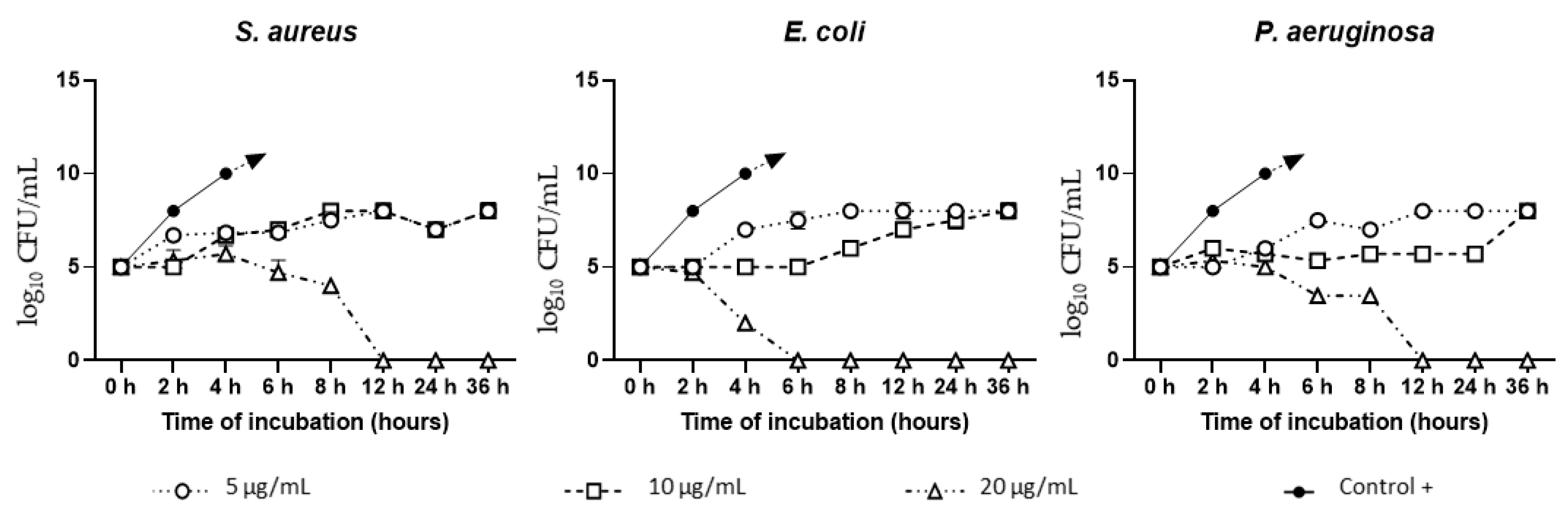
Minimum Inhibitory Concentration (MIC)
Time-Dependent Inhibition Test
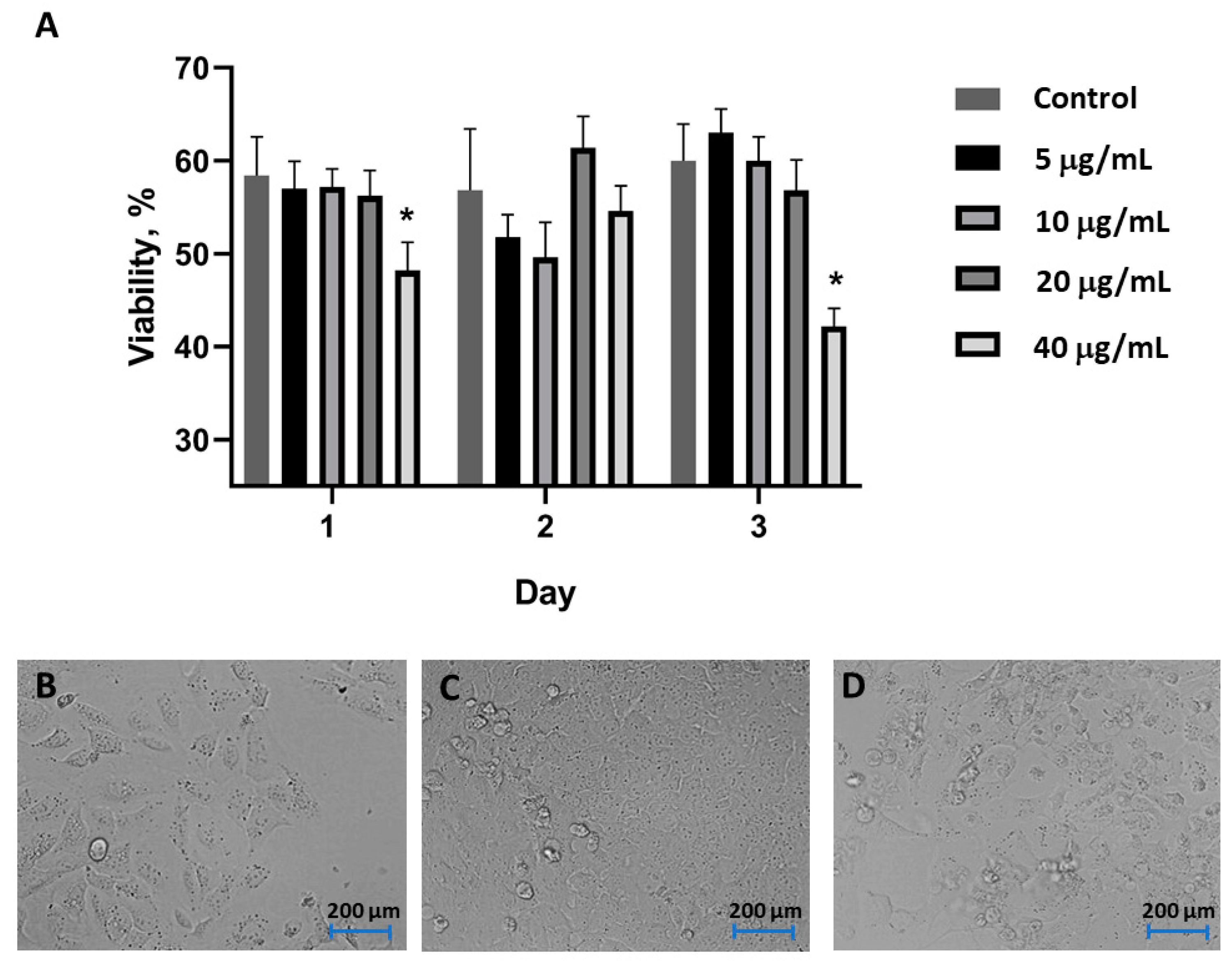
Cell Toxicity Experiment
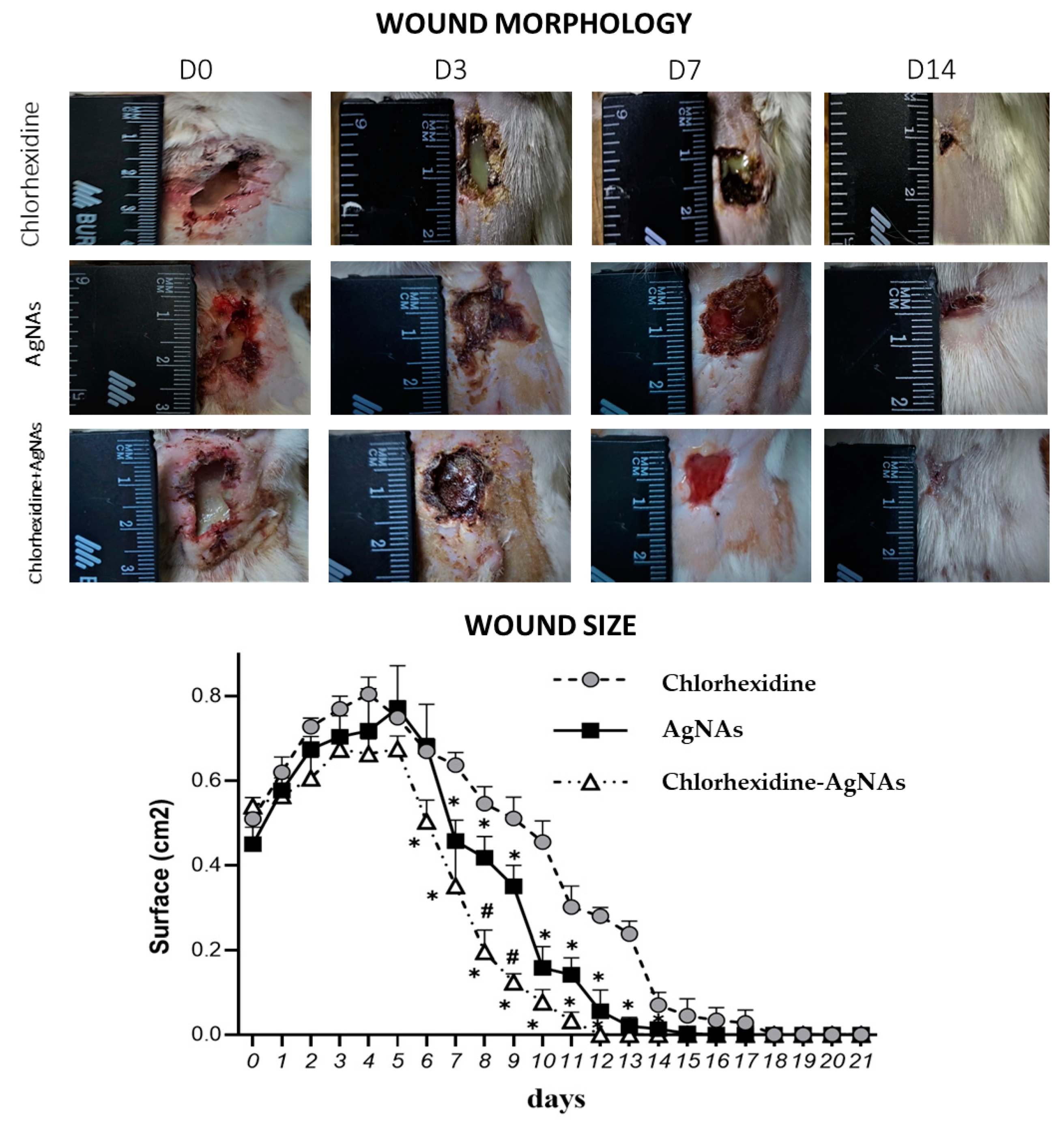
Animal Experiment Design
- Control (24 rats)—application of 0.05% chlorhexidine solution;
- “AgNAs” (24 rats)—application of 40 μg/mL solution of AgNAs;
- “Chlorhexidine–AgNAs” (24 rats)—combined application of 40 μg/mL solution of AgNAs in 0.05% chlorhexidine.
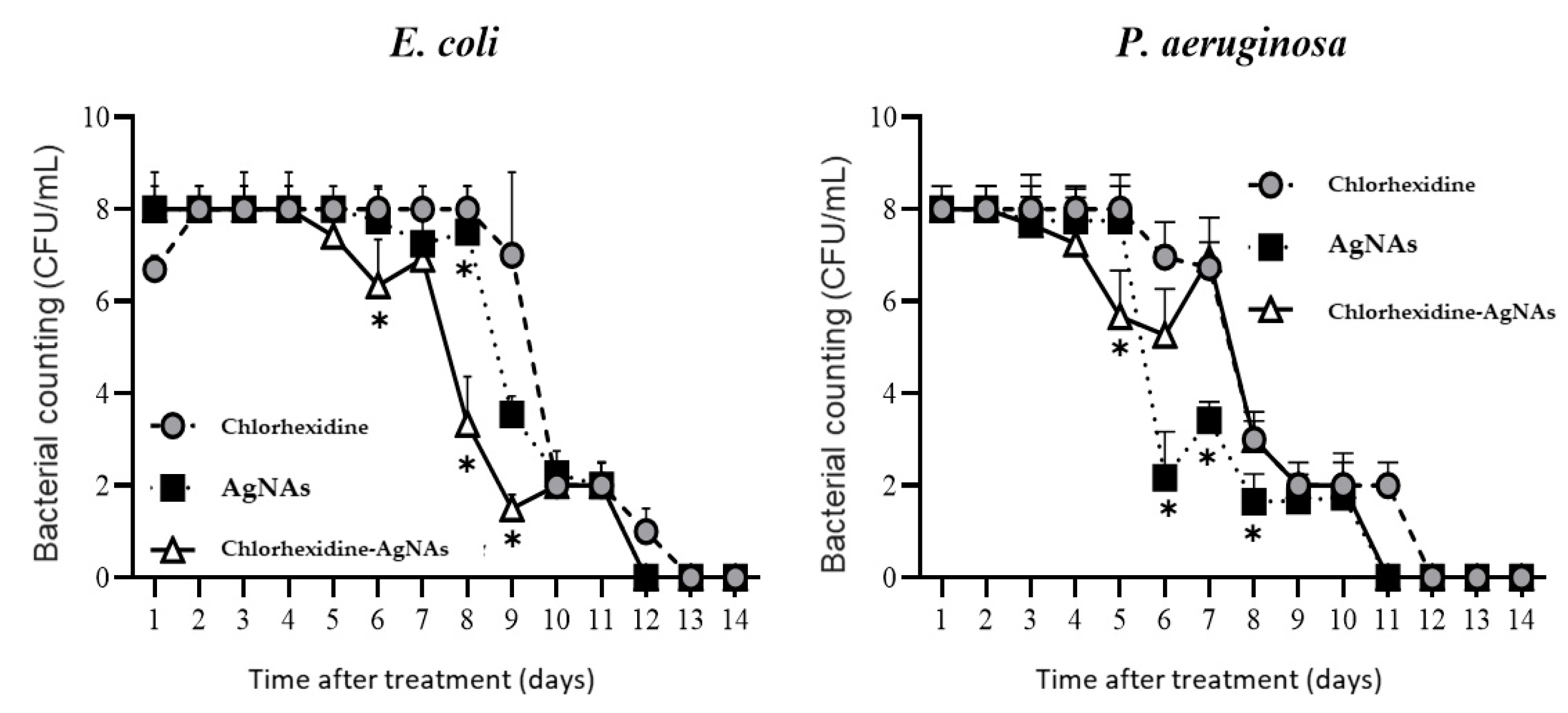
Wound Microbiology
Histological Analysis and Immunohistochemical Markers Assessment
Statistics
3. Results and Discussion
3.1. AgNAs Characterization
3.2. AgNAs In Vitro Antibacterial Study
3.3. Cell Toxicity
3.4. Animal Experiment
3.5. Histological Evaluation of Wound Healing
3.5.1. AgNAs Accelerated Wound Cleansing and Shortened the Inflammatory Phase of the Wound Process
3.5.2. AgNAs Application Facilitates Wound Healing
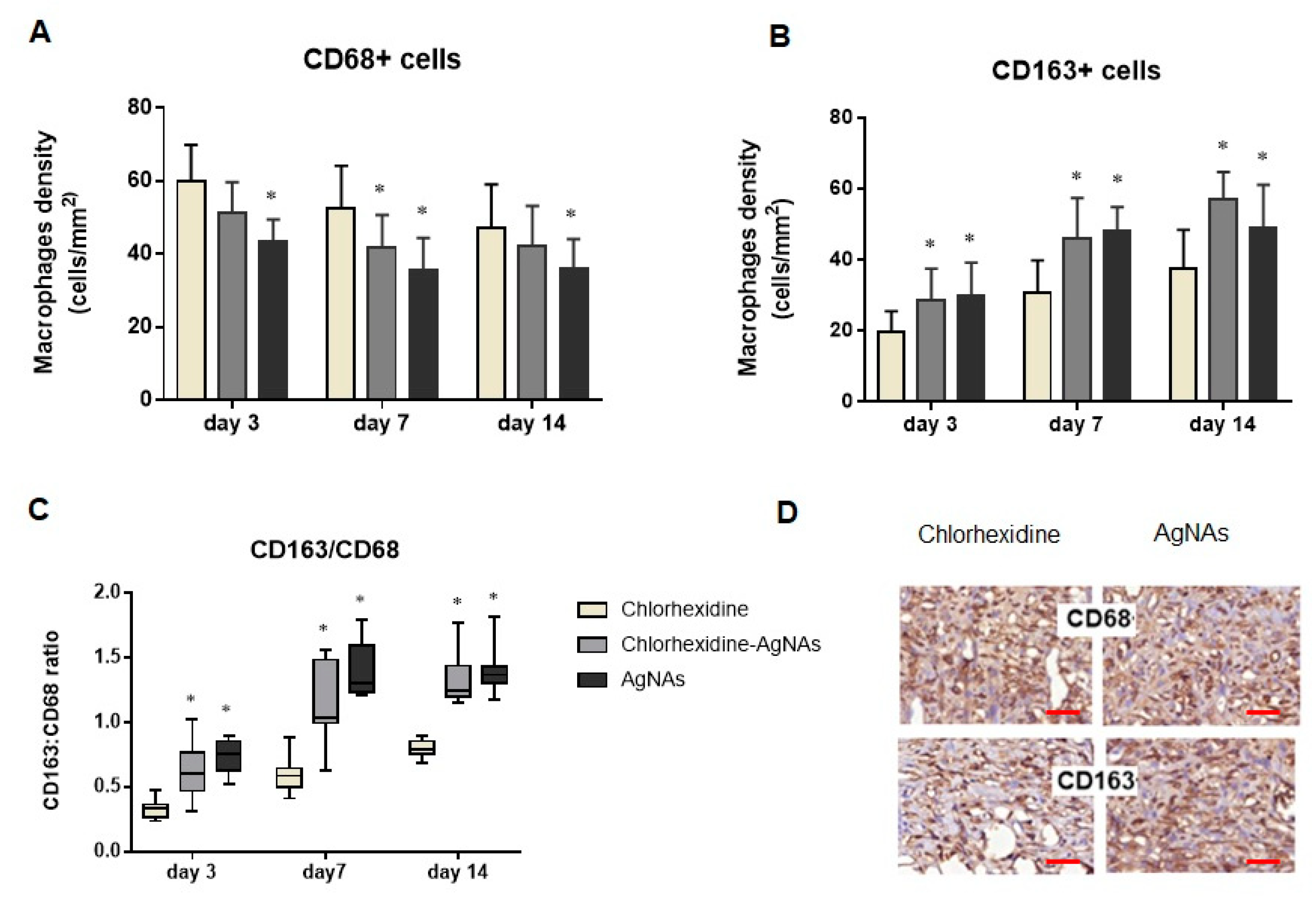
3.5.3. AgNAs Impact Macrophages Polarization Promoting Wound Phases Progress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mellinghoff, S.C.; Otto, C.; Cornely, O.A. Surgical Site Infections: Current Management and Role of New Antibiotics. Curr. Opin. Infect. Dis. 2019, 32, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.J.; Van Goor, H.; Reilly, J.; Petrosillo, N.; Geiss, H.K.; Torres, A.J.; Berger, A. Surgical Site Infection—A European Perspective of Incidence and Economic Burden. Int. Wound J. 2004, 1, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, G.S.; Fowler, V.G.; Kong, L.K.; McClelland, R.S.; Gopal, A.K.; Marr, K.A.; Li, J.; Sexton, D.J.; Glower, D.; Corey, G.R. Staphylococcus Aureus Bacteremia in the Surgical Patient: A Prospective Analysis of 73 Postoperative Patients Who Developed Staphylococcus Aureus Bacteremia at a Tertiary Care Facility. J. Am. Coll. Surg. 2000, 190, 50–57. [Google Scholar] [CrossRef]
- Ciorba, V.; Odone, A.; Veronesi, L.; Pasquarella, C.; Signorelli, C. Antibiotic Resistance as a Major Public Health Concern: Epidemiology and Economic Impact. Ann. Ig. 2015, 27, 562–579. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a Therapeutic Tool to Combat Microbial Resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Myronov, P.; Bugaiov, V.; Holubnycha, V.; Sikora, V.; Deineka, V.; Lyndin, M.; Opanasyuk, A.; Romaniuk, A.; Pogorielov, M. Low-Frequency Ultrasound Increase Effectiveness of Silver Nanoparticles in a Purulent Wound Model. Biomed. Eng. Lett. 2020, 10, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Hindi, K.M.; Ditto, A.J.; Panzner, M.J.; Medvetz, D.A.; Han, D.S.; Hovis, C.E.; Hilliard, J.K.; Taylor, J.B.; Yun, Y.H.; Cannon, C.L.; et al. The Antimicrobial Efficacy of Sustained Release Silver-Carbene Complex-Loaded l-Tyrosine Polyphosphate Nanoparticles: Characterization, in Vitro and in Vivo Studies. Biomaterials 2009, 30, 3771–3779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Zhang, H.; Yang, S.; Xi, Z.; Tang, T.; Yin, R.; Zhang, W. Electrospun PLGA membrane incorporated with andrographolide-loaded mesoporous silica nanoparticles for sustained antibacterial wound dressing. Nanomedicine 2018, 13, 2881–2899. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, L.; Yang, S.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; Tang, T. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater. 2018, 79, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ipe, D.S.; Kumar, P.T.S.; Love, R.M.; Hamlet, S.M. Silver Nanoparticles at Biocompatible Dosage Synergistically Increases Bacterial Susceptibility to Antibiotics. Front. Microbiol. 2020, 11, 1074. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef] [PubMed]
- Holubnycha, V.; Myronov, P.; Bugaiov, V.; Opanasyuk, A.; Dobrozhan, O.; Yanovska, A.; Pogorielov, M.; Kalinkevich, O. Effect of Ultrasound Treatment on Chitosan-Silver Nanoparticles Antimicrobial Activity. In Proceedings of the 2018 IEEE 8th International Conference on Nanomaterials: Applications and Properties, NAP 2018, Zatoka, Ukraine, 9–14 September 2018; Institute of Electrical and Electronics Engineers Inc.: Piscataway Township, NJ, USA, 2018. [Google Scholar] [CrossRef]
- He, J.; Qiao, Y.; Zhang, H.; Zhao, J.; Li, W.; Xie, T.; Zhong, D.; Wei, Q.; Hua, S.; Yu, Y.; et al. Gold–Silver Nanoshells Promote Wound Healing from Drug-Resistant Bacteria Infection and Enable Monitoring via Surface-Enhanced Raman Scattering Imaging. Biomaterials 2020, 234. [Google Scholar] [CrossRef]
- Vlamidis, Y.; Voliani, V. Bringing Again Noble Metal Nanoparticles to the Forefront of Cancer Therapy. Front. Bioeng. Biotechnol. 2018, 6, 143. [Google Scholar] [CrossRef]
- Cassano, D.; Mapanao, A.K.; Summa, M.; Vlamidis, Y.; Giannone, G.; Santi, M.; Guzzolino, E.; Pitto, L.; Poliseno, L.; Bertorelli, R.; et al. Biosafety and Biokinetics of Noble Metals: The Impact of Their Chemical Nature. ACS Appl. Bio Mater. 2019, 2, 4464–4470. [Google Scholar] [CrossRef]
- Pocovĺ-Martĺnez, S.; Cassano, D.; Voliani, V. Naked Nanoparticles in Silica Nanocapsules: A Versatile Family of Nanorattle Catalysts. ACS Appl. Nano Mater. 2018, 1, 1836–1840. [Google Scholar] [CrossRef]
- Cassano, D.; David, J.; Luin, S.; Voliani, V. Passion Fruit-like Nano-Architectures: A General Synthesis Route. Sci. Rep. 2017, 7, srep43795. [Google Scholar] [CrossRef] [PubMed]
- Mapanao, A.K.; Santi, M.; Faraci, P.; Cappello, V.; Cassano, D.; Voliani, V. Endogenously Triggerable Ultrasmall-in-Nano Architectures: Targeting Assessment on 3D Pancreatic Carcinoma Spheroids. ACS Omega 2018, 3, 11796–11801. [Google Scholar] [CrossRef]
- Santi, M.; Mapanao, A.K.; Cassano, D.; Vlamidis, Y.; Cappello, V.; Voliani, V. Endogenously-Activated Ultrasmall-in-Nano Therapeutics: Assessment on 3D Head and Neck Squamous Cell Carcinomas. Cancers 2020, 12, 1063. [Google Scholar] [CrossRef] [PubMed]
- Armanetti, P.; Pocoví-Martínez, S.; Flori, A.; Avigo, C.; Cassano, D.; Menichetti, L.; Voliani, V. Dual Photoacoustic/Ultrasound Multi-Parametric Imaging from Passion Fruit-like Nano-Architectures. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Cassano, D.; Santi, M.; Cappello, V.; Luin, S.; Signore, G.; Voliani, V. Biodegradable Passion Fruit-Like Nano-Architectures as Carriers for Cisplatin Prodrug. Part. Part. Syst. Charact. 2016, 33, 818–824. [Google Scholar] [CrossRef]
- Wood, T.E.; Howard, S.A.; Förster, A.; Nolan, L.M.; Manoli, E.; Bullen, N.P.; Yau, H.C.L.; Hachani, A.; Hayward, R.D.; Whitney, J.C.; et al. The Pseudomonas Aeruginosa T6SS Delivers a Periplasmic Toxin That Disrupts Bacterial Cell Morphology. Cell Rep. 2019, 29, 187–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.X.; Werner, J.; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Cytotoxicity Evaluation of Chlorhexidine Gluconate on Human Fibroblasts, Myoblasts, and Osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A.J.; Rumpf, D.A.H. Chlorhexidine-Induced Changes to Human Gingival Fibroblast Collagen and Non-Collagen Protein Production. J. Periodontol. 1999, 70, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Cheng, H.; Xu, J.; Li, F.; Gao, B.; Li, Z.; Gao, C.; Huo, K.; Fu, J.; Xiong, W. Silver-Loaded Nanotubular Structures Enhanced Bactericidal Efficiency of Antibiotics with Synergistic Effect in Vitro and in Vivo. Int. J. Nanomed. 2017, 12, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Cheng, H.; Xu, N.; Li, Y.; Chen, Y.; Wei, Y.; Gao, B.; Fu, J.; Huo, K.; Xiong, W. Poly(Dopamine) and Ag Nanoparticle-Loaded TiO2 Nanotubes with Optimized Antibacterial and ROS-Scavenging Bioactivities. Nanomedicine 2019, 14, 803–818. [Google Scholar] [CrossRef]
- Xue, Y.; Hong, X.; Gao, J.; Shen, R.; Ye, Z. Preparation and Biological Characterization of the Mixture of Poly(Lactic-Co-Glycolic Acid)/Chitosan/Ag Nanoparticles for Periodontal Tissue Engineering. Int. J. Nanomed. 2019, 14, 483–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carthy, J.M. TGFβ Signaling and the Control of Myofibroblast Differentiation: Implications for Chronic Inflammatory Disorders. J. Cell. Physiol. 2018, 233, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M.R.; Koster, K.M.; Murphy, P.S. Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses. J. Immunol. 2017, 198, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages That Have Ingested Apoptotic Cells in Vitro Inhibit Proinflammatory Cytokine Production through Autocrine/Paracrine Mechanisms Involving TGF-β, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, T.J.; DiPietro, L.A. Inflammation and Wound Healing: The Role of the Macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [Green Version]
- Brancato, S.K.; Albina, J.E. Wound Macrophages as Key Regulators of Repair: Origin, Phenotype, and Function. Am. J. Pathol. 2011, 178, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and Polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Guan, M.; Ren, R.; Gao, C.; Cheng, H.; Li, Y.; Gao, B.; Wei, Y.; Fu, J.; Sun, J.; et al. Improved Immunoregulation of Ultra-Low-Dose Silver Nanoparticle-Loaded TiO2 Nanotubes via M2 Macrophage Polarization by Regulating GLUT1 and Autophagy. Int. J. Nanomed. 2020, 15, 2011–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pernakov, M.; Ermini, M.L.; Sulaieva, O.; Cassano, D.; Santucci, M.; Husak, Y.; Korniienko, V.; Giannone, G.; Yusupova, A.; Liubchak, I.; et al. Complementary Effect of Non-Persistent Silver Nano-Architectures and Chlorhexidine on Infected Wound Healing. Biomedicines 2021, 9, 1215. https://doi.org/10.3390/biomedicines9091215
Pernakov M, Ermini ML, Sulaieva O, Cassano D, Santucci M, Husak Y, Korniienko V, Giannone G, Yusupova A, Liubchak I, et al. Complementary Effect of Non-Persistent Silver Nano-Architectures and Chlorhexidine on Infected Wound Healing. Biomedicines. 2021; 9(9):1215. https://doi.org/10.3390/biomedicines9091215
Chicago/Turabian StylePernakov, Mykola, Maria Laura Ermini, Oksana Sulaieva, Domenico Cassano, Marco Santucci, Yevhenia Husak, Viktoriia Korniienko, Giulia Giannone, Aziza Yusupova, Iryna Liubchak, and et al. 2021. "Complementary Effect of Non-Persistent Silver Nano-Architectures and Chlorhexidine on Infected Wound Healing" Biomedicines 9, no. 9: 1215. https://doi.org/10.3390/biomedicines9091215
APA StylePernakov, M., Ermini, M. L., Sulaieva, O., Cassano, D., Santucci, M., Husak, Y., Korniienko, V., Giannone, G., Yusupova, A., Liubchak, I., Hristova, M. T., Savchenko, A., Holubnycha, V., Voliani, V., & Pogorielov, M. (2021). Complementary Effect of Non-Persistent Silver Nano-Architectures and Chlorhexidine on Infected Wound Healing. Biomedicines, 9(9), 1215. https://doi.org/10.3390/biomedicines9091215







