Raloxifene Ameliorates Glucosamine-Induced Insulin Resistance in Ovariectomized Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals
2.3. Intraperitoneal Glucose Tolerance Test
2.4. Plasma Glucose and Insulin Concentrations
2.5. Determination of IR in Rats
2.6. Measurement of Islet Size
2.7. Immunofluorescence Stains for Insulin and Terminal Deoxynucleotidyl Transferase dUTP Nick End-Labeling in Pancreatic Islets
2.8. Western Blot Analysis for PEPCK in the Liver and GLUT-4 in the Soleus Muscle
2.9. Cell Culture and Compound Stimulation
2.10. Extracellular Insulin Levels
2.11. Cell Viability Analysis
2.12. Western Blot Analysis for Protein Expression Related to ER Stress
2.13. Statistical Analysis
3. Results
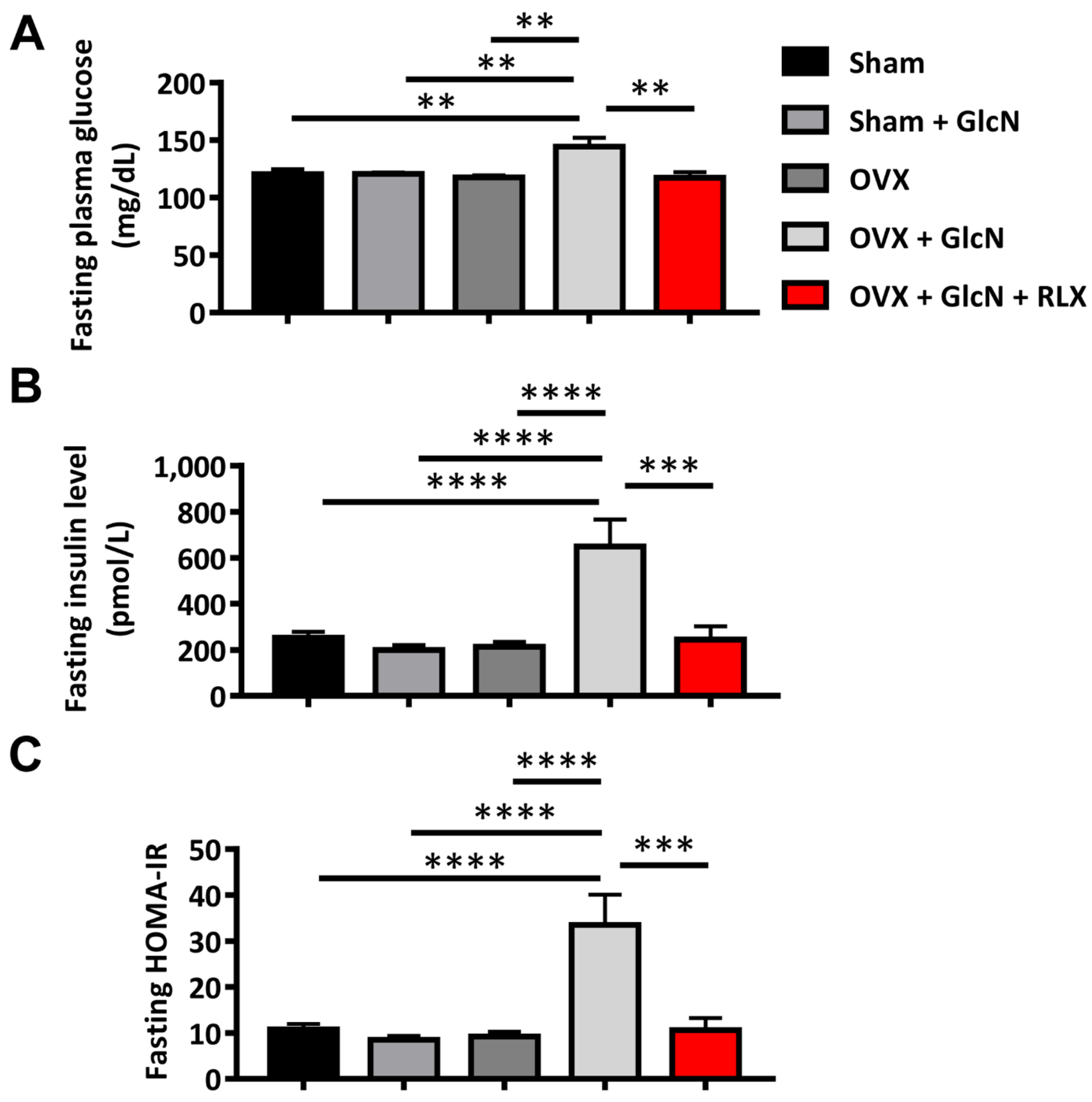
3.1. RLX Ameliorated Fasting Glucose, Insulin, and HOMA-IR in the OVX + GlcN Rats
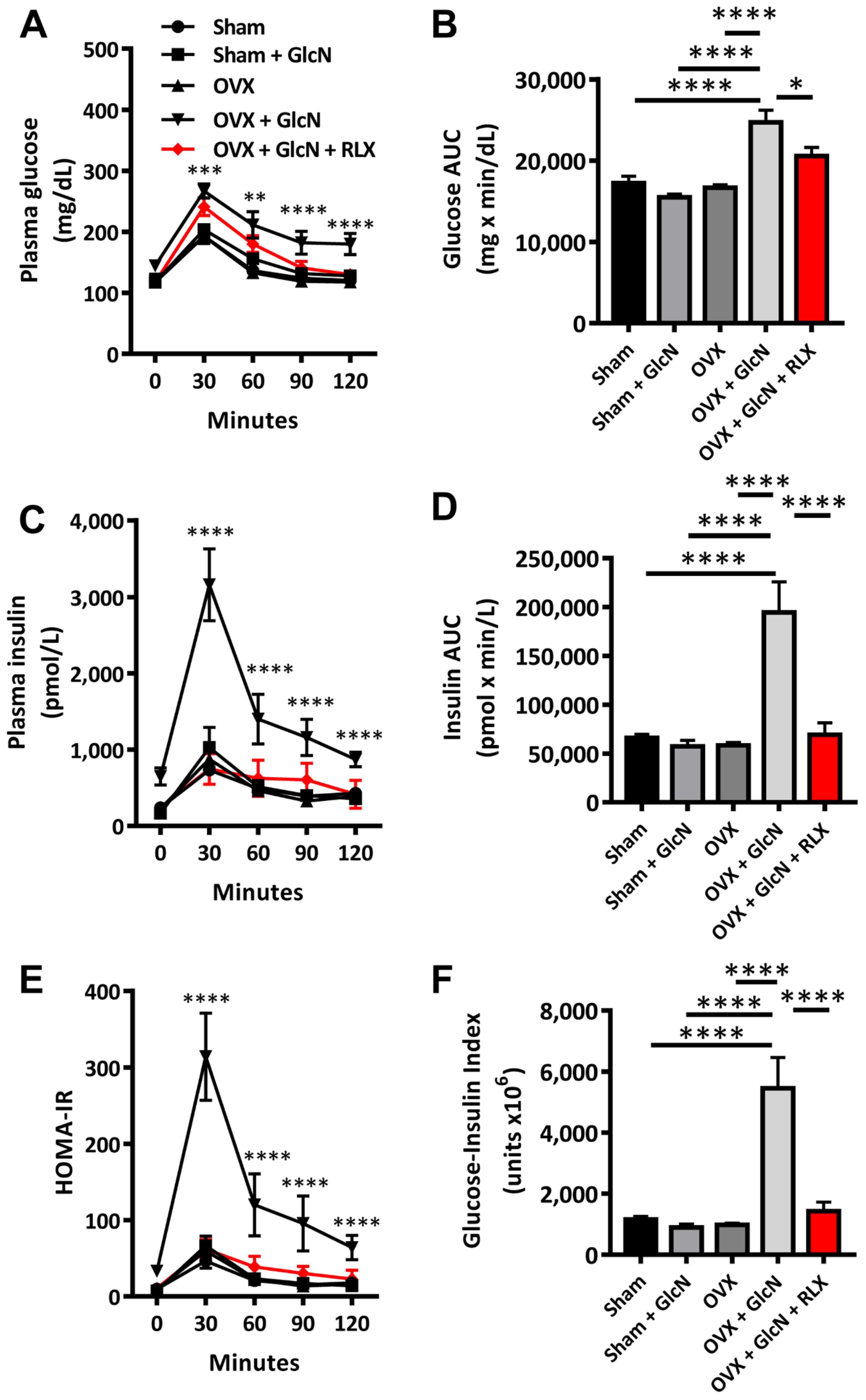
3.2. RLX Ameliorated Insulin, Glucose, Glucose-Insulin Index, and HOMA-IR in Plasma during IPGTT in the OVX + GlcN Rats
3.3. RLX Decreased Islet Size in the OVX + GlcN Rats
3.4. RLX Decreased Pancreatic Islet Apoptosis in TUNEL Stain in the OVX + GlcN Rats
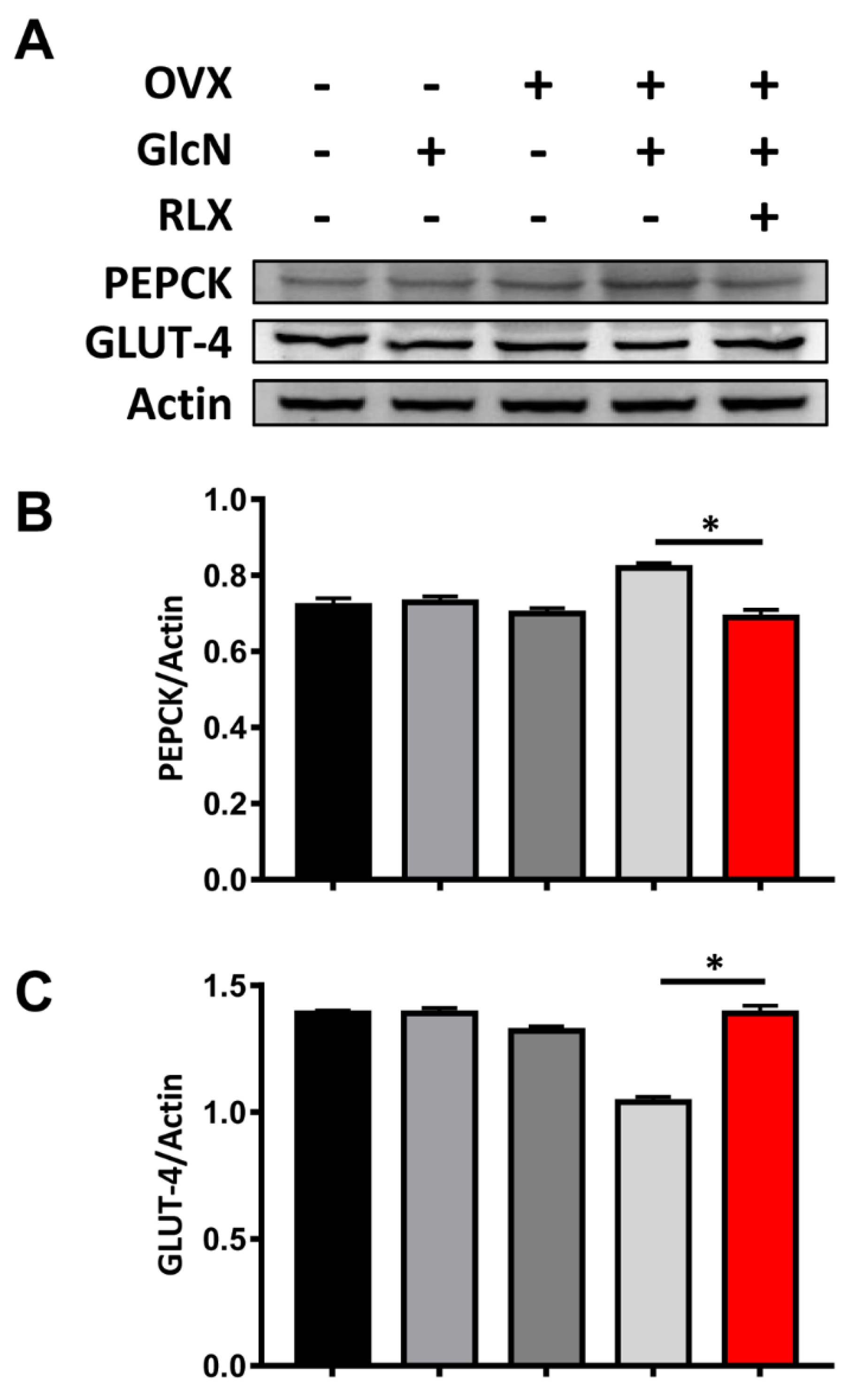
3.5. RLX Increased the Expression of PEPCK in the Liver and Decreased the Expression of GLUT-4 in the Soleus Muscle in the OVX + GlcN Rats
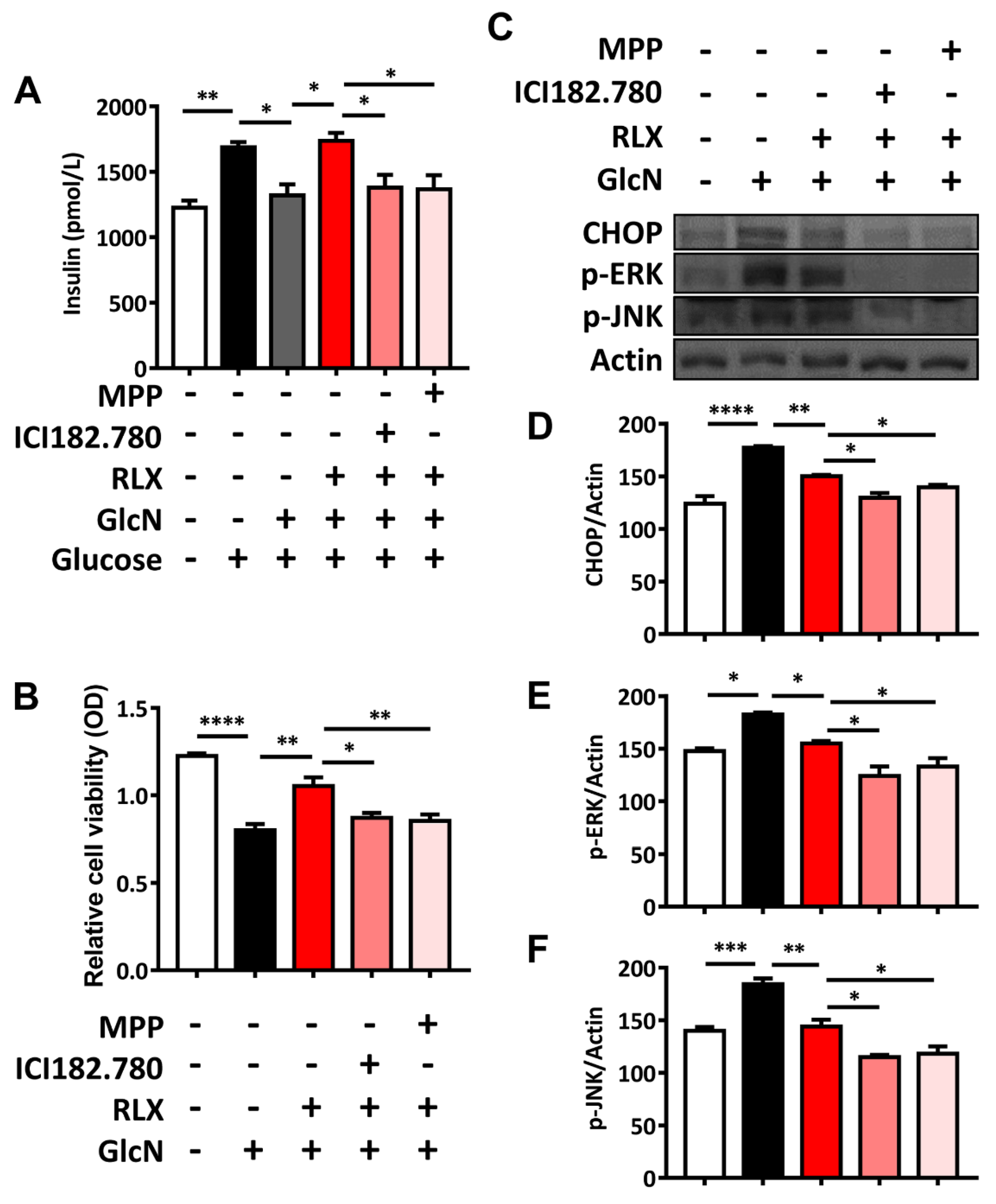
3.6. RXL Increased Extracellular Insulin Secretion and Cell Viability in MIN-6 Cells Treated with GlcN
3.7. RXL Decreased the Expression of ER Stress-Associated Proteins CHOP, p-ERK, and p-JUN in MIN-6 Cells Treated with GlcN in Western Blot Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiajue, R.; Qi, X.; Jiang, Y.; Wang, Q.; Wang, W.; Pei, Y.; Wang, X.; Huang, W.; Zheng, X.; Ning, Z.; et al. Incident Fracture Risk in Type 2 Diabetic Postmenopausal Women in Mainland China: Peking Vertebral Fracture Study. Calcif. Tissue Int. 2019, 105, 466–475. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Veronese, N.; Cooper, C.; Reginster, J.Y.; Hochberg, M.; Branco, J.; Bruyère, O.; Chapurlat, R.; Al-Daghri, N.; Dennison, E.; Herrero-Beaumont, G.; et al. Type 2 diabetes mellitus and osteoarthritis. Semin. Arthritis Rheum. 2019, 49, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Hsu, N.C.; Shih, C.L.; Huang, H.T.; Chen, C.H.; Chou, P.H. Medication-Taking Habit and Outcome of Glucosamine Sulfate for Osteoarthritis Patients Influenced by National Health Insurance Regulations in Taiwan. J. Clin. Med. 2019, 8, 1734. [Google Scholar] [CrossRef] [Green Version]
- Ghouri, A.; Conaghan, P.G. Prospects for Therapies in Osteoarthritis. Calcif. Tissue Int. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Hu, H.; Zhou, Y.; Yan, Y.; Wei, X.; Fan, X.; Yang, D.; He, H.; Oh, Y.; Chen, K.; et al. Glucosamine induces increased musclin gene expression through endoplasmic reticulum stress-induced unfolding protein response signaling pathways in mouse skeletal muscle cells. Food Chem. Toxicol. 2019, 125, 95–105. [Google Scholar] [CrossRef]
- Ciaraldi, T.P.; Carter, L.; Nikoulina, S.; Mudaliar, S.; McClain, D.A.; Henry, R.R. Glucosamine regulation of glucose metabolism in cultured human skeletal muscle cells: Divergent effects on glucose transport/phosphorylation and glycogen synthase in non-diabetic and type 2 diabetic subjects. Endocrinology 1999, 140, 3971–3980. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandris, C.; Andreozzi, F.; Federici, M.; Cardellini, M.; Brunetti, A.; Ranalli, M.; Del Guerra, S.; Lauro, D.; Del Prato, S.; Marchetti, P.; et al. Increased O-glycosylation of insulin signaling proteins results in their impaired activation and enhanced susceptibility to apoptosis in pancreatic beta-cells. FASEB J. 2004, 18, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Biggee, B.A.; Blinn, C.M.; Nuite, M.; Silbert, J.E.; McAlindon, T.E. Effects of oral glucosamine sulphate on serum glucose and insulin during an oral glucose tolerance test of subjects with osteoarthritis. Ann. Rheum. Dis. 2007, 66, 260–262. [Google Scholar] [CrossRef] [Green Version]
- Monauni, T.; Zenti, M.G.; Cretti, A.; Daniels, M.C.; Targher, G.; Caruso, B.; Caputo, M.; McClain, D.; Del Prato, S.; Giaccari, A.; et al. Effects of glucosamine infusion on insulin secretion and insulin action in humans. Diabetes 2000, 49, 926–935. [Google Scholar] [CrossRef] [Green Version]
- Muniyappa, R.; Karne, R.J.; Hall, G.; Crandon, S.K.; Bronstein, J.A.; Ver, M.R.; Hortin, G.L.; Quon, M.J. Oral glucosamine for 6 weeks at standard doses does not cause or worsen insulin resistance or endothelial dysfunction in lean or obese subjects. Diabetes 2006, 55, 3142–3150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scroggie, D.A.; Albright, A.; Harris, M.D. The effect of glucosamine-chondroitin supplementation on glycosylated hemoglobin levels in patients with type 2 diabetes mellitus: A placebo-controlled, double-blinded, randomized clinical trial. Arch. Intern. Med. 2003, 163, 1587–1590. [Google Scholar] [CrossRef] [Green Version]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le May, C.; Chu, K.; Hu, M.; Ortega, C.S.; Simpson, E.R.; Korach, K.S.; Tsai, M.J.; Mauvais-Jarvis, F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 9232–9237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riant, E.; Waget, A.; Cogo, H.; Arnal, J.F.; Burcelin, R.; Gourdy, P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 2009, 150, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Tiano, J.P.; Delghingaro-Augusto, V.; Le May, C.; Liu, S.; Kaw, M.K.; Khuder, S.S.; Latour, M.G.; Bhatt, S.A.; Korach, K.S.; Najjar, S.M.; et al. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J. Clin. Investig. 2011, 121, 3331–3342. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Le May, C.; Wong, W.P.; Ward, R.D.; Clegg, D.J.; Marcelli, M.; Korach, K.S.; Mauvais-Jarvis, F. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes 2009, 58, 2292–2302. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Patel, A.V.; Calle, E.E.; Jacob, E.J.; Thun, M.J. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. JAMA 2001, 285, 1460–1465. [Google Scholar] [CrossRef] [Green Version]
- Conley, R.B.; Adib, G.; Adler, R.A.; Akesson, K.E.; Alexander, I.M.; Amenta, K.C.; Blank, R.D.; Brox, W.T.; Carmody, E.E.; Chapman-Novakofski, K.; et al. Secondary Fracture Prevention: Consensus Clinical Recommendations from a Multistakeholder Coalition. J. Bone Miner. Res. 2020, 35, 36–52. [Google Scholar] [CrossRef] [Green Version]
- Tatangelo, G.; Watts, J.; Lim, K.; Connaughton, C.; Abimanyi-Ochom, J.; Borgstrom, F.; Nicholson, G.C.; Shore-Lorenti, C.; Stuart, A.L.; Iuliano-Burns, S.; et al. The Cost of Osteoporosis, Osteopenia, and Associated Fractures in Australia in 2017. J. Bone Miner. Res. 2019, 34, 616–625. [Google Scholar] [CrossRef]
- Shieh, A.; Greendale, G.A.; Cauley, J.A.; Karvonen-Gutierrez, C.; Crandall, C.J.; Karlamangla, A.S. Estradiol and Follicle-Stimulating Hormone as Predictors of Onset of Menopause Transition-Related Bone Loss in Pre- and Perimenopausal Women. J. Bone Miner. Res. 2019, 34, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Binkley, N.; Bilezikian, J.P. Treated Osteoporosis Is Still Osteoporosis. J. Bone Miner. Res. 2019, 34, 605–606. [Google Scholar] [CrossRef]
- Crandall, C.J.; Larson, J.; Manson, J.E.; Cauley, J.A.; LaCroix, A.Z.; Wactawski-Wende, J.; Datta, M.; Sattari, M.; Schousboe, J.T.; Leslie, W.D.; et al. A Comparison of US and Canadian Osteoporosis Screening and Treatment Strategies in Postmenopausal Women. J. Bone Miner. Res. 2019, 34, 607–615. [Google Scholar] [CrossRef]
- Chang, P.Y.; Feldman, D.; Stefanick, M.L.; McDonnell, D.P.; Thompson, B.M.; McDonald, J.G.; Lee, J.S. 27-Hydroxycholesterol, an Endogenous SERM, and Risk of Fracture in Postmenopausal Women: A Nested Case-Cohort Study in the Women’s Health Initiative. J. Bone Miner. Res. 2019, 34, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.H.; Hung, W.C.; Chang, I.L.; Tsai, T.T.; Chang, Y.F.; McCloskey, E.V.; Watts, N.B.; McClung, M.R.; Huang, C.F.; Chen, C.H.; et al. Pharmacologic intervention for prevention of fractures in osteopenic and osteoporotic postmenopausal women: Systemic review and meta-analysis. Bone Rep. 2020, 13, 100729. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, Y.F.; Chen, C.H.; Lewiecki, E.M.; Wuster, C.; Reid, I.; Tsai, K.S.; Matsumoto, T.; Mercado-Asis, L.B.; Chan, D.C.; et al. Consensus Statement on the Use of Bone Turnover Markers for Short-Term Monitoring of Osteoporosis Treatment in the Asia-Pacific Region. J. Clin. Densitom. 2021, 24, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Centofanti, F.; Santoro, M.; Marini, M.; Visconti, V.V.; Rinaldi, A.M.; Celi, M.; D’Arcangelo, G.; Novelli, G.; Orlandi, A.; Tancredi, V.; et al. Identification of Aberrantly-Expressed Long Non-Coding RNAs in Osteoblastic Cells from Osteoporotic Patients. Biomedicines 2020, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Taylor, E.A.; Donnelly, E.; Yao, X.; Johnson, M.L.; Amugongo, S.K.; Kimmel, D.B.; Lane, N.E. Sequential Treatment of Estrogen Deficient, Osteopenic Rats with Alendronate, Parathyroid Hormone (1-34), or Raloxifene Alters Cortical Bone Mineral and Matrix Composition. Calcif. Tissue Int. 2020, 106, 303–314. [Google Scholar] [CrossRef]
- Hozumi, Y.; Kawano, M.; Jordan, V.C. In vitro study of the effect of raloxifene on lipid metabolism compared with tamoxifen. Eur. J. Endocrinol. 2000, 143, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Tiano, J.; Mauvais-Jarvis, F. Selective estrogen receptor modulation in pancreatic beta-cells and the prevention of type 2 diabetes. Islets 2012, 4, 173–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernales, S.; Papa, F.R.; Walter, P. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2006, 22, 487–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, C.F.; Lai, G.Y.; Chen, C.H.; Chiu, C.C.; Hung, S.W.; Chang, C.F. 6,7-Dihydroxy-2-(4′-hydroxyphenyl)naphthalene induces HCT116 cell apoptosis through activation of endoplasmic reticulum stress and the extrinsic apoptotic pathway. Drug Des. Dev. Ther. 2019, 13, 1609–1621. [Google Scholar] [CrossRef] [Green Version]
- Gundamaraju, R.; Lu, W.; Azimi, I.; Eri, R.; Sohal, S.S. Endogenous Anti-Cancer Candidates in GPCR, ER Stress, and EMT. Biomedicines 2020, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Benameur, T.; Korashy, H.M.; Zeidan, A.; Agouni, A. Interplay between Endoplasmic Reticulum Stress and Large Extracellular Vesicles (Microparticles) in Endothelial Cell Dysfunction. Biomedicines 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Bugliani, M.; Lupi, R.; Marselli, L.; Masini, M.; Boggi, U.; Filipponi, F.; Weir, G.C.; Eizirik, D.L.; Cnop, M. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 2007, 50, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- Laybutt, D.R.; Preston, A.M.; Akerfeldt, M.C.; Kench, J.G.; Busch, A.K.; Biankin, A.V.; Biden, T.J. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 2007, 50, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Marciani, P.; Marku, A.; Ghislanzoni, S.; Bertuzzi, F.; Rossi, R.; Di Giancamillo, A.; Castagna, M.; Perego, C. Verbascoside Protects Pancreatic beta-Cells against ER-Stress. Biomedicines 2020, 8, 582. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.S.; Baek, W.K. Glucosamine induces autophagic cell death through the stimulation of ER stress in human glioma cancer cells. Biochem. Biophys. Res. Commun. 2010, 399, 111–116. [Google Scholar] [CrossRef]
- Morin, M.J.; Porter, C.W.; McKernan, P.; Bernacki, R.J. The biochemical and ultrastructural effects of tunicamycin and D-glucosamine in L1210 leukemic cells. J. Cell Physiol. 1983, 114, 162–172. [Google Scholar] [CrossRef]
- Kang, L.; Chen, C.H.; Cheng, Y.C.; Chang, C.H.; Lee, C.T.; Chang, J.K.; Cheng, J.T.; Chang, F.M. Glucosamine-induced insulin resistance in ovariectomized rats is relevant to decreasing the expression of glucose transport protein subtype 4 in the skeletal muscle and in increasing the size of pancreatic islets. Menopause 2012, 19, 496–502. [Google Scholar] [CrossRef]
- Kang, L.; Chen, C.H.; Wu, M.H.; Chang, J.K.; Chang, F.M.; Cheng, J.T. 17beta-estradiol protects against glucosamine-induced pancreatic beta-cell dysfunction. Menopause 2014, 21, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.L.; Chen, Y.H.; Liao, H.J.; Chen, C.H.; Hung, S.H.; Lee, M.J.; Fu, Y.C.; Wang, Y.H.; Wang, G.J.; Chang, J.K. Simvastatin increases osteoblasts and osteogenic proteins in ovariectomized rats. Eur. J. Clin. Investig. 2009, 39, 296–303. [Google Scholar] [CrossRef]
- Juan, Y.S.; Chuang, S.M.; Long, C.Y.; Chen, C.H.; Levin, R.M.; Liu, K.M.; Huang, C.H. Neuroprotection of green tea catechins on surgical menopause-induced overactive bladder in a rat model. Menopause 2012, 19, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kang, L.; Lin, R.W.; Fu, Y.C.; Lin, Y.S.; Chang, J.K.; Chen, H.T.; Chen, C.H.; Lin, S.Y.; Wang, G.J.; et al. (-)-Epigallocatechin-3-gallate improves bone microarchitecture in ovariectomized rats. Menopause 2013, 20, 687–694. [Google Scholar] [CrossRef]
- Chen, C.H.; Huang, T.H.; Cheng, T.L.; Chang, C.F.; Wang, C.Z.; Wu, M.H.; Kang, L. Exercise training ameliorates glucosamine-induced insulin resistance in ovariectomized rats. Menopause 2017, 24, 617–623. [Google Scholar] [CrossRef]
- Chen, C.H.; Ho, M.L.; Chang, L.H.; Kang, L.; Lin, Y.S.; Lin, S.Y.; Wu, S.C.; Chang, J.K. Parathyroid hormone-(1-34) ameliorated knee osteoarthritis in rats via autophagy. J. Appl. Physiol. 2018, 124, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.C.; Chen, C.H.; Chou, L.Y.; Cheng, T.L.; Kang, L.; Chuang, S.C.; Lin, Y.S.; Ho, M.L.; Wang, Y.H.; Lin, S.Y.; et al. Discoidin Domain Receptors 1 Inhibition Alleviates Osteoarthritis via Enhancing Autophagy. Int. J. Mol. Sci. 2020, 21, 6991. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kang, L.; Chang, L.H.; Cheng, T.L.; Lin, S.Y.; Wu, S.C.; Lin, Y.S.; Chuang, S.C.; Lee, T.C.; Chang, J.K.; et al. Intra-articular low-dose parathyroid hormone (1-34) improves mobility and articular cartilage quality in a preclinical age-related knee osteoarthritis model. Bone Jt. Res. 2021, 10, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Lee, Y.L.; Chen, J.C.; Chen, C.H.; Ho, P.S. Impact of type 2 diabetes on postoperative outcome after hip fracture: Nationwide population-based study in Taiwan. BMJ Open Diabetes Res. Care 2020, 8, e000843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Huang, P.J.; Huang, H.T.; Lin, S.Y.; Wang, H.Y.; Fang, T.J.; Lin, Y.C.; Ho, C.J.; Lee, T.C.; Lu, Y.M.; et al. Impact of orthogeriatric care, comorbidity, and complication on 1-year mortality in surgical hip fracture patients: An observational study. Medicine 2019, 98, e17912. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Chen, C.M.; Wang, C.Y.; Ko, P.W.; Chen, C.H.; Hsieh, C.P.; Chiu, H.C. Frailty is Associated with an Increased Risk of Major Adverse Outcomes in Elderly Patients Following Surgical Treatment of Hip Fracture. Sci. Rep. 2019, 9, 19135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Elsalmawy, A.H.; Ish-Shalom, S.; Lim, S.J.; Al-Ali, N.S.; Cunha-Borges, J.L.; Yang, H.; Casas, N.; Altan, L.; Moll, T.; et al. Study description and baseline characteristics of the population enrolled in a multinational, observational study of teriparatide in postmenopausal women with osteoporosis: The Asia and Latin America Fracture Observational Study (ALAFOS). Curr. Med. Res. Opin. 2019, 35, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Borhan, S.; Papaioannou, A.; Gajic-Veljanoski, O.; Kennedy, C.; Ioannidis, G.; Berger, C.; Goltzman, D.; Josse, R.; Kovacs, C.S.; Hanley, D.A.; et al. Incident Fragility Fractures Have a Long-Term Negative Impact on Health-Related Quality of Life of Older People: The Canadian Multicentre Osteoporosis Study. J. Bone Miner. Res. 2019, 34, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Bliuc, D.; Tran, T.; van Geel, T.; Adachi, J.D.; Berger, C.; van den Bergh, J.; Eisman, J.A.; Geusens, P.; Goltzman, D.; Hanley, D.A.; et al. Reduced Bone Loss Is Associated With Reduced Mortality Risk in Subjects Exposed to Nitrogen Bisphosphonates: A Mediation Analysis. J. Bone Miner. Res. 2019, 34, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Papapoulos, S.E.; Polyzos, S.A.; Appelman-Dijkstra, N.M.; Makras, P. Zoledronate for the Prevention of Bone Loss in Women Discontinuing Denosumab Treatment. A Prospective 2-Year Clinical Trial. J. Bone Miner. Res. 2019, 34, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Osagie-Clouard, L.; Sanghani-Kerai, A.; Coathup, M.; Meeson, R.; Briggs, T.; Blunn, G. The influence of parathyroid hormone 1-34 on the osteogenic characteristics of adipose- and bone-marrow-derived mesenchymal stem cells from juvenile and ovarectomized rats. Bone Jt. Res. 2019, 8, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Huang, H.T.; Cheng, T.L.; Lu, Y.M.; Lin, S.Y.; Ho, C.J.; Lee, T.C.; Hsu, C.H.; Huang, P.J.; Huang, H.H.; et al. Application of microrna in human osteoporosis and fragility fracture: A systemic review of literatures. Int. J. Mol. Sci. 2021, 22, 5232. [Google Scholar] [CrossRef] [PubMed]
- Saad, R.K.; Harb, H.; Bou-Orm, I.R.; Ammar, W.; El-Hajj Fuleihan, G. Secular Trends of Hip Fractures in Lebanon, 2006 to 2017: Implications for Clinical Practice and Public Health Policy in the Middle East Region. J. Bone Miner. Res. 2020, 35, 71–80. [Google Scholar] [CrossRef]
- Li, S.; Mao, Y.; Zhou, F.; Yang, H.; Shi, Q.; Meng, B. Gut microbiome and osteoporosis: A review. Bone Jt. Res. 2020, 9, 524–530. [Google Scholar] [CrossRef]
- Huang, H.T.; Cheng, T.L.; Lin, S.Y.; Ho, C.J.; Chyu, J.Y.; Yang, R.S.; Chen, C.H.; Shen, C.L. Osteoprotective Roles of Green Tea Catechins. Antioxidants 2020, 9, 1136. [Google Scholar] [CrossRef]
- Chou, Y.S.; Jiang, H.J.; Chen, C.H.; Ho, P.S.; Lee, T.C. Proton pump inhibitor use and risk of hip fracture in patients with type 2 diabetes. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dahl, C.; Holvik, K.; Meyer, H.E.; Stigum, H.; Solbakken, S.M.; Schei, B.; Sogaard, A.J.; Omsland, T.K. Increased Mortality in Hip Fracture Patients Living Alone: A NOREPOS Study. J. Bone Miner. Res. 2021, 36, 480–488. [Google Scholar] [CrossRef]
- Chen, C.H.; Lim, S.J.; Oh, J.K.; Huang, T.W.; Zeng, Y.H.; Wu, M.T.; Yang, H.L.; Cheung, J.P.; Kim, J.W.; Han, J.H.; et al. Teriparatide in East Asian Postmenopausal Women with Osteoporosis in a Real-World Setting: A Baseline Analysis of the Asia and Latin America Fracture Observational Study (ALAFOS). Clin. Interv. Aging 2020, 15, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.Y.; Huang, H.T.; Chou, S.H.; Ho, C.J.; Liu, Z.M.; Chen, C.H.; Lu, C.C. The Safety of Continuing Antiplatelet Medication Among Elderly Patients Undergoing Urgent Hip Fracture Surgery. Orthopedics 2019, 42, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.D.; Chang, L.Y.; Akesson, K.E.; Mitchell, P.; Chen, C.H.; Lewiecki, E.M.; Lee, J.K.; Lau, T.C.; Songpatanasilp, T.; Lee, K.B.; et al. Consensus on best practice standards for Fracture Liaison Service in the Asia-Pacific region. Arch. Osteoporos. 2018, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Tsai, K.S.; Peng, J.K.; Chen, C.H.; Lin, G.T.; Lin, C.H.; Tu, S.T.; Mao, I.C.; Gau, Y.L.; Liu, H.C.; et al. The development of Taiwan Fracture Liaison Service network. Osteoporos. Sarcopenia 2018, 4, 47–52. [Google Scholar] [CrossRef]
- Chen, C.H.; Elsalmawy, A.H.; Ish-Shalom, S.; Lim, S.J.; AlAli, N.S.; Cunha-Borges, J.L.; Yang, H.; Casas, N.; Altan, L.; Belaya, Z.; et al. The Effect of Teriparatide Treatment on the Risk of Fragility Fractures in Postmenopausal Women with Osteoporosis: Results from the Asian and Latin America Fracture Observational Study (ALAFOS). Calcif. Tissue Int. 2021, in press. [Google Scholar] [CrossRef]
- Lisk, R.; Yeong, K.; Fluck, D.; Fry, C.H.; Han, T.S. The Ability of the Nottingham Hip Fracture Score to Predict Mobility, Length of Stay and Mortality in Hospital, and Discharge Destination in Patients Admitted with a Hip Fracture. Calcif. Tissue Int. 2020, 107, 319–326. [Google Scholar] [CrossRef]
- Mortensen, S.J.; Mohamadi, A.; Wright, C.L.; Chan, J.J.; Weaver, M.J.; von Keudell, A.; Nazarian, A. Medications as a Risk Factor for Fragility Hip Fractures: A Systematic Review and Meta-analysis. Calcif. Tissue Int. 2020, 107, 1–9. [Google Scholar] [CrossRef]
- Tei, R.M.H.; Ramlau-Hansen, C.H.; Plana-Ripoll, O.; Brink, O.; Langdahl, B.L. OFELIA: Prevalence of Osteoporosis in Fragility Fracture Patients. Calcif. Tissue Int. 2019, 104, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.; Langdahl, B.L.; Fujiwara, S.; Saag, K.; Napoli, N.; Soen, S.; Enomoto, H.; Melby, T.E.; Disch, D.P.; Marin, F.; et al. Reduction of Hip and Other Fractures in Patients Receiving Teriparatide in Real-World Clinical Practice: Integrated Analysis of Four Prospective Observational Studies. Calcif. Tissue Int. 2019, 104, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef] [Green Version]
- Macias, I.; Alcorta-Sevillano, N.; Rodriguez, C.I.; Infante, A. Osteoporosis and the Potential of Cell-Based Therapeutic Strategies. Int. J. Mol. Sci. 2020, 21, 1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukon, Y.; Makino, T.; Kodama, J.; Tsukazaki, H.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. Molecular-Based Treatment Strategies for Osteoporosis: A Literature Review. Int. J. Mol. Sci. 2019, 20, 2557. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.Y.; Chen, L.R.; Chen, K.H. Osteoporosis in Patients with Chronic Kidney Diseases: A Systemic Review. Int. J. Mol. Sci. 2020, 21, 6864. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Rajawat, J. Skeletal Aging and Osteoporosis: Mechanisms and Therapeutics. Int. J. Mol. Sci. 2021, 22, 3553. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Zhang, Y.; Huang, L.; Shi, Q. Mesenchymal stem cells—A promising strategy for treating knee osteoarthritis. Bone Jt. Res. 2020, 9, 719–728. [Google Scholar] [CrossRef]
- Luk, H.Y.; Appell, C.; Chyu, M.C.; Chen, C.H.; Wang, C.Y.; Yang, R.S.; Shen, C.L. Impacts of Green Tea on Joint and Skeletal Muscle Health: Prospects of Translational Nutrition. Antioxidants 2020, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Hain, B.A.; Jude, B.; Xu, H.; Smuin, D.M.; Fox, E.J.; Elfar, J.C.; Waning, D.L. Zoledronic Acid Improves Muscle Function in Healthy Mice Treated with Chemotherapy. J. Bone Miner. Res. 2020, 35, 368–381. [Google Scholar] [CrossRef] [Green Version]
- Chakhtoura, M.; Dagher, H.; Sharara, S.; Ajjour, S.; Chamoun, N.; Cauley, J.; Mahfoud, Z.; Boudreau, R.; El Hajj Fuleihan, G. Systematic review of major osteoporotic fracture to hip fracture incidence rate ratios worldwide: Implications for Fracture Risk Assessment Tool (FRAX)-derived estimates. J. Bone Miner. Res. 2021. [Google Scholar] [CrossRef]
- Pickering, M.E.; Chapurlat, R. Where Two Common Conditions of Aging Meet: Osteoarthritis and Sarcopenia. Calcif. Tissue Int. 2020, 107, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Liu, X.; He, Z.; Han, X.; Yan, M.; Qu, X.; Li, X.; Yu, Z. Articular Cartilage Degradation and Aberrant Subchondral Bone Remodeling in Patients with Osteoarthritis and Osteoporosis. J. Bone Miner. Res. 2020, 35, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.T.; Gou, Y.; Fang, J.K.; Hu, Y.P.; Lian, Q.Q.; Zhang, Y.Y.; Wang, Y.D.; Tian, F.M.; Zhang, L. Parathyroid hormone (1-34) ameliorates cartilage degeneration and subchondral bone deterioration in collagenase-induced osteoarthritis model in mice. Bone Jt. Res. 2020, 9, 675–688. [Google Scholar] [CrossRef]
- Lingard, E.A.; Mitchell, S.Y.; Francis, R.M.; Rawlings, D.; Peaston, R.; Birrell, F.N.; McCaskie, A.W. The prevalence of osteoporosis in patients with severe hip and knee osteoarthritis awaiting joint arthroplasty. Age Ageing 2010, 39, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.P.; Wu, P.K.; Lee, C.H.; Shih, C.M.; Chiu, Y.C.; Hsu, C.E. Association of osteoporosis and varus inclination of the tibial plateau in postmenopausal women with advanced osteoarthritis of the knee. BMC Musculoskelet. Disord. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Kasher, M.; Williams, F.M.K.; Freidin, M.B.; Cherny, S.; Livshits, G. An in-depth study of the associations between osteoarthritis- and osteoporosis-related phenotypes at different skeletal locations. Osteoporos. Int. 2020, 31, 2197–2208. [Google Scholar] [CrossRef]
- Bei, M.J.; Tian, F.M.; Xiao, Y.P.; Cao, X.H.; Liu, N.; Zheng, Z.Y.; Dai, M.W.; Wang, W.Y.; Song, H.P.; Zhang, L. Raloxifene retards cartilage degradation and improves subchondral bone micro-architecture in ovariectomized rats with patella baja-induced-patellofemoral joint osteoarthritis. Osteoarthr. Cartil. 2020, 28, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.N.; Khaksari, M.; Sepehri, G.; Karam, G.A.; Raji-Amirhasani, A.; Azizian, H. The effects of alone and combination tamoxifen, raloxifene and estrogen on lipid profile and atherogenic index of ovariectomized type 2 diabetic rats. Life Sci. 2020, 263, 118573. [Google Scholar] [CrossRef]
- Nagamani, M.; Szymajda, A.; Sepilian, V.; Urban, R.J.; Gilkison, C. Effects of raloxifene on insulin sensitivity, beta-cell function, and hepatic insulin extraction in normal postmenopausal women. Fertil. Steril. 2008, 89, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Grover-Paez, F.; Zavalza-Gomez, A.B.; Anaya-Prado, R. Raloxifene modifies the insulin sensitivity and lipid profile of postmenopausal insulin resistant women. Gynecol. Endocrinol. 2013, 29, 674–677. [Google Scholar] [CrossRef]
- Qiu, S.; Vazquez, J.T.; Boulger, E.; Liu, H.; Xue, P.; Hussain, M.A.; Wolfe, A. Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hevener, A.L.; Ribas, V.; Moore, T.M.; Zhou, Z. The Impact of Skeletal Muscle ERα on Mitochondrial Function and Metabolic Health. Endocrinology 2020, 161, bqz017. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; Drew, B.G.; Zhou, Z.; Phun, J.; Kalajian, N.Y.; Soleymani, T.; Daraei, P.; Widjaja, K.; Wanagat, J.; de Aguiar Vallim, T.Q.; et al. Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci. Transl. Med. 2016, 8, 334ra54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryzgalova, G.; Gao, H.; Ahren, B.; Zierath, J.R.; Galuska, D.; Steiler, T.L.; Dahlman-Wright, K.; Nilsson, S.; Gustafsson, J.A.; Efendic, S.; et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: Insulin sensitivity in the liver. Diabetologia 2006, 49, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieli-Conwright, C.M.; Spektor, T.M.; Rice, J.C.; Todd Schroeder, E. Oestradiol and SERM treatments influence oestrogen receptor coregulator gene expression in human skeletal muscle cells. Acta Physiol. 2009, 197, 187–196. [Google Scholar] [CrossRef]
- Zhou, Z.; Ribas, V.; Rajbhandari, P.; Drew, B.G.; Moore, T.M.; Fluitt, A.H.; Reddish, B.R.; Whitney, K.A.; Georgia, S.; Vergnes, L.; et al. Estrogen receptor alpha protects pancreatic beta-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J. Biol. Chem. 2018, 293, 4735–4751. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Cheng, T.-L.; Chang, C.-F.; Huang, H.-T.; Lin, S.-Y.; Wu, M.-H.; Kang, L. Raloxifene Ameliorates Glucosamine-Induced Insulin Resistance in Ovariectomized Rats. Biomedicines 2021, 9, 1114. https://doi.org/10.3390/biomedicines9091114
Chen C-H, Cheng T-L, Chang C-F, Huang H-T, Lin S-Y, Wu M-H, Kang L. Raloxifene Ameliorates Glucosamine-Induced Insulin Resistance in Ovariectomized Rats. Biomedicines. 2021; 9(9):1114. https://doi.org/10.3390/biomedicines9091114
Chicago/Turabian StyleChen, Chung-Hwan, Tsung-Lin Cheng, Chi-Fen Chang, Hsuan-Ti Huang, Sung-Yen Lin, Meng-Hsing Wu, and Lin Kang. 2021. "Raloxifene Ameliorates Glucosamine-Induced Insulin Resistance in Ovariectomized Rats" Biomedicines 9, no. 9: 1114. https://doi.org/10.3390/biomedicines9091114






