Microbiome First Medicine in Health and Safety
Abstract
:1. Introduction
2. The Microimmunosome: A Systems Biology Therapeutic Target
3. The Immune System and Superorganism Integrity
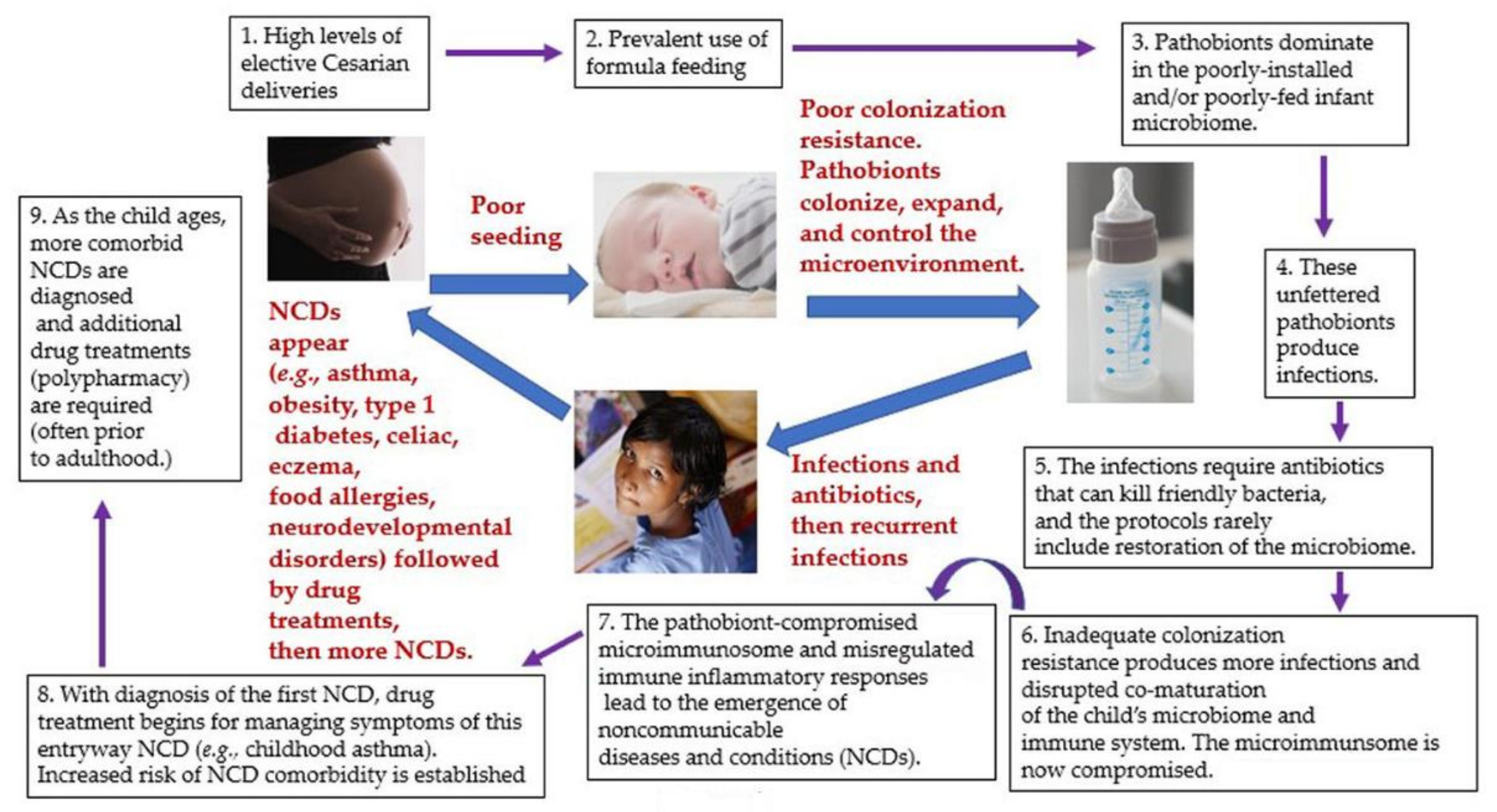
4. Introducing Microbiome First Medicine
5. NCDs: Human Illness and Death
6. The Immune System as a Manager of Superorganism Integrity
7. NCDs as Mainly Immune-Inflicted Diseases
8. Two High-Impact Examples of NCDs
8.1. Asthma
8.2. Obesity
9. Marching to Multimorbidity and Polypharmacy through a Web of NCDs
10. Drug Safety for the Human Superorganism
11. Microbe Management: Keystone Species and Cooperative Metabolic Communities
12. Blocking Pathobionts to Protect against NCDs
13. Critical Windows for Programming Health vs. Disease
14. Probiotics, Prebiotics and Targeted Rebiosis: Clinical and Preclinical Examples
15. Revisiting the Microimmunosome: Bidirectional Approaches for Homeostasis
16. Challenges for Microbiome First Medicine
17. Conclusions
- Knowledge of a patient’s prior and current microbiome status
- Probiotic installation in the patient to:
- A.
- Facilitate key developmental events during infant development (e.g., microbiota needed to digest human milk and reduce NCD-promoting inflammation)
- B.
- Provide enhanced colonization resistance and reduced risk of infections
- C.
- Correct a potential physiological imbalance (e.g., hormonal, immunological, neurological, G.I., hepatic, renal, or reproductive related)
- D.
- Reduce the risk of a comorbid NCDs
- E.
- Aid the effectiveness of a medication
- F.
- Reduce side effects of a medication
- G.
- Improve multi-system functionality in circumstances of neurological disorders (e.g., brain-gut)
- H.
- Reduce the risk of cytokine storm in the event of certain infections
- Dietary and/or prebiotic alterations to support the microbiome overall and any adjustments made to the microbiota
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, S.; Izard, J.; Walsh, E.; Batich, K.; Chongsathidkiet, P.; Clarke, G.; Sela, D.A.; Muller, A.J.; Mullin, J.M.; Albert, K.; et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Res. 2017, 77, 1783–1812. [Google Scholar] [CrossRef] [Green Version]
- Mohajeri, M.H.; La Fata, G.; Steinert, R.E.; Weber, P. Relationship between the gut microbiome and brain function. Nutr. Rev. 2018, 76, 481–496. [Google Scholar] [CrossRef]
- Fox, M.; Lee, S.M.; Wiley, K.S.; Lagishetty, V.; Sandman, C.A.; Jacobs, J.P.; Glynn, L.M. Development of the infant gut microbiome predicts temperament across the first year of life. Dev. Psychopathol. 2021, 1–12. [Google Scholar] [CrossRef]
- Planet of the microorganisms. Nat. Rev. Microbiol. 2018, 16, 257. [CrossRef] [PubMed]
- Spencer, S.P.; Fragiadakis, G.K.; Sonnenburg, J.L. Pursuing Human-Relevant Gut Microbiota-Immune Interactions. Immunity 2019, 51, 225–239. [Google Scholar] [CrossRef]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Vighi, G.; Marcucci, F.; Sensi, L.; Di Cara, G.; Frati, F. Allergy and the gastrointestinal system. Clin. Exp. Immunol. 2008, 153 (Suppl. S1), 3–6. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R. The microbiome-immune-host defense barrier complex (microimmunosome) and developmental programming of noncommunicable diseases. Reprod. Toxicol. 2017, 68, 49–58. [Google Scholar] [CrossRef]
- Schwierzeck, V.; Hülpüsch, C.; Reiger, M. Microbiome of Barrier Organs in Allergy: Who Runs the World? Germs! Handb. Exp. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Dixit, K.; Chaudhari, D.; Dhotre, D.; Shouche, Y.; Saroj, S. Restoration of dysbiotic human gut microbiome for homeostasis. Life Sci. 2021, 278, 119622. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Dietert, R.R.; Dietert, J.M. Twentieth Century Dogmas Prevent Sustainable Healthcare. Am. J. Biomed. Res. Sci. 2021, 13, 409–417. Available online: https://biomedgrid.com/pdf/AJBSR.MS.ID.001890.pdf (accessed on 24 July 2021).
- Dietert, R.R. A Focus on Microbiome Completeness and Optimized Colonization Resistance in Neonatology. NeoReviews 2018, 19, e78–e88. [Google Scholar] [CrossRef]
- Yang, X. More than 9,000,000 Unique Genes in Human Gut Bacterial Community: Estimating Gene Numbers Inside a Human Body. PLoS ONE 2009, 4, e6074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Noncommunicable Diseases. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 15 June 2021).
- Szmyd, B.; Rogut, M.; Białasiewicz, P.; Gabryelska, A. The impact of glucocorticoids and statins on sleep quality. Sleep Med. Rev. 2021, 55, 101380. [Google Scholar] [CrossRef]
- Sullivan, A.B.; Miller, D. Who is Taking Care of the Caregiver? J. Patient Exp. 2015, 2, 7–12. [Google Scholar] [CrossRef]
- Montesó-Curto, P.; Cubí-Guillen, M.T.; Llàdser Navarro, A.N.; Puig Llobet, M.; Toussaint, L. Family perceptions and experiences of living with patients with fibromyalgia syndrome. Disabil. Rehabil. 2021, 7, 1–8. [Google Scholar] [CrossRef]
- Dembic, Z. Immune system protects integrity of tissues. Mol. Immunol. 2000, 37, 563–569. [Google Scholar] [CrossRef]
- Dietert, R.R.; Dietert, J.M. The Microbiome and Sustainable Healthcare. Healthcare 2015, 3, 100–129. [Google Scholar] [CrossRef] [Green Version]
- Ogra, P.L. Respiratory syncytial virus: The virus, the disease and the immune response. Paediatr. Respir. Rev. 2004, 5, S119–S126. [Google Scholar] [CrossRef]
- Egan, M.; Bunyavanich, S. Allergic rhinitis: The “Ghost Diagnosis” in patients with asthma. Asthma Res. Pract. 2015, 1, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, L.; Keil, T.; Kostev, K. Comorbid disorders associated with asthma in children in Germany—National analysis of pediatric primary care data. Pediatr. Allergy Immunol. 2016, 27, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.C.; Hsu, J.; Gower, W.A. Comorbidities of asthma in U.S. children. Respir. Med. 2016, 116, 34–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foong, R.X.; du Toit, G.; Fox, A.T. Asthma, Food Allergy, and How They Relate to Each Other. Front. Pediatr. 2017, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Weatherburn, C.J.; Guthrie, B.; Mercer, S.W.; Morales, D.R. Comorbidities in adults with asthma: Population-based cross-sectional analysis of 1.4 million adults in Scotland. Clin. Exp. Allergy 2017, 47, 1246–1252. [Google Scholar] [CrossRef]
- Ussavarungsi, K.; Limsuwat, C.; Berdine, G.; Nugent, K. Is the sinonasal questionnaire a useful screening instrument for chronic sinonasal diseases in pulmonary clinics? Chronic Respir. Dis. 2013, 10, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Tiotiu, A.; Plavec, D.; Novakova, S.; Mihaicuta, S.; Novakova, P.; Labor, M.; Bikov, A. Current opinions for the management of asthma associated with ear, nose and throat comorbidities. Eur. Respir. Rev. 2018, 27, 180056. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Berhane, K.; Urman, R.; Lida Chatzi, V.; Breton, C.; Gilliland, F.D. The Dynamic Relationship between Asthma and Obesity in Schoolchildren. Am. J. Epidemiol. 2020, 189, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Salsman, M.L.; Nordberg, H.O.; Wittchen, H.U.; Klotsche, J.; Mühlig, S.; Riedel, O.; Ritz, T. Extrapulmonary symptoms of patients with asthma treated in specialist pulmonary care. J. Psychosom. Res. 2021, 148, 110538. [Google Scholar] [CrossRef]
- Chai, P.H.; Chang, S.; Cawthorpe, D. The Temporal Hyper-Morbidity of Asthma and Attention Deficit Disorder: Implications for Interpretation Based on Comparison of Prospective and Cross-Sectional Population Samples. Psychiatry Investig. 2021, 18, 166–171. [Google Scholar] [CrossRef]
- Havemann, B.D.; Henderson, C.A.; El-Serag, H.B. The association between gastro-oesophageal reflux disease and asthma: A systematic review. Gut 2007, 56, 1654–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, H.K.; Song, P.; Lee, J.H. Novel association between asthma and osteoarthritis: A nationwide health and nutrition examination survey. BMC Pulm. Med. 2021, 21, 59. [Google Scholar] [CrossRef]
- Fretzayas, A.; Moustaki, M.; Loukou, I.; Douros, K. Differentiating vocal cord dysfunction from asthma. Asthma Allergy 2017, 10, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, J.; Patterson, C.C.; Menzies-Gow, A.; Niven, R.M.; Mansur, A.H.; Bucknall, C.; Chaudhuri, R.; Price, D.; Brightling, C.E.; Heaney, L.G.; et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: Cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016, 71, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, R.; Lipworth, B. Impact of nasal polyps on endotype and phenotype in patients with moderate to severe asthma. Ann. Allergy Asthma Immunol. 2021, 18, S1081–S1206. [Google Scholar] [CrossRef]
- Landré, B.; Nadif, R.; Goldberg, M.; Gourmelen, J.; Zins, M.; Ankri, J.; Herr, M. Asthma is associated with frailty among community-dwelling adults: The GAZEL cohort. BMJ Open Respir. Res. 2020, 7, e000526. [Google Scholar] [CrossRef] [PubMed]
- Zolotareva, O.; Saik, O.V.; Königs, C.; Bragina, E.Y.; Goncharova, I.A.; Freidin, M.B.; Dosenko, V.E.; Ivanisenko, V.A.; Hofestädt, R. Comorbidity of asthma and hypertension may be mediated by shared genetic dysregulation and drug side effects. Sci. Rep. 2019, 9, 16302. [Google Scholar] [CrossRef] [Green Version]
- Qaisar, R.; Qayum, M.; Muhammad, T. Reduced sarcoplasmic reticulum Ca2+ ATPase activity underlies skeletal muscle wasting in asthma. Life Sci. 2021, 273, 119296. [Google Scholar] [CrossRef]
- Lyons, J.J.; Yi, T. Mast cell tryptases in allergic inflammation and immediate hypersensitivity. Curr. Opin. Immunol. 2021, 72, 94–106. [Google Scholar] [CrossRef]
- Keselman, A.; Heller, N. Estrogen Signaling Modulates Allergic Inflammation and Contributes to Sex Differences in Asthma. Front. Immunol. 2015, 6, 568. [Google Scholar] [CrossRef] [Green Version]
- Tesse, R.; Schieck, M.; Kabesch, M. Asthma and endocrine disorders: Shared mechanisms and genetic pleiotropy. Mol. Cell. Endocrinol. 2011, 333, 103–111. [Google Scholar] [CrossRef]
- Mueller, N.T.; Koh, W.P.; Odegaard, A.O.; Gross, M.D.; Yuan, J.M.; Pereira, M.A. Asthma and the risk of type 2 diabetes in the Singapore Chinese Health Study. Diabetes Res. Clin. Pract. 2013, 99, 192–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, R. Asthma and obstructive sleep apnea: More than an association! Lung India 2018, 35, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.; Jackson, D.J.; Kent, B.D. Sleep and asthma. Curr. Opin. Pulm. Med. 2018, 24, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Luyster, F.S.; Ritterband, L.M.; Sereika, S.M.; Buysse, D.J.; Wenzel, S.E.; Strollo, P.J. Internet-Based Cognitive-Behavioral Therapy for Insomnia in Adults With Asthma: A Pilot Study. Behav. Sleep Med. 2020, 18, 10–22. [Google Scholar] [CrossRef]
- Wee, J.H.; Park, M.W.; Min, C.; Byun, S.H.; Park, B.; Choi, H.G. Association between asthma and cardiovascular disease. Eur. J. Clin. Investig. 2021, 51, e13396. [Google Scholar] [CrossRef]
- Page, L.K.; Staples, K.J.; Spalluto, C.M.; Watson, A.; Wilkinson, T.M.A. Influence of Hypoxia on the Epithelial-Pathogen Interactions in the Lung: Implications for Respiratory Disease. Front. Immunol. 2021, 12, 653969. [Google Scholar] [CrossRef]
- Corlateanu, A.; Stratan, I.; Covantev, S.; Botnaru, V.; Corlateanu, O.; Siafakas, N. Asthma and stroke: A narrative review. Asthma Res. Pract. 2021, 7, 3. [Google Scholar] [CrossRef]
- Raita, Y.; Camargo, C.A., Jr.; Faridi, M.K.; Brown, D.F.M.; Shimada, Y.J.; Hasegawa, K. Risk of Acute Myocardial Infarction and Ischemic Stroke in Patients with Asthma Exacerbation: A Population-Based, Self-Controlled Case Series Study. J. Allergy Clin. Immunol. Pract. 2020, 8, 188–194.e8. [Google Scholar] [CrossRef]
- Johnson, E.; McAlees, J.; Lewkowich, I.; Sah, R. Asthma and Posttraumatic Stress Disorder (PTSD): Emerging links, potential models and mechanisms. Brain Behav. Immun. 2021, 6, S0889–S1591. [Google Scholar] [CrossRef]
- Yingchoncharoen, P.; Charoenngam, N.; Ponvilawan, B.; Thongpiya, J.; Chaikijurajai, T.; Ungprasert, P. The Association between Asthma and Risk of Myasthenia Gravis: A Systematic Review and Meta-analysis. Lung 2021, 199, 273–280. [Google Scholar] [CrossRef]
- Charokopos, A.; Braman, S.S.; Brown, S.A.W.; Mhango, G.; de-Torres, J.P.; Zulueta, J.J.; Sharma, S.; Holguin, F.; Sigel, K.M.; Powell, C.A.; et al. Lung Cancer Risk Among Patients with Asthma-Chronic Obstructive Pulmonary Disease Overlap. Ann. Am. Thorac. Soc. 2021. [Google Scholar] [CrossRef]
- Pashley, C.H.; Wardlaw, A.J. Allergic fungal airways disease (AFAD): An under-recognised asthma endotype. Mycopathologia 2021. [Google Scholar] [CrossRef]
- Sgrazzutti, L.; Sansone, F.; Attanasi, M.; Di Pillo, S.; Chiarelli, F. Coaggregation of Asthma and Type 1 Diabetes in Children: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 5757. [Google Scholar] [CrossRef]
- Bekaert, S.; Rocks, N.; Vanwinge, C.; Noel, A.; Cataldo, D. Asthma-related inflammation promotes lung metastasis of breast cancer cells through CCL11-CCR3 pathway. Respir. Res. 2021, 22, 61. [Google Scholar] [CrossRef]
- Berti, A.; Cornec, D.; Casal Moura, M.; Smyth, R.J.; Dagna, L.; Specks, U.; Keogh, K.A. Eosinophilic Granulomatosis with Polyangiitis: Clinical Predictors of Long-term Asthma Severity. Chest 2020, 157, 1086–1099. [Google Scholar] [CrossRef]
- Wang, L.; Gao, S.; Yu, M.; Sheng, Z.; Tan, W. Association of asthma with coronary heart disease: A meta analysis of 11 trials. PLoS ONE 2017, 12, e0179335. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Lang, A.; Teo, S.M.; Judd, L.M.; Gangnon, R.; Evans, M.D.; Lee, K.E.; Vrtis, R.; Holt, P.G.; Lemanske, R.F.; et al. Developmental patterns in the nasopharyngeal microbiome during infancy are associated with asthma risk. J. Allergy Clin. Immunol. 2021, 147, 1683–1691. [Google Scholar] [CrossRef]
- Lee, D.C.; Kananurak, A.; Tran, M.T.; Connolly, P.A.; Polage, C.R.; Iwase, T.; Bevins, C.L.; Underwood, M.A. Bacterial Colonization of the Hospitalized Newborn: Competition between Staphylococcus aureus and Staphylococcus epidermidis. Pediatr. Infect. Dis. J. 2019, 38, 682–686. [Google Scholar] [CrossRef]
- Cole, A.L.; Sundar, M.; Lopez, A.; Forsman, A.; Yooseph, S.; Cole, A.M. Identification of Nasal Gammaproteobacteria with Potent Activity against Staphylococcus aureus: Novel Insights into the “Noncarrier” State. mSphere 2021, 6, e01015–e01020. [Google Scholar] [CrossRef]
- Dietert, R. The Human Superorganism: How the Microbiome Is Revolutionizing the Pursuit of a Healthy Life, 1st ed.; Dutton Penguin Random House: New York, NY, USA, 2016; pp. 122–127. [Google Scholar]
- Ribeiro, J.C.; Duarte, J.G.; Gomes, G.A.O.; Costa-Guarisco, L.P.; de Jesus, I.T.M.; Nascimento, C.M.C.; Santos-Orlandi, A.A.; Orlandi, F.S.; Vasilceac, F.A.; Zazzetta, M.S.; et al. Associations between inflammatory markers and muscle strength in older adults according to the presence or absence of obesity. Exp. Gerontol. 2021, 151, 111409. [Google Scholar] [CrossRef]
- Abbasi, A.; Juszczyk, D.; van Jaarsveld, C.H.M.; Gulliford, M.C. Body Mass Index and Incident Type 1 and Type 2 Diabetes in Children and Young Adults: A Retrospective Cohort Study. J. Endocr. Soc. 2017, 1, 524–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.E.; Mouton, A.J.; da Silva, A.A.; Omoto, A.C.M.; Wang, Z.; Li, X.; do Carmo, J.M. Obesity, kidney dysfunction, and inflammation: Interactions in hypertension. Cardiovasc. Res. 2021, 117, 1859–1876. [Google Scholar] [CrossRef]
- Jahangir, E.; De Schutter, A.; Lavie, C.J. The relationship between obesity and coronary artery disease. Transl. Res. 2014, 164, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Rychter, A.M.; Naskręt, D.; Zawada, A.; Ratajczak, A.E.; Dobrowolska, A.; Krela-Kaźmierczak, I. Can We Change in Diet and Behaviour in Order to Decrease Carotid Intima-Media Thickness in Patients with Obesity? J. Personal. Med. 2021, 11, 505. [Google Scholar] [CrossRef]
- Moore, J.M.; Waldrop, S.W.; Cree-Green, M. Weight Management in Adolescents with Polycystic Ovary Syndrome. Curr. Obes. Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.J.; Strain, M.; Gomelsky, A.; Rothschild, J.; Dmochowski, R. Obesity and female stress urinary incontinence. Urology 2013, 82, 759–763. [Google Scholar] [CrossRef] [PubMed]
- King, L.K.; March, L.; Anandacoomarasamy, A. Obesity & osteoarthritis. Indian J. Med. Res. 2013, 138, 185–193. [Google Scholar]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med. Dis. 2017, 1, 00019. [Google Scholar]
- Zhao, B.; Sun, S.; He, X.; Yang, J.; Ma, X.; Yan, B. Sleep fragmentation and the risk of obesity: The Sleep Heart Health Study. Obesity 2021, 29, 1387–1393. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Festi, D.; Colecchia, A.; Sacco, T.; Bondi, M.; Roda, E.; Marchesini, G. Hepatic steatosis in obese patients: Clinical aspects and prognostic significance. Obes. Rev. 2004, 5, 27–42. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, L.; Bai, C.; Chen, O. Association between abdominal obesity and asthma: A meta-analysis. Allergy Asthma Clin. Immunol. 2019, 15, 16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Johansson, L.; Andersson-Assarsson, J.; Taube, M.; Peltonen, M.; Svensson, P.A.; Herder, C.; Rudin, A.; Carlsson, L.; Rantapää-Dahlqvist, S.; et al. Adiponectin Associates with Rheumatoid Arthritis Risk in Overweight and Obesity Independently of Other Adipokines. J. Clin. Med. 2021, 10, 2791. [Google Scholar] [CrossRef]
- Mendall, M.; Harpsøe, M.C.; Kumar, D.; Andersson, M.; Jess, T. Relation of body mass index to risk of developing inflammatory bowel disease amongst women in the Danish National Birth Cohort. PLoS ONE 2018, 13, e0190600. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.R.; Hidayat, K.; Chen, C.L.; Li, Y.H.; Xu, J.Y.; Qin, L.Q. Body mass index, waist circumference, and risk of hearing loss: A meta-analysis and systematic review of observational study. Environ. Health Prev. Med. 2020, 25, 25. [Google Scholar] [CrossRef]
- Blokhin, I.O.; Lentz, S.R. Mechanisms of thrombosis in obesity. Curr. Opin. Hematol. 2013, 20, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Dağ, Z.Ö.; Dilbaz, B. Impact of obesity on infertility in women. J. Turk. Ger. Gynecol. Assoc. 2015, 16, 111–117. [Google Scholar] [CrossRef]
- Crow, R.S.; Lohman, M.C.; Titus, A.J.; Cook, S.B.; Bruce, M.L.; Mackenzie, T.A.; Bartels, S.J.; Batsis, J.A. Association of Obesity and Frailty in Older Adults: NHANES 1999–2004. J. Nutr. Health Aging 2019, 23, 138–144. [Google Scholar] [CrossRef]
- Lloret, A.; Monllor, P.; Esteve, D.; Cervera-Ferri, A.; Lloret, M.A. Obesity as a Risk Factor for Alzheimer’s Disease: Implication of Leptin and Glutamate. Front. Neurosci. 2019, 13, 508. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.Y.; Lee, J.H.; Jung, S.M.; Suh, Y.S.; Koh, J.H.; Kwok, S.K.; Ju, J.H.; Park, K.S.; Park, S.H. Visceral fat obesity is highly associated with primary gout in a metabolically obese but normal weighted population: A case control study. Arthritis Res. Ther. 2015, 17, 79. [Google Scholar] [CrossRef] [Green Version]
- Song, R.H.; Wang, B.; Yao, Q.M.; Li, Q.; Jia, X.; Zhang, J.A. The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10, 2349. [Google Scholar] [CrossRef]
- Ma, Y.; Ajnakina, O.; Steptoe, A.; Cadar, D. Higher risk of dementia in English older individuals who are overweight or obese. Int. J. Epidemiol. 2020, 49, 1353–1365. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Genes, T.M. Obesity and Multiple Sclerosis—A Multifaceted Association. J. Clin. Med. 2021, 10, 2689. [Google Scholar] [CrossRef]
- McWhinney, S.; Kolenic, M.; Franke, K.; Fialova, M.; Knytl, P.; Matejka, M.; Spaniel, F.; Hajek, T. Obesity as a Risk Factor for Accelerated Brain Ageing in First-Episode Psychosis—A Longitudinal Study. Schizophr. Bull. 2021. [Google Scholar] [CrossRef]
- Ramos-Nino, M.E.; MacLean, C.D.; Littenberg, B. Association between prevalence of obstructive lung disease and obesity: Results from The Vermont Diabetes Information System. Asthma Res. Pract. 2021, 7, 6. [Google Scholar] [CrossRef]
- Opel, N.; Narr, K.L.; Abbott, C.; Argyelan, M.; Espinoza, R.; Emsell, L.; Bouckaert, F.; Sienaert, P.; Vandenbulcke, M.; Nordanskog, P.; et al. Elevated body weight modulates subcortical volume change and associated clinical response following electroconvulsive therapy. J. Psychiatry Neurosci. 2021, 46, E418–E426. [Google Scholar] [CrossRef]
- Vander Velden, J.W.; Osborne, D.M. Obesity Prevents S-Adenosylmethionine-Mediated Improvements in Age-Related Peripheral and Hippocampal Outcomes. Nutrients 2021, 13, 1201. [Google Scholar] [CrossRef]
- Norden, A.; Rekhtman, S.; Strunk, A.; Garg, A. Risk of psoriasis according to body mass index: A retrospective cohort analysis. J. Am. Acad. Dermatol. 2021, 9, S0190–S9622. [Google Scholar] [CrossRef]
- Fast, K.; Björk, A.; Strandberg, M.; Johannesson, E.; Wentz, E.; Dahlgren, J. Half of the children with overweight or obesity and attention-deficit/hyperactivity disorder reach normal weight with stimulants. Acta Paediatr. 2021. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Zoccali, C.; Ikizler, T.A. Obesity in CKD—What should nephrologists know? J. Am. Soc. Nephrol. 2013, 24, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Nussbaum, B.M.; Mathew, M.S.; Atem, F.; Barlow, S.E.; Gupta, O.T.; Messiah, S.E. Distribution of comorbidities as primary diagnoses by obesity class among patients in a large US paediatric healthcare system. Clin. Obes. 2021, e12478. [Google Scholar] [CrossRef]
- Martin-Taboada, M.; Vila-Bedmar, R.; Medina-Gómez, G. From Obesity to Chronic Kidney Disease: How Can Adipose Tissue Affect Renal Function? Nephron 2021, 21, 1–5. [Google Scholar] [CrossRef]
- Yuan, L.; Chang, M.; Wang, J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: A systematic review and meta-analysis. Age Ageing 2021, 50, 1118–1128. [Google Scholar] [CrossRef]
- Paccoud, R.; Saint-Laurent, C.; Piccolo, E.; Tajan, M.; Dortignac, A.; Pereira, O.; Le Gonidec, S.; Baba, I.; Gélineau, A.; Askia, H.; et al. SHP2 drives inflammation-triggered insulin resistance by reshaping tissue macrophage populations. Sci. Transl. Med. 2021, 13, eabe2587. [Google Scholar] [CrossRef]
- Connolly, K.D.; Rees, D.A.; James, P.E. Role of adipocyte-derived extracellular vesicles in vascular inflammation. Free Radic. Biol. Med. 2021, M172, 58–64. [Google Scholar] [CrossRef]
- Dietert, R.R.; DeWitt, J.C.; Germolec, D.R.; & Zelikoff, J.T. Breaking patterns of environmentally influenced disease for health risk reduction: Immune perspectives. Environ. Health Perspect. 2010, 118, 1091–1099. [Google Scholar] [CrossRef]
- Halling, M.L.; Kjeldsen, J.; Knudsen, T.; Nielsen, J.; Hansen, L.K. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J. Gastroenterol. 2017, 23, 6137–6146. [Google Scholar] [CrossRef] [Green Version]
- Butwicka, A.; Olén, O.; Larsson, H.; Halfvarson, J.; Almqvist, C.; Lichtenstein, P.; Serlachius, E.; Frisén, L.; Ludvigsson, J.F. Association of Childhood-Onset Inflammatory Bowel Disease with Risk of Psychiatric Disorders and Suicide Attempt. JAMA Pediatr. 2019, 173, 969–978. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, D.E.; Xiang, J.; Pilkerton, C.S. Multimorbidity Trends in United States Adults, 1988–2014. J. Am. Board Fam. Med. JABFM 2018, 31, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Aljeaidi, M.S.; Tan, E.C. The association between polypharmacy and cognitive ability in older adults: A national cohort study. Res. Soc. Adm. Pharm. 2021, 5, S1551–S7411. [Google Scholar] [CrossRef]
- Dietert, R.R.; Silbergeld, E.K. Biomarkers for the 21st century: Listening to the microbiome. Toxicol. Sci. 2015, 144, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Saha, J.R.; Butler, V.P., Jr.; Neu, H.C.; Lindenbaum, J. Digoxin-inactivating bacteria: Identification in human gut flora. Science 1983, 220, 325–327. [Google Scholar] [CrossRef]
- Tuteja, S.; Ferguson, J.F. Gut Microbiome and Response to Cardiovascular Drugs. Circ. Genomic Prec. Med. 2019, 12, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haiser, H.J.; Seim, K.L.; Balskus, E.P.; Turnbaugh, P.J. Mechanistic insight into digoxin inactivation by Eggerthella lenta augments our understanding of its pharmacokinetics. Gut Microbes 2014, 5, 233–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, M.; Aronoff, D.M. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin. Microbiol. Infect. 2016, 22, 178.e1–178.e9. [Google Scholar] [CrossRef] [Green Version]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Cifone, M.G.; Lombardi, F.; Pergolizzi, J.V.; Varrassi, G. Influence of Microbiota on NSAID Enteropathy: A Systematic Review of Current Knowledge and the Role of Probiotics. Adv. Ther. 2020, 37, 1933–1945. [Google Scholar] [CrossRef] [Green Version]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imhann, F.; Vich Vila, A.; Bonder, M.J.; Lopez Manosalva, A.G.; Koonen, D.P.Y.; Fu, J.; Wijmenga, C.; Zhernakova, A.; Weersma, R.K. The influence of proton pump inhibitors and other commonly used medication on the gut microbiota. Gut Microbes 2017, 8, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Lukić, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O. Elliott, E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 2019, 9, 133. [Google Scholar] [CrossRef]
- Sjöstedt, P.; Jesper Enander, J.; Isung, J. Serotonin Reuptake Inhibitors and the Gut Microbiome: Significance of the Gut Microbiome in Relation to Mechanism of Action, Treatment Response, Side Effects, and Tachyphylaxis. Front. Psychiatry 2021, 12, 778. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Tropini, C.; Moss, E.L.; Merrill, B.D.; Ng, K.M.; Higginbottom, S.K.; Casavant, E.P.; Gonzalez, C.G.; Fremin, B.; Bouley, D.M.; Elias, J.E.; et al. Transient Osmotic Perturbation Causes Long-Term Alteration to the Gut Microbiota. Cell 2018, 173, 1742–1754.e17. [Google Scholar] [CrossRef] [Green Version]
- Pequegnat, B.; Monteiro, M.A. Carbohydrate Scaffolds for the Study of the Autism-associated Bacterium, Clostridium bolteae. Curr. Med. Chem. 2019, 26, 6341–6348. [Google Scholar] [CrossRef]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef]
- Eitan, S.; Madison, C.A.; Kuempel, J. The self-serving benefits of being a good host: A role for our micro-inhabitants in shaping opioids’ function. Neurosci. Biobehav. Rev. 2021, 127, 284–295. [Google Scholar] [CrossRef]
- O’Sullivan, S.J.; Schwaber, J.S. Similarities in alcohol and opioid withdrawal syndromes suggest common negative reinforcement mechanisms involving the interoceptive antireward pathway. Neurosci. Biobehav. Rev. 2021, 125, 355–364. [Google Scholar] [CrossRef]
- Goubet, A.-G.; Daillère, R.; Routy, B.; Derosa, L.M.; Roberti, P.; Zitvogel, L. The impact of the intestinal microbiota in therapeutic responses against cancer. C. R. Biol. 2018, 341, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Hally, A.; Leonetti, A.; Gregori, A.; Tiseo, M.; Deng, D.M.; Giovannetti, E.; Peters, G.J. The Role of the Microbiome in Cancer and Therapy Efficacy: Focus on Lung Cancer. Anticancer Res. 2020, 40, 4807–4818. [Google Scholar] [CrossRef] [PubMed]
- Haiser, H.J.; Gootenberg, D.B.; Chatman, K.; Sirasani, G.; Balskus, E.P.; Turnbaugh, P.J. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science 2013, 341, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Maseda, D.; Zackular, J.P.; Trindade, B.; Kirk, L.; Roxas, J.L.; Rogers, L.M.; Washington, M.K.; Du, L.; Koyama, T.; Viswanathan, V.K.; et al. Nonsteroidal Anti-inflammatory Drugs Alter the Microbiota and Exacerbate Clostridium difficile Colitis while Dysregulating the Inflammatory Response. mBio 2019, 10, e02282-e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, G.; Zaccari, P.; Rocco, G.; Scalese, G.; Panetta, C.; Porowska, B.; Pontone, S.; Severi, C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J. Gastroenterol. 2019, 25, 2706–2719. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Berthelot, L.; Soulillou, J.P.; Montassier, E. Impact of non-antibiotic drugs on the human intestinal microbiome. Expert Rev. Mol. Diagn. 2021, 15, 1–14. [Google Scholar] [CrossRef]
- Baltz, R.H. Genome mining for drug discovery: Progress at the front end. J. Ind. Microbiol. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Henrick, B.M.; Chew, S.; Casaburi, G.; Brown, H.K.; Frese, S.A.; Zhou, Y.; Underwood, M.A.; Smilowitz, J.T. Colonization by B. infantis EVC001 modulates enteric inflammation in exclusively breastfed infants. Pediatr. Res. 2019, 86, 749–757. [Google Scholar] [CrossRef]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898. [Google Scholar] [CrossRef]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Finlay, B.B.; CIFAR Humans; Microbiome. Are noncommunicable diseases communicable? Science 2020, 367, 250–251. [Google Scholar] [CrossRef]
- Dietert, R.R. Lessons for Human Holobiont Medicine in the Era of SARS-Cov-2. Am. J. Biomed. Res. Sci. 2021, 13, 152–156. [Google Scholar]
- Teufelberger, A.R.; Bröker, B.M.; Krysko, D.V.; Bachert, C.; Krysko, O. Staphylococcus aureus Orchestrates Type 2 Airway Diseases. Trends Mol. Med. 2019, 25, 696–707. [Google Scholar] [CrossRef] [Green Version]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Rud, T.; Karch, A.; Pieper, D.H.; Vital, M. Pathogenic functions of host microbiota. Microbiome 2018, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Rud, T.; Pieper, D.H.; Vital, M. Potential TMA-Producing Bacteria Are Ubiquitously Found in Mammalia. Front. Microbiol. 2020, 10, 2966. [Google Scholar] [CrossRef]
- Dalla Via, A.; Gargari, G.; Taverniti, V.; Rondini, G.; Velardi, I.; Gambaro, V.; Visconti, G.L.; De Vitis, V.; Gardana, C.; Ragg, E.; et al. Urinary TMAO Levels Are Associated with the Taxonomic Composition of the Gut Microbiota and with the Choline TMA-Lyase Gene (cutC) Harbored by Enterobacteriaceae. Nutrients 2019, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Dietert, R.R.; Etzel, R.A.; Chen, D.; Halonen, M.; Holladay, S.D.; Jarabek, A.M.; Landreth, K.; Peden, D.B.; Pinkerton, K.; Smialowicz, R.J.; et al. Workshop to identify critical windows of exposure for children’s health: Immune and respiratory systems work group summary. Environ. Health Perspect. 2000, 108, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Heindel, J.J. The developmental basis of disease: Update on environmental exposures and animal models. Basic Clin. Pharmacol. Toxicol. 2019, 125, 5–13. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ Br. Med. J. 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heindel, J.J.; Vandenberg, L.N. Developmental origins of health and disease: A paradigm for understanding disease cause and prevention. Curr. Opin. Pediatr. 2015, 27, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. MMBR 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, M.; Holdbrooks, H.; Mishra, P.; Abrantes, M.A.; Eskew, S.; Garma, M.; Oca, C.G.; McGuckin, C.; Hein, C.B.; Mitchell, R.D.; et al. Impact of Probiotic, B. infantis EVC001 Feeding in Premature Infants on the Gut Microbiome, Nosocomially Acquired Antibiotic Resistance, and Enteric Inflammation. Front. Pediat. 2021, 9, 618009. [Google Scholar] [CrossRef]
- Duar, R.M.; Henrick, B.M.; Casaburi, G.; Frese, S.A. Integrating the Ecosystem Services Framework to Define Dysbiosis of the Breastfed Infant Gut: The Role of B. infantis and Human Milk Oligosaccharides. Front. Nutr. 2020, 7, 33. [Google Scholar] [CrossRef]
- Liao, W.; Chen, C.; Wen, T.; Zhao, Q. Probiotics for the Prevention of Antibiotic-associated Diarrhea in Adults: A Meta-Analysis of Randomized Placebo-Controlled Trials. J. Clin. Gastroenterol. 2021, 55, 469–480. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Mańkowska-Wierzbicka, D.; Mardas, M.; Stelmach-Mardas, M. Role of Probiotics in Modulating Human Gut Microbiota Populations and Activities in Patients with Colorectal Cancer—A Systematic Review of Clinical Trials. Nutrients 2021, 13, 1160. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, C.; Li, W.; Wei, J.; Hong, H.; Li, J.; Feng, L.; Wei, H.; Xin, H.; Chen, T. A Phase II Randomized Clinical Trial and Mechanistic Studies Using Improved Probiotics to Prevent Oral Mucositis Induced by Concurrent Radiotherapy and Chemotherapy in Nasopharyngeal Carcinoma. Front. Immunol. 2021, 12, 618150. [Google Scholar] [CrossRef]
- Rodriguez-Arrastia, M.; Martinez-Ortigosa, A.; Rueda-Ruzafa, L.; Folch Ayora, A.; Ropero-Padilla, C. Probiotic Supplements on Oncology Patients’ Treatment-Related Side Effects: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 4265. [Google Scholar] [CrossRef]
- Yu, J.; Cao, G.; Yuan, S.; Luo, C.; Yu, J.; Cai, M. Probiotic supplements and bone health in postmenopausal women: A meta-analysis of randomised controlled trials. BMJ Open 2021, 11, e041393. [Google Scholar] [CrossRef]
- Schreiber, C.; Tamir, S.; Golan, R.; Weinstein, A.; Weinstein, Y. The effect of probiotic supplementation on performance, inflammatory markers and gastro-intestinal symptoms in elite road cyclists. J. Int. Soc. Sports Nutr. 2021, 18, 36. [Google Scholar] [CrossRef]
- Piwat, S.; Teanpaisan, R.; Manmontri, C.; Wattanarat, O.; Pahumunto, N.; Makeudom, A.; Krisanaprakornkit, S.; Nirunsittirat, A. Efficacy of Probiotic Milk for Caries Regression in Preschool Children: A Multicenter Randomized Controlled Trial. Caries Res. 2020, 54, 491–501. [Google Scholar] [CrossRef]
- Shen, N.T.; Maw, A.; Tmanova, L.L.; Pino, A.; Ancy, K.; Crawford, C.V.; Simon, M.S.; Evans, A.T. Timely Use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review with Meta-Regression Analysis. Gastroenterology 2017, 152, 1889–1900.e9. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 12, CD006095. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Luo, Y.; Walsh, S.; Grinspan, A. Oral Fecal Microbiota Transplant Capsules Are Safe and Effective for Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2021, 55, 300–308. [Google Scholar] [CrossRef]
- Beghetti, I.; Panizza, D.; Lenzi, J.; Gori, D.; Martini, S.; Corvaglia, L.; Aceti, A. Probiotics for Preventing Necrotizing Enterocolitis in Preterm Infants: A Network Meta-Analysis. Nutrients 2021, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Pitsillides, L.; Pellino, G.; Tekkis, P.; Kontovounisios, C. The Effect of Perioperative Administration of Probiotics on Colorectal Cancer Surgery Outcomes. Nutrients 2021, 13, 1451. [Google Scholar] [CrossRef]
- Rashidi, K.; Razi, B.; Darand, M.; Dehghani, A.; Janmohammadi, P.; Alizadeh, S. Effect of probiotic fermented dairy products on incidence of respiratory tract infections: A systematic review and meta-analysis of randomized clinical trials. Nutr. J. 2021, 20, 61. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Adams, J.B.; Borody, T.J.; Kang, D.W.; Khoruts, A.; Krajmalnik-Brown, R.; Sadowsky, M.J. Microbiota transplant therapy and autism: Lessons for the clinic. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1033–1037. [Google Scholar] [CrossRef]
- Qureshi, F.; Adams, J.; Hanagan, K.; Kang, D.W.; Krajmalnik-Brown, R.; Hahn, J. Multivariate Analysis of Fecal Metabolites from Children with Autism Spectrum Disorder and Gastrointestinal Symptoms before and after Microbiota Transfer Therapy. J. Pers. Med. 2020, 10, 152. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Vargason, T.; Santiago, M.; Hahn, J.; Krajmalnik-Brown, R. Distinct Fecal and Plasma Metabolites in Children with Autism Spectrum Disorders and Their Modulation after Microbiota Transfer Therapy. mSphere 2020, 5, e00314–e00320. [Google Scholar] [CrossRef]
- Solito, A.; Bozzi Cionci, N.; Calgaro, M.; Caputo, M.; Vannini, L.; Hasballa, I.; Archero, F.; Giglione, E.; Ricotti, R.; Walker, G.E.; et al. Supplementation with Bifidobacterium breve BR03 and B632 strains improved insulin sensitivity in children and adolescents with obesity in a cross-over, randomized double-blind placebo-controlled trial. Clin. Nutr. 2021, 40, 4585–4594. [Google Scholar] [CrossRef]
- Schellekens, H.; Torres-Fuentes, C.; van de Wouw, M.; Long-Smith, C.M.; Mitchell, A.; Strain, C.; Berding, K.; Bastiaanssen, T.; Rea, K.; Golubeva, A.V.; et al. Bifidobacterium longum counters the effects of obesity: Partial successful translation from rodent to human. EBioMedicine 2021, 63, 103176. [Google Scholar] [CrossRef]
- Déchelotte, P.; Breton, J.; Trotin-Picolo, C.; Grube, B.; Erlenbeck, C.; Bothe, G.; Fetissov, S.O.; Lambert, G. The Probiotic Strain, H. alvei HA4597® Improves Weight Loss in Overweight Subjects under Moderate Hypocaloric Diet: A Proof-of-Concept, Multicenter Randomized, Double-Blind Placebo-Controlled Study. Nutrients 2021, 13, 1902. [Google Scholar] [CrossRef]
- Moludi, J.; Kafil, H.S.; Qaisar, S.A.; Gholizadeh, P.; Alizadeh, M.; Vayghyan, H.J. Effect of probiotic supplementation along with calorie restriction on metabolic endotoxemia, and inflammation markers in coronary artery disease patients: A double blind placebo controlled randomized clinical trial. Nutr. J. 2021, 20, 47. [Google Scholar] [CrossRef]
- Rittiphairoj, T.; Pongpirul, K.; Janchot, K.; Mueller, N.T.; Li, T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 722–734. [Google Scholar] [CrossRef]
- Toejing, P.; Khampithum, N.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Influence of Lactobacillus paracasei HII01 Supplementation on Glycemia and Inflammatory Biomarkers in Type 2 Diabetes: A Randomized Clinical Trial. Foods 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, R.; Rohilla, L.; Jacob, N.; Yadav, J.; Sachdeva, N. A high potency multi-strain probiotic improves glycemic control in children with new-onset type 1 diabetes mellitus: A randomized, double-blind, and placebo-controlled pilot study. Pediatr. Diabetes 2021. [Google Scholar] [CrossRef]
- Rufino, M.N.; da Costa, A.L.; Jorge, E.N.; Paiano, V.F.; Camparoto, M.L.; Keller, R.; Bremer-Neto, H. Synbiotics improve clinical indicators of ulcerative colitis: Systematic review with meta-analysis. Nutr. Rev. 2021, nuab017. [Google Scholar] [CrossRef]
- Chen, H.T.; Huang, H.L.; Xu, H.M.; Luo, Q.L.; He, J.; Li, Y.Q.; Zhou, Y.L.; Nie, Y.Q.; Zhou, Y.J. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp. Ther. Med. 2020, 19, 2650–2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, I.K.; Yen, T.H.; Hsieh, P.S.; Ho, H.H.; Kuo, Y.W.; Huang, Y.Y.; Kuo, Y.L.; Li, C.Y.; Lin, H.C.; Wang, J.Y. Effect of a Probiotic Combination in an Experimental Mouse Model and Clinical Patients With Chronic Kidney Disease: A Pilot Study. Front. Nutr. 2021, 8, 661794. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.; Park, S.H.; Lee, J.S.; Byeon, J.H.; Kim, S.H.; Lim, J.; Yoo, Y. Probiotic mixture reduces gut inflammation and microbial dysbiosis in children with atopic dermatitis. Australas. J. Dermatol. 2021, 62, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.; Ponsonby, A.L.; Orsini, F.; Tey, D.; Robinson, M.; Su, E.L.; Licciardi, P.; Burks, W.; Donath, S. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. J. Allergy Clin. Immunol. 2015, 135, 737–744.e8. [Google Scholar] [CrossRef] [PubMed]
- Dunn Galvin, A.; McMahon, S.; Ponsonby, A.L.; Hsiao, K.C.; Tang, M.L.K.; PPOIT Study Team. The longitudinal impact of probiotic and peanut oral immunotherapy on health-related quality of life. Allergy 2018, 73, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Liu, Q.; Wang, W. Bifidobacterium bifidum TMC3115 ameliorates milk protein allergy in by affecting gut microbiota: A randomized double-blind control trial. J. Food Biochem. 2020, 44, e13489. [Google Scholar] [CrossRef]
- Anania, C.; Di Marino, V.P.; Olivero, F.; De Canditiis, D.; Brindisi, G.; Iannilli, F.; De Castro, G.; Zicari, A.M.; Duse, M. Treatment with a Probiotic Mixture Containing Bifidobacterium animalis Subsp. Lactis BB12 and Enterococcus faecium L3 for the Prevention of Allergic Rhinitis Symptoms in Children: A Randomized Controlled Trial. Nutrients 2021, 13, 1315. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef] [Green Version]
- Ahanchian, H.; Jafari, S.A.; Ansari, E.; Ganji, T.; Kiani, M.A.; Khalesi, M.; Momen, T.; Kianifar, H. A multi-strain Synbiotic may reduce viral respiratory infections in asthmatic children: A randomized controlled trial. Electron. Physician 2016, 8, 2833–2839. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, F.; Faleiros, S.; Cabello, R.; Díaz-Dosque, M.; Rodríguez, G.; Escobar, A. The consumption of milk supplemented with probiotics decreases the occurrence of caries and the salivary concentration of hβD-3 in children. Clin. Oral Investig. 2021, 25, 3823–3830. [Google Scholar] [CrossRef]
- Duysburgh, C.; Van den Abbeele, P.; Morera, M.; Marzorati, M. Lacticaseibacillus rhamnosus GG and Saccharomyces cerevisiae boulardii supplementation exert protective effects on human gut microbiome following antibiotic administration in vitro. Benef. Microbes 2021, 30, 1–16. [Google Scholar] [CrossRef]
- Hu, W.; Lu, W.; Li, L.; Zhang, H.; Lee, Y.K.; Chen, W.; Zhao, J. Both living and dead Faecalibacterium prausnitzii alleviate house dust mite-induced allergic asthma through the modulation of gut microbiota and short-chain fatty acid production. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef]
- Wang, W.; Luo, X.; Zhang, Q.; He, X.; Zhang, Z.; Wang, X. Bifidobacterium infantis Relieves Allergic Asthma in Mice by Regulating Th1/Th2. Med. Sci. Monit. 2020, 26, e920583. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Li, Q.; Su, H.; Sun, X. Exploration of the effect of mixed probiotics on microbiota of allergic asthma mice. Cell. Immunol. 2021, 367, 104399. [Google Scholar] [CrossRef]
- Xue, J.M.; Ma, F.; An, Y.F.; Suo, L.M.; Geng, X.R.; Song, Y.N.; Mo, L.H.; Luo, X.Q.; Zhang, X.W.; Liu, D.B.; et al. Probiotic extracts ameliorate nasal allergy by inducing interleukin-35-producing dendritic cells in mice. Int. Forum Allergy Rhinol. 2019, 9, 1289–1296. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, X.Y.; Cao, Y.J.; Zheng, T.T.; Cheng, W.J.; Chen, L.J.; Lv, X.C.; Ni, L.; Rao, P.F.; Liang, P. The beneficial effects of Lactobacillus brevis FZU0713-fermented Laminaria japonica on lipid metabolism and intestinal microbiota in hyperlipidemic rats fed with a high-fat diet. Food Funct. 2021, 12, 7145–7160. [Google Scholar] [CrossRef]
- Okada, Y.; Tsuzuki, Y.; Sugihara, N.; Nishii, S.; Shibuya, N.; Mizoguchi, A.; Itoh, S.; Tanemoto, R.; Inaba, K.; Hanawa, Y.; et al. Novel probiotic yeast from Miso promotes regulatory dendritic cell IL-10 production and attenuates DSS-induced colitis in mice. J. Gastroenterol. 2021, 56, 829–842. [Google Scholar] [CrossRef]
- O’Morain, V.L.l.; Chan, Y.H.; Williams, J.O.; Alotibi, R.; Alahmadi, A.; Rodrigues, N.P.; Plummer, S.F.; Hughes, T.R.; Michael, D.R.; Ramji, D.P. The Lab4P Consortium of Probiotics Attenuates Atherosclerosis in LDL Receptor Deficient Mice Fed a High Fat Diet and Causes Plaque Stabilization by Inhibiting Inflammation and Several Pro-atherogenic Processes. Mol. Nutr. Food Res. 2021, e2100214. [Google Scholar] [CrossRef]
- Mahajan, E.; Sinha, S.; Bhatia, A.; Sehgal, R.; Medhi, B. Evaluation of the effect of probiotic as add-on therapy with conventional therapy and alone in malaria induced mice. BMC Res. Notes 2021, 14, 246. [Google Scholar] [CrossRef]
- Scott, B.M.; Gutiérrez-Vázquez, C.; Sanmarco, L.M.; da Silva Pereira, J.A.; Li, Z.; Plasencia, A.; Hewson, P.; Cox, L.M.; O’Brien, M.; Chen, S.K.; et al. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat. Med. 2021, 27, 1212–1222. [Google Scholar] [CrossRef]
- Huang, H.; Li, K.; Lee, Y.; Chen, M. Preventive Effects of Lactobacillus Mixture against Chronic Kidney Disease Progression through Enhancement of Beneficial Bacteria and Downregulation of Gut-Derived Uremic Toxins. J. Agric. Food Chem. 2021, 69, 7353–7366. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Huang, Z.; He, Z.; Yue, Y.; Zhou, Y.; Ross, R.P.; Stanton, C.; Zhang, H.; Zhao, J.; Chen, W. Protective effect of Bifidobacterium bifidum FSDJN7O5 and Bifidobacterium breve FHNFQ23M3 on diarrhea caused by enterotoxigenic Escherichia coli. Food Funct. 2021, 12, 7271–7282. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fukui, H.; Ran, Y.; Xu, X.; Ebisutani, N.; Nakanishi, T.; Tanaka, Y.; Maeda, A.; Makizaki, Y.; Tomita, T.; et al. Probiotic Bifidobacterium bifidum G9-1 Has a Preventive Effect on the Acceleration of Colonic Permeability and M1 Macrophage Population in Maternally Separated Rats. Biomedicines 2021, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Beller, A.; Kruglov, A.; Durek, P.; von Goetze, V.; Werner, K.; Heinz, G.A.; Ninnemann, J.; Lehmann, K.; Maier, R.; Hoffmann, U.; et al. Specific microbiota enhances intestinal IgA levels by inducing TGF-β in T follicular helper cells of Peyer’s patches in mice. Eur. J. Immunol. 2020, 50, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, T.; Ohno, H. Reciprocal regulation of IgA and the gut microbiota: A key mutualism in the intestine. Int. Immunol. 2021, dxab049. [Google Scholar] [CrossRef]
- Huus, K.E.; Peterse, C.; Finlay, B.B. Diversity and dynamism of IgA-microbiota interactions. Nat. Rev. Immunol. 2021, 21, 514–525. [Google Scholar] [CrossRef]
- Nakajima, A.; Vogelzang, A.; Maruya, M.; Miyajima, M.; Murata, M.; Son, A.; Kuwahara, T.; Tsuruyama, T.; Yamada, S.; Matsuura, M.; et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J. Exp. Med. 2018, 215, 2019–2034. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, J.M.; de Zoete, M.R.; Palm, N.W.; Laenen, Y.; Bright, R.; Mallette, M.; Bu, K.; Bielecka, A.A.; Xu, F.; Hurtado-Lorenzo, A.; et al. Immunoglobulin A Targets a Unique Subset of the Microbiota in Inflammatory Bowel Disease. Cell Host Microbe 2021, 29, 83–93.e3. [Google Scholar] [CrossRef]
- Pabst, O.; Slack, E. IgA and the intestinal microbiota: The importance of being specific. Mucosal Immunol. 2020, 13, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef] [Green Version]
- Kawamoto, S.; Maruya, M.; Kato, L.M.; Suda, W.; Atarashi, K.; Doi, Y.; Tsutsui, Y.; Qin, H.; Honda, K.; Okada, T. Foxp3+ T cells regulate immunoglobulin A selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 2014, 41, 152–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, K.; Ohno, H.; Satoh-Takayama, N. Innate Lymphoid Cells: Important Regulators of Host-Bacteria Interaction for Border Defense. Microorganisms 2020, 8, 1342. [Google Scholar] [CrossRef] [PubMed]
- Hardman, C.S.; Chen, Y.L.; Salimi, M.; Jarrett, R.; Johnson, D.; Järvinen, V.J.; Owens, R.J.; Repapi, E.; Cousins, D.J.; Barlow, J.L.; et al. CD1a presentation of endogenous antigens by group 2 innate lymphoid cells. Sci. Immunol. 2017, 2, eaan5918. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, Y.; Sailike, J.; Sun, X.; Abuduwaili, N.; Tuoliuhan, H.; Yusufu, M.; Nabi, X.H. Fourteen composite probiotics alleviate type 2 diabetes through modulating gut microbiota and modifying M1/M2 phenotype macrophage in db/db mice. Pharmacol. Res. 2020, 161, 105150. [Google Scholar] [CrossRef]
- Zhang, Q.; Hao, H.; Xie, Z.; Cheng, Y.; Yin, Y.; Xie, M.; Huang, H.; Gao, J.; Liu, H.; Tong, C.; et al. M2 macrophages infusion ameliorates obesity and insulin resistance by remodeling inflammatory/macrophages’ homeostasis in obese mice. Mol. Cell. Endocrinol. 2017, 443, 63–71. [Google Scholar] [CrossRef]
- Orliaguet, L.; Ejlalmanesh, T.; Alzaid, F. Metabolic and Molecular Mechanisms of Macrophage Polarisation and Adipose Tissue Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 5731. [Google Scholar] [CrossRef]
- Vega-Galaviz, D.; Vecchyo-Tenorio, G.D.; Alcántara-Suárez, R.; Méndez-García, L.A.; Sánchez-Del Real, A.L.; Villalobos-Molina, R.; Fragoso, J.M.; León-Cabrera, S.; Ostoa-Saloma, P.; Pérez-Tamayo, R.; et al. M2 macrophage immunotherapy abolishes glucose intolerance by increasing IL-10 expression and AKT activation. Immunotherapy 2020, 12, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Ðanić, M.; Stanimirov, B.; Pavlović, N.; Goločorbin-Kon, S.; Al-Salami, H.; Stankov, K.; Mikov, M. Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome. Front. Pharmacol. 2018, 9, 1382. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Z.; Zhang, X.; Zhu, F.; Liu, Y.; Jin, C.; Du, X.; Xu, C.; Chen, Y.; Cai, W.; et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes 2020, 12, 1819155. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Huang, F.; Zhao, L.; Zhang, Y.; Yang, W.; Wang, S.; Li, M.; Han, X.; Ge, K.; Qu, C.; et al. A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. EBioMedicine 2020, 55, 102766. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C. Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 2008, 20, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R.; Piepenbrink, M.S. Lead and immune function. Crit. Rev. Toxicol. 2006, 36, 359–385. [Google Scholar] [CrossRef]
- Bellinger, D.C. Lead Contamination in Flint—An Abject Failure to Protect Public Health. N. Engl. J. Med. 2016, 374, 1101–1103. [Google Scholar] [CrossRef]
- Hanna-Attisha, M.; Lanphear, B.; Landrigan, P. Lead Poisoning in the 21st Century: The Silent Epidemic Continues. Am. J. Public Health 2018, 108, 1430. [Google Scholar] [CrossRef]
- Bertók, L. Effect of sulfhydryl compound on the lead acetate-induced endotoxin hypersensitivity of rats. J. Bacteriol. 1968, 95, 1974–1975. [Google Scholar] [CrossRef] [Green Version]
- Faith, R.E.; Luster, M.I.; Kimmel, C.A. Effect of chronic developmental lead exposure on cell-mediated immune functions. Clin. Exp. Immunol. 1979, 35, 413–420. [Google Scholar]
- Miller, T.E.; Golemboski, K.A.; Ha, R.S.; Bunn, T.; Sanders, F.S.; Dietert, R.R. Developmental exposure to lead causes persistent immunotoxicity in Fischer 344 rats. Toxicol. Sci. 1998, 42, 129–135. [Google Scholar] [CrossRef]
- Bunn, T.L.; Parsons, P.J.; Kao, E.; Dietert, R.R. Exposure to lead during critical windows of embryonic development: Differential immunotoxic outcome based on stage of exposure and gender. Toxicol. Sci. 2001, 64, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Di Lenardo, T.Z.; Ward, B.J.; Pillet, S.; Mann, K.; Bornman, R.; Obida, M.; Chevrier, J. Exposure to lead and vaccine-specific IgG titers in South African children participating in the Venda Health Examination of Mothers, Babies and their Environment (VHEMBE): A longitudinal study. Environ. Res. 2020, 180, 108794. [Google Scholar] [CrossRef]
- Liu, W.; Feng, H.; Zheng, S.; Xu, S.; Massey, I.Y.; Zhang, C.; Wang, X.; Yang, F. Pb Toxicity on Gut Physiology and Microbiota. Front. Physiol. 2021, 12, 574913. [Google Scholar] [CrossRef]
- Zhai, Q.; Qu, D.; Feng, S.; Yu, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Oral Supplementation of Lead-Intolerant Intestinal Microbes Protects Against Lead (Pb) Toxicity in Mice. Front. Microbiol. 2020, 10, 3161. [Google Scholar] [CrossRef]
- Finlay, B.B.; Amato, K.R.; Azad, M.; Blaser, M.J.; Bosch, T.C.G.; Chu, H.; Dominguez-Bello, M.G.; Ehrlich, S.D.; Elinav, E.; Geva-Zatorsky, N.; et al. The hygiene hypothesis, the COVID pandemic, and consequences for the human microbiome. Proc. Natl. Acad. Sci. USA 2021, 118, e2010217118. [Google Scholar] [CrossRef]
- Dietert, R.R. The microbiological basis of human superorganism freedom. Am. J. Biomed. Sci. Res. 2021, 13, 653–661. [Google Scholar] [CrossRef]
- Blaser, M.J. Missing Microbes: How the Overuse of Antibiotics Is Fueling Our Modern Plagues; Henry Holt and Company: New York, NY, USA, 2014; 288p, ISBN 978-0805098105. [Google Scholar]
- Bilello, J.; Okereke, I. Impact of Environmental and Pharmacologic Changes on the Upper Gastrointestinal Microbiome. Biomedicines 2021, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Boem, F.; Nannini, G.; Amedei, A. Not just ‘immunity’: How the microbiota can reshape our approach to cancer immunotherapy. Immunotherapy 2020, 12, 407–416. [Google Scholar] [CrossRef]
| Disease, Condition, and/or Extrapulmonary Symptom | Reference(s) |
|---|---|
| Allergic rhinitis | [22] |
| Atopic dermatitis | [23] |
| Food allergies | [24,25] |
| Chronic obstructive pulmonary disease (COPD) | [26] |
| Chronic sinusitis | [27,28] |
| Obesity | [29] |
| Depression | [26] |
| Anxiety | [26] |
| Mood swings | [30] |
| Attention Deficit Disorder | [31] |
| Fatigue | [30] |
| Gastro-esophageal reflux disease (GERD) | [32] |
| Osteoarthritis | [33] |
| Vocal cord dysfunction | [34] |
| Stomach ulcers | [35] |
| Nasal polyps | [36] |
| Frailty | [37] |
| Hypertension | [38] |
| Skeletal muscle wasting/Sarcopenia | [39] |
| Mast cell activation syndrome | [40] |
| Hormone imbalances/disorders | [41,42] |
| Type 2 diabetes | [43] |
| Obstructive sleep apnea | [44] |
| Insomnia/sleep disorders | [45,46] |
| Ischemic heart disease | [47] |
| Hypoxia | [48] |
| Stroke | [49] |
| Acute myocardial infarction | [50] |
| Posttraumatic Stress Disorder (PTSD) | [51] |
| Myasthenia gravis | [52] |
| Lung cancer | [53] |
| Allergic bronchopulmonary aspergillosis (ABPA) | [54] |
| Type 1 diabetes 1 | [55] |
| Metastasized breast cancer 2 | [56] |
| Eosinophilic granulomatosis with polyangiitis (vasculitis) 3 | [57] |
| Coronary heart disease 4 | [58] |
| Disease or Condition | Reference |
|---|---|
| Type 2 diabetes | [64] |
| Hypertension | [65] |
| Coronary artery disease | [66] |
| Atherosclerosis | [67] |
| Polycystic ovary syndrome (PCOS) | [68] |
| Urinary stress incontinence | [69] |
| Osteoarthritis | [70] |
| Dyslipidemia | [71] |
| Obstructive sleep apnea | [72] |
| Sleep fragmentation | [73] |
| Nonalcoholic fatty liver disease (NAFLD) | [74] |
| Nonalcoholic hepatic steatosis (NAHD) | [75] |
| Asthma | [76] |
| Rheumatoid arthritis | [77] |
| Crohn’s disease (in women) | [78] |
| Hearing loss | [79] |
| Deep vein thrombosis | [80] |
| Infertility | [81] |
| Frailty | [82] |
| Alzheimer’s disease | [83] |
| Gout | [84] |
| Hypothyroidism | [85] |
| Dementia | [86] |
| Multiple sclerosis | [87] |
| Schizophrenia (cortical thickness reduction) | [88] |
| Chronic obstructive pulmonary disease (COPD) | [89] |
| Depression | [90] |
| Anxiety | [91] |
| Psoriasis | [92] |
| Attention Deficit Disorder | [93] |
| Chronic kidney disease | [94] |
| Leukemia | [95] |
| Uterine cancer | [95] |
| Gallbladder cancer | [95] |
| Thyroid cancer | [95] |
| Cancer of the cervix | [95] |
| Hepatic cancer | [95] |
| Ovarian cancer | [95] |
| Postmenopausal breast cancer | [95] |
| Colon cancer | [95] |
| Kidney cancer | [95] |
| Pancreatic cancer | [95] |
| Rectal cancer | [95] |
| Drug/Drug Category | Damages and/or Interaction(s) | Reference(s) |
|---|---|---|
| Digoxin (Cardiovascular) | Internal drug dose affected by gut bacterium, Eggerthella lenta | [108,109,110] |
| Non-steroidal anti-inflammatory drugs (NSAIDs) | Produces NSAID-specific enteropathy, damage to specific microbiota that protect against gastric enteropathy; Probiotics may help functionality | [111,112,113] |
| Proton Pump Inhibitors | Damages approximately 20% of the gut microbiota; increases the risk of enteric infections | [112,114,115] |
| SSRI Antidepressants | SSRIs act like antibiotics completely restructuring the gut microbiome causing loss of some needed species and overgrowth of others | [112,116,117] |
| Oral steroids | Can cause overgrowth of obesogenic, methogenic bacteria | [112] |
| Metformin (Type 2 diabetes) | Increased growth of E. coli species with increased risk from pathobionts | [112,118] |
| Laxatives (with polyethylene glycol) | Reduced diversity of the gut microbiome | [119] |
| Beta Blockers | Increase in pathobiont for dental caries, Streptococcus mutans | [118] |
| H1 inhibitor antihistamines | Increase in Clostridium bolteae a pathobiont associated with both gut and neurotoxic metabolites | [118,120] |
| Platelet aggregation inhibitors (aspirin) | Increases in several Streptoccocus and Clostridial pathobionts and reduction in a major GABA producer (Bifidobacterium adolescentis) | [118,121] |
| Opioids (Pain management) | The gut microbiome becomes dysbiotic and can lock in the dependency. | [122,123] |
| Cancer therapeutics | The gut microbiome can be altered by cancer therapeutics and this can subsequently affect immune anti-tumor effectiveness. Also the cancer drug, cyclophosphamide, has a better prognosis for lung cancer patients when the patients carry the bacterium, Enterococcus hirae | [124,125] |
| Probiotic/Rebiosis Strategy [Reference(s) in brackets] | Disease Prevention/ Therapy | Specific Effects |
|---|---|---|
| Bifidobacterium longum ssp infantis EVC001 [131] | Protection against pathobiont-induced enteric inflammation in the infant | Protection against enteric inflammation and reduction in proinflammatory cytokine levels promoted by Clostridiaceae and Enterobacteriaceae |
| Multiple different probiotics [148] | Protection against antibiotic-associateddiarrhea (AAD) | Meta-analysis of 36 studies including 9312 participants showing a 38% reduction in disease incidence. |
| Multiple probiotic regimes [149] | Protection against post-operative adverse outcomes from colon cancer surgery | Meta analysis of six studies with 457 subjects. Probiotic administration improved gut barrier and colon function, reduced inflammatory markers, and increased gut microbiome diversity with increased colonization resistance post- surgery |
| Probiotic cocktail (L. plantarum MH-301, B. animalis subsp. Lactis LPL-RH, L. rhamnosus LGG-18, and L. acidophilus) [150] | Protection against oral mucositis (OM) following nasopharyngeal cancer chemoradio-therapy | In a clinical trial of 77 patients, probiotic supplementation resulted in a significantly lower prevalence of OM and a reduced severity grade. |
| Multiple probiotic regimes [151] | Protection against side effects of chemo and radiation cancer therapies | Meta-analysis of 20 studies, 17 studies produced positive results in reducing side effects while three studies found no significant differences. |
| Multiple probiotic regimes [152] | Protection against osteoporosis in post-menopausal women | Meta analysis of five randomized controlled trials with 497 participants. Probiotic supplements significantly increased bone mineral density in the lumbar spine. |
| Multi-strain probiotic [153] | Protection against gastrointestinal symptoms among elite athletes | Reduced gastrointestinal symptoms and distress following intense training sessions competition |
| Reconstituted milk powder containing the probiotic, Lactobacillus paracasei SD1 [154] | Protection against new dental caries and Reduction in existing dental carieslesions | Beneficial preventative and therapeutic oral health effects in preschool children |
| Multiple probiotic regimes [155] | Protection against hospital acquired Clostridioides difficile infection | Meta-analysis of 19 prevention studies with 6261 subjects. The infection rate was decreased by greater than 50% from controls. |
| Multiple probiotic regimes [156] | Protection against Clostridioides difficile-associated diarrhea in children and adults | Meta analysis with complete case analysis of 31 trials with 8672 participants. The risk of C. difficile infection was reduced by 60%. |
| Oral Fecal Microbiota Transplant (FMT) [157] | Protection against recurrent Clostridioides difficile infection and Long term treatment of C. difficile infected patients | Meta analysis of efficacy of oral FMT capsules. 15 studies with 753 patients had an efficacy rate of 82.1% |
| Multiple probiotic regimes [158] | Protection against Necrotizing enterocolitis (NEC) in premature infants | Meta-analysis of 51 studies. Lactobacillus acidophilus LB was the most successful of probiotics used in reducing the risk of NEC |
| Different probiotic combinations across the trials [159] | Protection against complications following colorectal cancer surgery | Meta analysis of 15 trials, Improved mucosal protection/function and microbial diversity following antibiotics |
| Probiotic fermented dairy products [160] | Protection against respiratory infections | Meta analysis of 22 clinical trials with 10, 290 participants. Overall infection rate was decreased by 18–21% across children (18%), adults (19%), and elderly (21%) |
| Microbiota transfer therapy [161,162,163,164] | Therapy for autism spectrum disorders | Significant improvements in gastrointestinal symptoms, autism functionality and gut microbiome metabolism and diversity |
| Bifidobacterium breve BR03 and B632 strains [165] | Therapy for obesity/insulin resistance | Improved metabolic parameters; decreased weight; improved insulin sensitivity |
| Bifidobacterium longum APC1472 [166] | Therapy for obesity | Improved fasting blood glucose levels |
| Hafnia alvei probiotic strain HA4597® [167] | Therapy for obesity | Significantly improved weight loss; feeling of fullness; reduction in hip circumference; Fasting glycemia |
| Lactobacillus rhamnosus GG (LGG) [168] | Therapy for coronary artery disease (CAD) | Reduced metabolic endotoxemia and mega inflammation |
| Multiple probiotic regimes [169] | Therapy for type 2 diabetes | Meta-analysis of 26 studies with 1947 participants. Probiotics significantly reduced the glycemic index in type 2 diabetics. |
| Lactobacillus paracasei HII01 [170] | Therapy for type 2 diabetes | Decreased plasma blood glucose levels with reduced inflammatory markers and restored gut microbiota profile and function |
| Multi-strain probiotics [171] | Therapy for type 1 diabetes (children) | Improved glycemic control |
| Multiple different synbiotics, Meta-analysis [172] | Therapy for ulcerative colitis | Beneficial reduction in inflammation; Reduced inflammatory cytokines andC-reactive protein levels; elevated levels of anti-inflammatory cytokines |
| Fecal Microbiota Transplantation [173] | Therapy for active ulcerative colitis | Following three treatments, increased levels of gut Faecalibacterium prausnitzii bacterium and a significantly reduced Mayo Clinic score for UC. |
| Mix of Lactobacillus acidophilus (TYCA06), Bifidobacterium longum subspecies infantis (BLI-02), and B. bifidum (VDD088) [174] | Therapy for chronic kidney disease | Attenuation of renal function deterioration and reduction in inflammation |
| Mixture of Lactobacilli and Bifidobacteria strains [175] | Therapy for atopic dermatitis | Reduced clinical severity; reduced intestinal inflammation |
| Lactobacillus rhamnosus CGMCC 1.3724 [176,177] | Therapy for food allergy (peanuts) | Sustained tolerance when combined with peanut oral immunotherapy |
| Bifidobacterium bifidum TMC3115 [178] | Therapy for food allergy (Cow’s milk) | Probiotic treatment of infants ameliorated the allergy with lower allergy scores, elevated anti-inflammatory responses, lower serum IgE and higher IgG2 |
| Bifidobacterium animalis Subsp., Lactis BB12 and Enterococcus faecium L3 [179] | Therapy for allergic rhinitis (AR) | Prevention of signs and of required use of medications among children. With prophylactic probiotic treatment begun three months before allergy season, the signs and symptoms of AR were significantly reduced as was use of drugs, including oral antihistamines and local corticosteroids. |
| Bifidobacterium mixture (B. longum BB536, B. infantis M-63, B. breve (M-16V) [180] | Therapy for allergic rhinitis and intermittent asthma | Improved symptoms and quality of life |
| Multi-strain Synbiotic [181] | Therapy for viral infections in asthmatic children | Reduced number of viral infections vs. placebo group |
| Lactobacillus rhamnosus SP1 [182] | Prevention of and therapy for dental caries | Consumption of probiotic-laden milk reduced the prevalence of dental caries in children |
| Lacticaseibacillus rhamnosus GG and Saccharomyces cerevisiae boulardii [183] | Used for restoration of the gut microbiome following antibiotic treatment | Increases in major bacteria groups and short chain fatty acid production postantibiotics |
| Probiotic/Rebiosis Strategy (Species) [Reference(s)] | Disease Prevention/Therapy | Specific Effects |
|---|---|---|
| Faecalibacterium prausnitzii (mouse) [184] | Allergic asthma | Immune cell and cytokine normalization, reduced airway pathology, increase short chain fatty acid production; enhance gut microbiome diversity |
| Bifidobacterium longum ssp infantis (mouse) [185] | Allergic asthma | Probiotic supplementation reduced ovalbumin-specific IgE antibodies, reduced infiltration by inflammatory cells, and shifted cytokine levels from Th2 to Th1. |
| Mixed probiotics (mouse) [186] | Allergic asthma | Immune-based alleviation of allergic asthma, reduced inflammation with restoration of the gut microbiome |
| Bifidobacterium longum ssp infantis (mouse) [187] | Nasal allergy | Probiotic promotion of IL-10 producing dendritic cells that suppressed the nasal allergy. |
| Lactobacillus brevis FZU0713-fermented Laminaria japonica (rat) [188] | Obesity | Significantly inhibited obesity and improved serum and hepatic biochemical parameters in High fat diet-fed rats. Changes in both gut microbiota composition and short chain fatty acid production |
| Miso-derived Zygosaccharomyces sapae strain I-6 -yeast (mouse) [189] | Colitis | Significant reduction in inflammation by production of IL-10 from dendritic cells |
| Consortium of probiotics (mouse) [190] | Atherosclerosis | Blocked atherogenic processes via immunomodulation |
| Lactobacillus casei adjunct therapy (mouse) [191] | Malaria | Blocked parasitemia |
| Yeast-based engineered, self- tunable probiotics (mouse) [192] | Inflammatory bowel disease | Reduced inflammation, intestinal fibrosis and dysbiosis |
| Lactobacillus paracasei and Lactobacillus plantarum (mouse) [193] | Chronic kidney disease | Improved kidney function with reduction in kidney injury and fibrotic-related proteins and restoration of gut microbiota |
| Bifidobacterium bifidum FSDJN7O5 and Bifidobacterium breve FHNFQ23M3 (mouse) [194] | Diarrhea caused by enterotoxigenic Escherichia coli | Alleviation of symptoms with restoration of gut function and physiology |
| Bifidobacterium bifidum G9-1 (BBG9-1) (rat) [195] | Maternal separation used as a model of the colonic mucosal problems as seen with irritated bowel; M1-macrophage driven inflammation | Probiotic administration protected against M1 macrophage-driven adverse effects. There were reduced numbers of M1 macrophages with increased CD-80 positive cells; reduced inflammatory cytokine production; the probiotic administration protected and stabilized the colonic mucosa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietert, R.R. Microbiome First Medicine in Health and Safety. Biomedicines 2021, 9, 1099. https://doi.org/10.3390/biomedicines9091099
Dietert RR. Microbiome First Medicine in Health and Safety. Biomedicines. 2021; 9(9):1099. https://doi.org/10.3390/biomedicines9091099
Chicago/Turabian StyleDietert, Rodney R. 2021. "Microbiome First Medicine in Health and Safety" Biomedicines 9, no. 9: 1099. https://doi.org/10.3390/biomedicines9091099
APA StyleDietert, R. R. (2021). Microbiome First Medicine in Health and Safety. Biomedicines, 9(9), 1099. https://doi.org/10.3390/biomedicines9091099





