Vulvar Lichen Sclerosus from Pathophysiology to Therapeutic Approaches: Evidence and Prospects
Abstract
1. Introduction
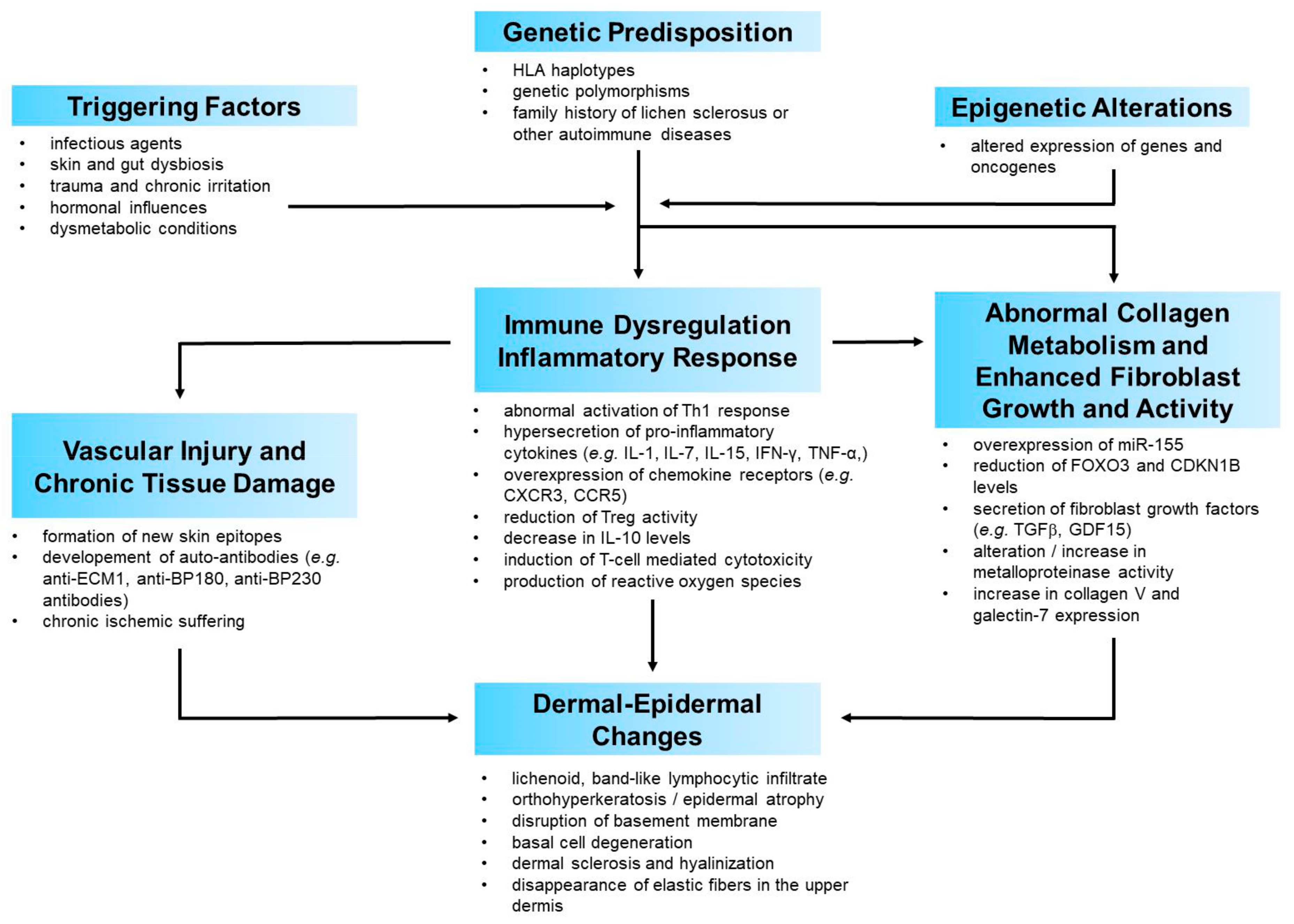
2. Update on VLS Etiopathogenesis
2.1. Predisposing Background and Genetics
2.2. Immune Dysregulation and Inflammatory Response
2.3. Abnormal Collagen Metabolism
2.4. Triggering Factors
2.5. A Possible Pathogenetic Model
3. Treatment Options
- (1)
- auto-immunogenic mechanisms, and subsequent inflammation and oxidative stress.
- (2)
- sclerotic tissue formation.
3.1. Treatments Mainly Acting on Immune Dysreactivity and Inflammatory Response
3.1.1. Topical Corticosteroids
- (1)
- (2)
- (3)
3.1.2. Topical Calcineurin Inhibitors
3.1.3. Miscellaneous Topical Treatments
3.1.4. Systemic Treatments
3.2. Treatments Mainly Acting on Abnormal Fibroblast and Collagen Metabolism
3.2.1. Topical Retinoids
3.2.2. Miscellaneous Topical Treatments
3.2.3. Physical Treatments
Phototherapy
Photodynamic Therapy
Laser
3.2.4. Injective Treatments
Adipose-Derived Stem Cells and Platelet-Rich Plasma
Heterologous Type I Collagen
3.2.5. Systemic Treatments
4. Concluding Remarks and Research Agenda
- (1)
- The exact sequence of events underlying VLS pathogenesis;
- (2)
- The key mediators involved in VLS immune response and those which, more than others, trigger an abnormal fibroblast and collagen metabolism; in other words, the agents that convert inflammation into fibrosis;
- (3)
- To what extent keratinocytes and fibroblasts actively participate in VLS pathogenesis and how they interact; and
- (4)
- How a genetic background predisposes certain individuals to an abnormal release of pro-inflammatory and pro-fibrotic mediators, in response to still not fully understood triggers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Treatment | Posology | Notes |
|---|---|---|
| Topical treatments | ||
| Topical Corticosteroids - Clobetasol Propionate 0.05% Ointment or Cream - Mometasone Furoate 0.1% Ointment or Cream | Once or twice a day for 12 weeks | - first line treatment in the active phase - anti-inflammatory and immunosuppressive activity - effectiveness on both symptoms and objective features - tachyphylaxis and dose-dependent side effects may be avoided by tapering regimens - ointment formulation seems to be more effective in comparison with cream - intralesional corticosteroid injection in recalcitrant forms - long-term maintenance treatment (reactive, continuative or proactive regimens) |
| Topical Calcineurin Inhibitors - Tacrolimus 0.1% Ointment - Pimecrolimus 1% Cream | Twice a day for 8 to 24 weeks | - second-line choice with lower effectiveness than ultra-potent corticosteroid - immunosuppressive activity - effectiveness on both symptoms and objective features - possible transient burning sensation during the first weeks of treatment |
| Calcipotriol 0.005% Ointment | Once to twice a day for 16 weeks | - inhibition of inflammatory response - attenuation of abnormal keratinocyte proliferation and differentiation - effectiveness on symptoms - alternative to standard treatment (weak evidence) |
| Oxatomide 5% gel | Twice a day for periods of 14-days | - antihistamine and anti-inflammatory properties - effectiveness on both symptoms and objective features - alternative to standard treatment (weak evidence) |
| Human Fibroblast Lysate Cream | Twice daily for 12 weeks | - presence of anti-inflammatory cytokines and wound-healing grow factors - no more effective than placebo |
| Systemic treatments | ||
| Oral Cyclosporine | 3–4 mg/kg/day for 12 weeks | - immunosuppressive effect - regression of symptoms and improvement of clinical features in resistant case - weak evidence |
| Oral or Subcutaneous Metothrexate | 10 to 15 mg/week | - immunosuppressive effect - regression of symptoms and improvement of clinical features in resistant case - weak evidence |
| Baricitinib | - inhibition of JAK 1/2 - anecdotal reports | |
| Treatment | Posology | Notes |
|---|---|---|
| Topical treatments | ||
| Topical Retinoids - Tretinoin 0.025% or 0.05% cream - cis-retinoic acid 0.5% Ointment | Daily application for 5 days per week or every other day, for 6 to 12 months | - normalizing keratinization process and collagen metabolism - mild anti-inflammatory effect - effectiveness on both symptoms and objective features - frequent irritant reaction with mild erythema and burning sensation - third-line choice |
| Cream Containing Avocado and Soybean Extracts | Daily application for 16–24 weeks | - modification of dermal connective tissue components - anti-inflammatory effect - effectiveness on both symptoms and objective features in mild-to-moderate forms |
| Emollients and Moisturizers | Daily application for months | - no effect on clinical and histopatological changes - preservation of skin integrity - effectiveness on symptoms - long-term maintenance therapy |
| Physical treatments | ||
| UVA1 Phototherapy | medium-dose UV-A1, 4 times weekly for 12 weeks | - inhibition of collagen synthesis and upregulation of collagenase activity - induction of repigmentation - effectiveness on both symptoms and objective features - alternative to topical potent and ultra-potent corticosteroids |
| Photodynamic Therapy | 2-week intervals for 6–8 weeks and irradiation with red (630–635 nm) or green light (495–570 nm) | - induction of apoptosis of lymphocytes and keratinocytes - alteration of cytokines and metalloproteinases expression - promotion of skin remodeling - effectiveness on both symptoms and objective features - mild-to-moderate pain or burning during irradiation - alternative to topical potent and ultra-potent corticosteroids |
| Laser | heterogeneous treatment schemes of non-ablative (Nd:YAG) and ablative (CO2) lasers | - induction of collagen remodelling due to neovascularization, neocollagenogenesis, elastogenesis, restoration of the trabecular architecture - improvement of epithelial degeneration and atrophy - effectiveness on both symptoms and objective features - post-treatment pain - alternative or complementary approach to topical potent and ultra-potent corticosteroids |
| Injective treatments | ||
| Adipose-Derived Stem Cells | heterogeneous treatment schemes and protocols | - inhibition of fibrosis - regeneration of damaged tissue - anti-inflammatory and immune-modulatory activity - effectiveness on scarring, atrophy and the other sequelae of VLS which are poorly responsive to topical therapies - alternative or complementary approach to topical potent and ultra-potent corticosteroids |
| Platelet-Rich Plasma | heterogeneous treatment schemes and protocols | - promotion of mesenchymal cell proliferation, tissue repair and angiogenesis - reduction of inflammation - effectiveness on scarring, atrophy and the other sequelae of VLS which are poorly responsive to topical therapies - alternative or complementary approach to topical potent and ultra-potent corticosteroids |
| Heterologous Type I Collagen | four injective treatments at 2-week intervals; then, every 2 months, as maintenance | - promotion of fibroblast proliferation and collagen synthesis - weak evidence |
| Systemic treatments | ||
| Acitretin | 20 to 30 mg/day for 16 weeks | - improvement in itching and clinical signs - alternative in recalcitrant forms - weak evidence |
| Potassium Para-Aminobenzoate (PABA) | 3g/day for 8 weeks | - improvement of skin fibroses - no more effective than placebo |
References
- Kirtschig, G.; Becker, K.L.; Gunthert, A.R.; Jasaitiene, D.; Cooper, S.; Chi, C.-C.; Kreuter, A.; Rall, K.; Aberer, W.; Riechardt, S.; et al. Evidence-based (S3) Guideline on (anogenital) Lichen sclerosus. J. Eur. Acad. Dermatol. Venereol. 2015, 29, e1–e43. [Google Scholar] [CrossRef]
- Fistarol, S.K.; Itin, P.H. Diagnosis and Treatment of Lichen Sclerosus. Am. J. Clin. Dermatol. 2012, 14, 27–47. [Google Scholar] [CrossRef]
- Nelson, D.M.; Peterson, A.C. Lichen Sclerosus: Epidemiological Distribution in an Equal Access Health Care System. J. Urol. 2011, 185, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.T.; Marinoff, S.C.; Christopher, K.; Srodon, M. Prevalence of vulvar lichen sclerosus in a general gynecology practice. J. Reprod. Med. Obs. Gynecol. 2005, 50, 477–480. [Google Scholar]
- Carli, P.; Cattaneo, A.; De Magnis, A.; Biggeri, A.; Taddei, G.; Giannotti, B. Squamous cell carcinoma arising in vulval lichen sclerosus. Eur. J. Cancer Prev. 1995, 4, 491–496. [Google Scholar] [CrossRef]
- Bleeker, M.C.; Visser, P.J.; Overbeek, L.I.; Van Beurden, M.; Berkhof, J. Lichen Sclerosus: Incidence and Risk of Vulvar Squamous Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1224–1230. [Google Scholar] [CrossRef]
- Halonen, P.M.; Jakobsson, M.I.; Heikinheimo, M.A.O.; Riska, A.E.; Gissler, M.V.; Pukkala, E.I. Lichen sclerosus and risk of cancer. Int. J. Cancer 2017, 140, 1998–2002. [Google Scholar] [CrossRef] [PubMed]
- Corazza, M.; Borghi, A.; Gafà, R.; Ghirardi, C.; Ferretti, S. Risk of vulvar carcinoma in women affected with lichen sclerosus: Results of a cohort study. J. Dtsch. Dermatol. Ges. 2019, 17, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Niamh, L.; Naveen, S.; Hazel, B. Diagnosis of Vulval Inflammatory Dermatoses: A Pathological Study with Clinical Correlation. Int. J. Gynecol. Pathol. 2009, 28, 554–558. [Google Scholar] [CrossRef]
- Regauer, S.; Liegl, B.; Reich, O. Early vulvar lichen sclerosus: A histopathological challenge. Histopathology 2005, 47, 340–347. [Google Scholar] [CrossRef]
- Borghi, A.; Corazza, M.; Minghetti, S.; Bianchini, E.; Virgili, A. Dermoscopic Features of Vulvar Lichen Sclerosus in the Setting of a Prospective Cohort of Patients: New Observations. Dermatology 2015, 232, 71–77. [Google Scholar] [CrossRef]
- Borghi, A.; Virgili, A.; Corazza, M. Dermoscopy of Inflammatory Genital Diseases. Dermatol. Clin. 2018, 36, 451–461. [Google Scholar] [CrossRef]
- Cooper, S.M.; Gao, X.-H.; Powell, J.J.; Wojnarowska, F. Does Treatment of Vulvar Lichen Sclerosus Influence Its Prognosis? Arch. Dermatol. 2004, 140, 702–706. [Google Scholar] [CrossRef]
- Lee, A.; Bradford, J.; Fischer, G. Long-term Management of Adult Vulvar Lichen Sclerosus. JAMA Dermatol. 2015, 151, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Virgili, A.; Borghi, A.; Toni, G.; Minghetti, S.; Corazza, M. Prospective Clinical and Epidemiologic Study of Vulvar Lichen Sclerosus: Analysis of Prevalence and Severity of Clinical Features, together with Historical and Demographic Associations. Dermatology 2014, 228, 145–151. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Ceausu, I.; Depypere, H.; Erel, C.T.; Lambrinoudaki, I.; Rees, M.; Schenck-Gustafsson, K.; Tremollieres, F.; van der Schouw, Y.T.; Simoncini, T. EMAS clinical guide: Vulvar lichen sclerosus in peri and postmenopausal women. Maturitas 2013, 74, 279–282. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Naldi, L.; Virgili, A.; Di Landro, A.; Simon, D.; Corazza, M.; Borghi, A. The other members of the GLS Italian Study Group An original exploration of genital lichen sclerosus: The semantic connectivity map. J. Eur. Acad. Dermatol. Venereol. 2018, 33, e59–e62. [Google Scholar] [CrossRef]
- Lee, A.; Fischer, G. Diagnosis and Treatment of Vulvar Lichen Sclerosus: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, K.L. Effect of lichen sclerosus on sexual function and parturition. J. Reprod. Med. 1995, 40, 351–354. [Google Scholar] [PubMed]
- Burrows, L.J.; Creasey, A.; Goldstein, A. The Treatment of Vulvar Lichen Sclerosus and Female Sexual Dysfunction. J. Sex. Med. 2011, 8, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, C.; Hondel, K.V.D.; Korfage, I.; Van Gestel, M.; Van Der Meijden, W. Quality of life in Dutch women with lichen sclerosus. Br. J. Dermatol. 2013, 168, 787–793. [Google Scholar] [CrossRef]
- Felmingham, C.; Chan, L.; Doyle, L.W.; Veysey, E. The Vulval Disease Quality of Life Index in women with vulval lichen sclerosus correlates with clinician and symptom scores. Australas. J. Dermatol. 2019, 61, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hickey, S.; Bell, H. Quality of Life in the Vulvar Clinic. J. Low. Genit. Tract Dis. 2010, 14, 225–229. [Google Scholar] [CrossRef]
- Kelekçi, K.H.; Ozyurt, S.; Ozkan, B.; Karaca, S.; Karakuzu, A.; Bilgin, I. The Impact of Inflammatory and Infectious Diseases of Vulvar on Quality of Life. J. Menopausal Med. 2016, 22, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Sadownik, L.A.; Koert, E.; Maher, C.; Smith, K.B. A Qualitative Exploration of Women’s Experiences of Living with Chronic Vulvar Dermatoses. J. Sex. Med. 2020, 17, 1740–1750. [Google Scholar] [CrossRef]
- Corazza, M.; Virgili, A.; Toni, G.; Valpiani, G.; Morotti, C.; Borghi, A. Pictorial Representation of Illness and Self-Measure to assess the perceived burden in patients with chronic inflammatory vulvar diseases: An observational study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2645–2651. [Google Scholar] [CrossRef]
- Trietsch, M.D.; Nooij, L.S.; Gaarenstroom, K.; van Poelgeest, M.I. Genetic and epigenetic changes in vulvar squamous cell carcinoma and its precursor lesions: A review of the current literature. Gynecol. Oncol. 2015, 136, 143–157. [Google Scholar] [CrossRef]
- Tran, D.A.; Tan, X.; Macri, C.J.; Goldstein, A.T.; Fu, S.W. Lichen Sclerosus: An autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int. J. Biol. Sci. 2019, 15, 1429–1439. [Google Scholar] [CrossRef]
- Virgili, A.; Cazzaniga, S.; Naldi, L.; Minghetti, S.; Verrone, A.; Stroppiana, E.; Caproni, M.; Nasca, M.; D’Antuono, A.; Papini, M.; et al. New insights into potential risk factors and associations in genital lichen sclerosus: Data from a multicentre Italian study on 729 consecutive cases. J. Eur. Acad. Dermatol. Venereol. 2016, 31, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, F.M.N.P.; Marques, M.T.Q.; Matos, H.J.; Pontes, L.F.S.; Porto, L.C.S.; Azevedo, L.M.S.; Filgueira, A.L. HLA markers in familial Lichen Sclerosus. J. Dtsch. Dermatol. Ges. 2006, 4, 842–847. [Google Scholar] [CrossRef]
- Higgins, C.A.; Cruickshank, M.E. A population-based case–control study of aetiological factors associated with vulval lichen sclerosus. J. Obstet. Gynaecol. 2012, 32, 271–275. [Google Scholar] [CrossRef]
- Sherman, V.; McPherson, T.; Baldo, M.; Salim, A.; Gao, X.H.; Wojnarowska, F. The high rate of familial lichen sclerosus suggests a genetic contribution: An observational cohort study. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Stingeni, L.; Bianchi, L.; Hansel, K.; Corazza, M.; Gallo, R.; Guarneri, F.; Patruno, C.; Rigano, L.; Romita, P.; Pigatto, P.D.; et al. Italian Guidelines in Patch Testing-adapted from the European Society of Contact Dermatitis (ESCD). G. Ital. Dermatol. Venereol. 2019, 154, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Doulaveri, G.; Armira, K.; Kouris, A.; Karypidis, D.; Potouridou, I. Genital Vulvar Lichen Sclerosus in Monozygotic Twin Women: A Case Report and Review of the Literature. Case Rep. Dermatol. 2013, 5, 321–325. [Google Scholar] [CrossRef]
- Bataille, V.; Lens, M.; Spector, T. The use of the twin model to investigate the genetics and epigenetics of skin diseases with genomic, transcriptomic and methylation data. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1067–1073. [Google Scholar] [CrossRef]
- Gao, X.-H.; Barnardo, M.C.; Winsey, S.; Ahmad, T.; Cook, J.; Agudelo, J.D.; Zhai, N.; Powell, J.J.; Fuggle, S.V.; Wojnarowska, F. The Association Between HLA DR, DQ Antigens, and Vulval Lichen Sclerosus in the UK: HLA DRB1*12 and its Associated DRB1*12/DQB1*0301/04/09/010 Haplotype Confers Susceptibility to Vulval Lichen Sclerosus, and HLA DRB1*0301/04 and its Associated DRB1*0301/04/DQB1*0201/02/03 Haplotype Protects from Vulval Lichen Sclerosus. J. Investig. Dermatol. 2005, 125, 895–899. [Google Scholar] [CrossRef]
- Şentürk, N.; Aydın, F.; Birinci, A.; Yildiz, L.; Cantürk, T.; Durupınar, B.; Turanlı, A.Y. Coexistence of HLA-B*08 and HLA-B*18 in Four Siblings with Lichen sclerosus. Dermatology 2004, 208, 64–66. [Google Scholar] [CrossRef]
- Liu, G.; Cao, F.; Zhao, M.; Shi, J.; Liu, S. Associations between HLA-A\B\DRB1 polymorphisms and risks of vulvar lichen sclerosus or squamous cell hyperplasia of the vulva. Genet. Mol. Res. 2015, 14, 15962–15971. [Google Scholar] [CrossRef]
- Haefner, H.K.; Welch, K.C.; Rolston, A.M.; Koeppe, E.S.; Stoffel, E.M.; Kiel, M.J.; Berger, M.B. Genomic Profiling of Vulvar Lichen Sclerosus Patients Shows Possible Pathogenetic Disease Mechanisms. J. Low. Genit. Tract Dis. 2019, 23, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Vanin, K.; Scurry, J.; Thorne, H.; Yuen, K.; Ramsay, R.G. Overexpression of Wild-type p53 in Lichen Sclerosus adjacent to Human Papillomavirus-negative Vulvar Cancer. J. Investig. Dermatol. 2002, 119, 1027–1033. [Google Scholar] [CrossRef][Green Version]
- Rotondo, J.C.; Borghi, A.; Selvatici, R.; Magri, E.; Bianchini, E.; Montinari, E.; Corazza, M.; Virgili, A.; Tognon, M.; Martini, F. Hypermethylation-Induced Inactivation of theIRF6Gene as a Possible Early Event in Progression of Vulvar Squamous Cell Carcinoma Associated With Lichen Sclerosus. JAMA Dermatol. 2016, 152, 928–933. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Borghi, A.; Selvatici, R.; Mazzoni, E.; Bononi, I.; Corazza, M.; Kussini, J.; Montinari, E.; Gafà, R.; Tognon, M.; et al. Association of Retinoic Acid Receptor β Gene With Onset and Progression of Lichen Sclerosus–Associated Vulvar Squamous Cell Carcinoma. JAMA Dermatol. 2018, 154, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Lerma, E.; Esteller, M.; Herman, J.G.; Prat, J. Alterations of the p16INK4a/Rb/cyclin-D1 pathway in vulvar carcinoma, vulvar intraepithelial neoplasia, and lichen sclerosus. Hum. Pathol. 2002, 33, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Soufir, N.; Queille, S.; Liboutet, M.; Thibaudeau, O.; Bachelier, F.; Delestaing, G.; Balloy, B.; Breuer, J.; Janin, A.; Dubertret, L.; et al. Inactivation of the CDKN2A and the p53 tumour suppressor genes in external genital carcinomas and their precursors. Br. J. Dermatol. 2006, 156, 448–453. [Google Scholar] [CrossRef]
- Aidé, S.; Lattario, F.R.; Almeida, G.; Val, I.C.D.; Carvalho, M.D.G. Promoter Hypermethylation of Death-Associated Protein Kinase and p16 Genes in Vulvar Lichen Sclerosus. J. Low. Genit. Tract Dis. 2012, 16, 133–139. [Google Scholar] [CrossRef]
- Guerrero, D.; Guarch, R.; Ojer, A.; Casas, J.M.; Méndez-Meca, C.; Esteller, M.; Barba-Ramos, E.; García-Bragado, F.; Puras, A. Differential hypermethylation of genes in vulvar cancer and lichen sclerosus coexisting or not with vulvar cancer. Int. J. Cancer 2010, 128, 2853–2864. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; Lin, M.-C.; Sheets, E.E.; Muto, M.G.; Sun, D.; Crum, C.P. Allelic Imbalance in Lichen Sclerosus, Hyperplasia, and Intraepithelial Neoplasia of the Vulva. Gynecol. Oncol. 2000, 77, 171–176. [Google Scholar] [CrossRef]
- Aidé, S.; Lattario, F.R.; Almeida, G.; Val, I.C.D.; Carvalho, M.D.G. Promoter Hypermethylation Patterns of Death-Associated Protein Kinase and p16 Genes in Vulvar Lichen Sclerosus. J. Low. Genit. Tract Dis. 2010, 14, 282–286. [Google Scholar] [CrossRef]
- Guerrero-Setas, D.; Perez-Janices, N.; Ojer, A.; Blanco-Fernandez, L.; Guarch-Troyas, C.; Guarch, R. Differential gene hypermethylation in genital lichen sclerosus and cancer: A comparative study. Histopathology 2013, 63, 659–669. [Google Scholar] [CrossRef]
- Gambichler, T.; Terras, S.; Kreuter, A.; Skrygan, M. Altered global methylation and hydroxymethylation status in vulvar lichen sclerosus: Further support for epigenetic mechanisms. Br. J. Dermatol. 2014, 170, 687–693. [Google Scholar] [CrossRef]
- Ren, L.; Zhao, Y.; Huo, X.; Wu, X. MiR-155-5p promotes fibroblast cell proliferation and inhibits FOXO signaling pathway in vulvar lichen sclerosis by targeting FOXO3 and CDKN1B. Gene 2018, 653, 43–50. [Google Scholar] [CrossRef]
- Wang, L.; Yi, J.; Chen, H.; Wang, P.; Shen, Y. Level of Foxp3, DNMTs, methylation of Foxp3 promoter region, and CD4 + CD25 + CD127low regulatory T cells in vulvar lichen sclerosus. Kaohsiung J. Med. Sci. 2021, 37, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Ridley, C.; McGibbon, D.; Black, M. Lichen sclerosus et atrophicus and autoimmunity—A study of 350 women. Br. J. Dermatol. 1988, 118, 41–46. [Google Scholar] [CrossRef]
- Cooper, S.M.; Ali, I.; Baldo, M.; Wojnarowska, F. The Association of Lichen Sclerosus and Erosive Lichen Planus of the Vulva with Autoimmune Disease. Arch. Dermatol. 2008, 144, 1432–1435. [Google Scholar] [CrossRef]
- Thiers, B. High Prevalence of Thyroid Disease in Patients with Lichen Sclerosus. Yearb. Dermatol. Dermatol. Surg. 2008, 2008, 111. [Google Scholar] [CrossRef]
- Leese, G.P.; Flynn, R.V.; Jung, R.T.; Macdonald, T.M.; Murphy, M.J.; Morris, A.D. Increasing prevalence and incidence of thyroid disease in Tayside, Scotland: The Thyroid Epidemiology Audit and Research Study (TEARS). Clin. Endocrinol. 2007, 68, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.; Wojnarowska, F.; Winsey, S.; Marren, P.; Welsh, K. Lichen sclerosus premenarche: Autoimmunity and immunogenetics. Br. J. Dermatol. 2000, 142, 481–484. [Google Scholar] [CrossRef]
- Kreuter, A.; Kryvosheyeva, Y.; Terras, S.; Moritz, R.; Möllenhoff, K.; Altmeyer, P.; Scola, N.; Gambichler, T. Association of Autoimmune Diseases with Lichen Sclerosus in 532 Male and Female Patients. Acta Derm. Venereol. 2013, 93, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Bieber, A.K.; Steuer, A.B.; Melnick, L.E.; Wong, P.W.; Pomeranz, M.K. Autoimmune and dermatologic conditions associated with lichen sclerosus. J. Am. Acad. Dermatol. 2021, 85, 228–229. [Google Scholar] [CrossRef]
- Grassi, S.; Cicogna, G.T.; Magri, F.; Fortuna, M.C.; Caro, G.; Pernazza, A.; Soda, G.; Miraglia, E.; Giustini, S.; Carlesimo, M.; et al. Frontal fibrosing alopecia and genital Lichen sclerosus: Single-center experience. J. Cosmet. Dermatol. 2021, 20, 615–620. [Google Scholar] [CrossRef]
- Terlou, A.; Santegoets, L.A.; van der Meijden, W.I.; Heijmans-Antonissen, C.; Swagemakers, S.M.; van der Spek, P.J.; Ewing, P.C.; van Beurden, M.; Helmerhorst, T.J.; Blok, L.J. An Autoimmune Phenotype in Vulvar Lichen Sclerosus and Lichen Planus: A Th1 Response and High Levels of MicroRNA-155. J. Investig. Dermatol. 2012, 132, 658–666. [Google Scholar] [CrossRef]
- Farrell, A.; Dean, D.; Millard, P.; Charnock, F.; Wojnarowska, F. Cytokine alterations in lichen sclerosus: An immunohistochemical study. Br. J. Dermatol. 2006, 155, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Belz, D.; Terras, S.; Kreuter, A. Humoral and cell-mediated autoimmunity in lichen sclerosus. Br. J. Dermatol. 2013, 169, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Tchórzewski, H.; Rotsztejn, H.; Banasik, M.; Lewkowicz, P.; Głowacka, E. The involvement of immunoregulatory T cells in the pathogenesis of lichen sclerosus. Med. Sci. Monit. 2005, 11, 39–43. [Google Scholar]
- Gross, T.; Wagner, A.; Ugurel, S.; Tilgen, W.; Reinhold, U. Identification of TIA-1+ and Granzyme B+ Cytotoxic T Cells in Lichen sclerosus et atrophicus. Dermatology 2001, 202, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.; Wiechert, A.; Merkel, C.; Bieber, T.; Tüting, T. IP10/CXCL10-CXCR3 Interaction: A Potential Self-recruiting Mechanism for Cytotoxic Lymphocytes in Lichen Sclerosus et Atrophicus. Acta Derm. Venereol. 2007, 87, 112–117. [Google Scholar] [CrossRef]
- Rosenthal, A.N.; Ryan, A.; Hopster, D.; Surentheran, T.; Jacobs, I. High frequency of loss of heterozygosity in vulval intraepithelial neoplasia (VIN) is associated with invasive vulval squamous cell carcinoma (VSCC). Int. J. Cancer 2001, 94, 896–900. [Google Scholar] [CrossRef]
- Sander, C.; Ali, I.; Dean, D.; Thiele, J.; Wojnarowska, F. Oxidative stress is implicated in the pathogenesis of lichen sclerosus. Br. J. Dermatol. 2004, 151, 627–635. [Google Scholar] [CrossRef]
- Oyama, N.; Chan, I.; Neill, S.M.; Hamada, T.; South, A.P.; Wessagowit, V.; Wojnarowska, F.; D’Cruz, D.; Hughes, G.J.; Black, M.M.; et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet 2003, 362, 118–123. [Google Scholar] [CrossRef]
- Howard, A.; Dean, D.; Cooper, S.; Kirtshig, G.; Wojnarowska, F. Circulating basement membrane zone antibodies are found in lichen sclerosus of the vulva. Australas. J. Dermatol. 2004, 45, 12–15. [Google Scholar] [CrossRef]
- Edmonds, E.; Oyama, N.; Chan, I.; Francis, N.; McGrath, J.; Bunker, C. Extracellular matrix protein 1 autoantibodies in male genital lichen sclerosus. Br. J. Dermatol. 2011, 165, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Baldo, M.; Bailey, A.; Bhogal, B.; Groves, R.W.; Ogg, G.; Wojnarowska, F. T cells reactive with the NC16A domain of BP180 are present in vulval lichen sclerosus and lichen planus. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Baldo, M.; Bhogal, B.; Groves, R.W.; Powell, J.; Wojnarowska, F. Childhood vulval lichen sclerosus: Autoimmunity to the basement membrane zone protein BP180 and its relationship to autoimmunity. Clin. Exp. Dermatol. 2010, 35, 543–545. [Google Scholar] [CrossRef]
- Fujimoto, N.; Terlizzi, J.; Aho, S.; Brittingham, R.; Fertala, A.; Oyama, N.; McGrath, J.; Uitto, J. Extracellular matrix protein 1 inhibits the activity of matrix metalloproteinase 9 through high-affinity protein/protein interactions. Exp. Dermatol. 2006, 15, 300–307. [Google Scholar] [CrossRef]
- Hamada, T. Lipoid proteinosis. Clin. Exp. Dermatol. 2002, 27, 624–629. [Google Scholar] [CrossRef]
- Carli, P.; Moretti, S.; Spallanzani, A.; Berti, E.; Cattaneo, A. Fibrogenic cytokines in vulvar lichen sclerosus: An immunohisto-chemical study. J. Reprod. Med. Obstet. Gynecol. 1997, 42, 161–165. [Google Scholar]
- Farrell, A.M.; Dean, D.; Charnock, M.; Woinarowska, F. Distribution of transforming growth factor-β isoforms TGF-β1, TGF-β2 and TGF- β3 and vascular endothelial growth factor in vulvar lichen sclerosus. J. Reprod. Med. Obs. Gynecol. 2001, 46, 117–124. [Google Scholar]
- Tsunemi, Y.; Ihn, H.; Saeki, H.; Tamaki, K. A case of lichen sclerosus et atrophicus with marked fibrosis in the dermis: Analysis of fibrogenetic cytokines by reverse transcriptase-polymerase chain reaction. J. Dermatol. 2004, 31, 142–145. [Google Scholar] [CrossRef]
- Ishige, T.; Nishimura, M.; Satoh, M.; Fujimoto, M.; Fukuyo, M.; Semba, T.; Kado, S.; Tsuchida, S.; Sawai, S.; Matsushita, K.; et al. Combined Secretomics and Transcriptomics Revealed Cancer-Derived GDF15 is Involved in Diffuse-Type Gastric Cancer Progression and Fibroblast Activation. Sci. Rep. 2016, 6, 21681. [Google Scholar] [CrossRef]
- Ünal, B.; Alan, S.; Başsorgun, C.; Karakaş, A.A.; Elpek, G.; Çiftçioğlu, M.A. The divergent roles of growth differentiation factor-15 (GDF-15) in benign and malignant skin pathologies. Arch. Dermatol. Res. 2015, 307, 551–557. [Google Scholar] [CrossRef]
- Corazza, M.; Oton-Gonzalez, L.; Scuderi, V.; Rotondo, J.C.; Lanzillotti, C.; Di Mauro, G.; Tognon, M.; Martini, F.; Borghi, A. Tissue cytokine/chemokine profile in vulvar lichen sclerosus: An observational study on keratinocyte and fibroblast cultures. J. Dermatol. Sci. 2020, 100, 223–226. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.A.P.; De Almeida, M.P.; Soares, F.A.; Filho, G.L.D.A.; Takiya, C.M.; Otazu, I.B.; Nasciutti, L.E. Metalloproteinases 2 and 9 and their tissue inhibitors 1 and 2 are increased in vulvar lichen sclerosus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 161, 96–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corrêa, A.C.; Azevedo, L.; Almeida, G.; Val, I.D.; Cuzzi, T.; Takiya, C.M. Decorin and chondroitin sulfate distribution in vulvar lichen sclerosus: Correlation with distinct histopathologic stages. J. Reprod. Med. 2007, 52, 38–42. [Google Scholar]
- Godoy, C.A.; Teodoro, W.R.; Velosa, A.P.P.; Garippo, A.L.; Eher, E.M.; Parra, E.R.; Sotto, M.; Capelozzi, V.L. Unusual remodeling of the hyalinization band in vulval lichen sclerosus by type V collagen and ECM 1 protein. Clinics 2015, 70, 356–362. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, S.; Li, H.; Qin, X.; Wu, X. Expression of galectin-7 in vulvar lichen sclerosus and its effect on dermal fibroblasts. Oncol. Lett. 2018, 16, 2559–2564. [Google Scholar] [CrossRef]
- Edmonds, E.; Mavin, S.; Francis, N.; Ho-Yen, D.; Bvunker, C. Borrelia burgdorferiis not associated with genital lichen sclerosus in men. Br. J. Dermatol. 2009, 160, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Eisendle, K.; Grabner, T.; Kutzner, H.; Zelger, B. Possible Role of Borreliaburgdorferi Sensu Lato Infection in Lichen Sclerosus. Arch. Dermatol. 2008, 144, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.; Strauss, S.; Gray, J.; Wojnarowska, F. Genital Carriage of Human Papilloma Virus (HPV) DNA in Prepubertal Girls with and without Vulval Disease. Pediatr. Dermatol. 2003, 20, 191–194. [Google Scholar] [CrossRef]
- Bunker, C.B.; Shim, T.N. Male Genital Lichen Sclerosus. Indian J. Dermatol. 2015, 60, 111–117. [Google Scholar] [CrossRef]
- Aidé, S.; Lattario, F.R.; Almeida, G.; Val, I.C.D.; Carvalho, M.D.G. Epstein-Barr Virus and Human Papillomavirus Infection in Vulvar Lichen Sclerosus. J. Low. Genit. Tract Dis. 2010, 14, 319–322. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Arnold, J.D.; Malayil, L.; Hittle, L.; Mongodin, E.F.; Marathe, K.S.; Gomez-Lobo, V.; Sapkota, A.R. Potential role of the skin and gut microbiota in premenarchal vulvar lichen sclerosus: A pilot case-control study. PLoS ONE 2021, 16, e0245243. [Google Scholar] [CrossRef]
- Meylan, P.; Lang, C.; Mermoud, S.; Johannsen, A.; Norrenberg, S.; Hohl, D.; Vial, Y.; Prod’Hom, G.; Greub, G.; Kypriotou, M.; et al. Skin Colonization by Staphylococcus aureus Precedes the Clinical Diagnosis of Atopic Dermatitis in Infancy. J. Investig. Dermatol. 2017, 137, 2497–2504. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. BioEssays 2016, 38, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.; Halpern, S.; Kirby, J.; Pembroke, A. Lichen sclerosus and the Kobner phenomenon. Clin. Exp. Dermatol. 1994, 19, 262–263. [Google Scholar] [CrossRef]
- Tegner, I.V.E. Lichen Sclerosus et Atrophicus Appearing in Old Scars of Burns from Welding Sparks. Acta Derm. Venereol. 2001, 81, 211. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaky, A.M.; Aluru, P.; Keegan, P.; Greene, D.R. Development of male genital lichen sclerosus in penile reconstruction skin grafts after cancer surgery: An unreported complication. BJU Int. 2011, 109, 776–779. [Google Scholar] [CrossRef]
- Gupta, S.; Malhotra, A.; Ajith, C. Lichen sclerosus: Role of occlusion of the genital skin in the pathogenesis. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.; Dragonetti, E.; Grande, M.; Bove, P.; Sansalone, S.; Rulli, F.; Tambucci, R.; Tucci, G.; Baldi, A. Skin Phototype and Local Trauma in the Onset of Balanitis Xerotica Obliterans (BXO) in Circumcised Patients. In Vivo 2012, 26, 143–146. [Google Scholar] [PubMed]
- Tournillac, I.; Dandurand, M.; Guillot, B. Lichen sclereux bulleux radiotherapie. Ann. Derm. Venereol. 1998, 125, 121–123. [Google Scholar] [PubMed]
- De Giorgi, V.; Scarfì, F.; Silvestri, F.; Maida, P.; Venturi, F.; Trane, L.; Gori, A. Genital piercing: A warning for the risk of vulvar lichen sclerosus. Dermatol. Ther. 2021, 34, e14703. [Google Scholar] [CrossRef]
- Edmonds, E.; Hunt, S.; Hawkins, D.; Dinneen, M.; Francis, N.; Bunker, C. Clinical parameters in male genital lichen sclerosus: A case series of 329 patients. J. Eur. Acad. Dermatol. Venereol. 2011, 26, 730–737. [Google Scholar] [CrossRef]
- Friedrich, E.G.; Kalra, P.S. Serum Levels of Sex Hormones in Vulvar Lichen Sclerosus, and the Effect of Topical Testosterone. N. Engl. J. Med. 1984, 310, 488–491. [Google Scholar] [CrossRef]
- Clifton, M.; Garner, I.B.B.; Kohler, S.; Smoller, B.R. Immunohistochemical evaluation of androgen receptors in genital and extragenital lichen sclerosus: Evidence for loss of androgen receptors in lesional epidermis. J. Am. Acad. Dermatol. 1999, 41, 43–46. [Google Scholar] [CrossRef]
- Günthert, A.R.; Faber, M.; Knappe, G.; Hellriegel, S.; Emons, G. Early onset vulvar Lichen Sclerosus in premenopausal women and oral contraceptives. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 56–60. [Google Scholar] [CrossRef]
- Chan, M.P.; Zimarowski, M.J. Vulvar dermatoses: A histopathologic review and classification of 183 cases. J. Cutan. Pathol. 2015, 42, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Fancher, K.; Gardner, J.M.; Shalin, S.C. Elastophagocytosis and interstitial granulomatous infiltrate are more common in extragenital vs genital lichen sclerosus. J. Cutan. Pathol. 2020, 47, 903–912. [Google Scholar] [CrossRef]
- Dalziel, K.L.; Millard, P.R.; Wojnarowska, F. The treatment of vulval lichen sclerosus with a very potent topical steroid (clobetasol Propionate 0.05%) cream. Br. J. Dermatol. 1991, 124, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Bieber, K.; Kridin, K.; Emtenani, S.; Boch, K.; Schmidt, E.; Ludwig, R.J. Milestones in Personalized Medicine in Pemphigus and Pemphigoid. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Białynicki-Birula, R.; Schwartz, R.A.; Janniger, C. Lichen planus: An update and review. Cutis 2012, 90, 17–23. [Google Scholar]
- Zouboulis, C.C.; Benhadou, F.; Byrd, A.S.; Chandran, N.S.; Giamarellos-Bourboulis, E.J.; Fabbrocini, G.; Frew, J.W.; Fujita, H.; González-López, M.A.; Guillem, P.; et al. What causes hidradenitis suppurativa?—15 years after. Exp. Dermatol. 2020, 29, 1154–1170. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Pfützner, W. Cutaneous drug hypersensitivity: Developments and controversies. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 308–318. [Google Scholar] [CrossRef]
- Camargo, C.M.D.S.; Brotas, A.M.; Ramos-E-Silva, M.; Carneiro, S. Isomorphic phenomenon of Koebner: Facts and controversies. Clin. Dermatol. 2013, 31, 741–749. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Yadav, T.C.; Khera, H.K.; Mishra, P.; Raghuwanshi, N.; Pruthi, V.; Prasad, R. Insights into interplay of immunopathophysiological events and molecular mechanistic cascades in psoriasis and its associated comorbidities. J. Autoimmun. 2021, 118, 102614. [Google Scholar] [CrossRef]
- Gunter, N.V.; Yap, B.J.M.; Chua, C.L.L.; Yap, W.H. Combining Understanding of Immunological Mechanisms and Genetic Variants Toward Development of Personalized Medicine for Psoriasis Patients. Front. Genet. 2019, 10, 395. [Google Scholar] [CrossRef]
- Ogawa, K.; Okada, Y. The current landscape of psoriasis genetics in 2020. J. Dermatol. Sci. 2020, 99, 2–8. [Google Scholar] [CrossRef]
- Vellaichamy, G.; Dimitrion, P.; Zhou, L.; Ozog, D.; Lim, H.W.; Liao, W.; Hamzavi, I.H.; Mi, Q.-S. Insights from γ-Secretase: Functional Genetics of Hidradenitis Suppurativa. J. Investig. Dermatol. 2021, 141, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Join-Lambert, O.; Sabat, R. Aetiology and pathogenesis of hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Koneczny, I.; Yilmaz, V.; Lazaridis, K.; Tzartos, J.; Lenz, T.L.; Tzartos, S.; Tüzün, E.; Leypoldt, F. Common Denominators in the Immunobiology of IgG4 Autoimmune Diseases: What Do Glomerulonephritis, Pemphigus Vulgaris, Myasthenia Gravis, Thrombotic Thrombocytopenic Purpura and Autoimmune Encephalitis Have in Common? Front. Immunol. 2021, 11. [Google Scholar] [CrossRef]
- Olbrich, M.; Künstner, A.; Witte, M.; Busch, H.; Fähnrich, A. Genetics and Omics Analysis of Autoimmune Skin Blistering Diseases. Front. Immunol. 2019, 10, 2327. [Google Scholar] [CrossRef]
- Spritz, R.A. Six Decades of Vitiligo Genetics: Genome-Wide Studies Provide Insights into Autoimmune Pathogenesis. J. Investig. Dermatol. 2012, 132, 268–273. [Google Scholar] [CrossRef]
- Wolf, B.; Horn, L.-C.; Höckel, M. Anogenital lichen sclerosus: Change of tissue position as pathogenetic factor. Gynecol. Oncol. Rep. 2017, 20, 73–74. [Google Scholar] [CrossRef]
- Kaushik, A.; Mahajan, R.; De, D.; Handa, S. Paediatric morphoea: A holistic review. Part 1: Epidemiology, aetiopathogenesis and clinical classification. Clin. Exp. Dermatol. 2020, 45, 673–678. [Google Scholar] [CrossRef]
- Torok, K.S.; Li, S.C.; Jacobe, H.M.; Taber, S.F.; Stevens, A.M.; Zulian, F.; Lu, T.T. Immunopathogenesis of Pediatric Localized Scleroderma. Front. Immunol. 2019, 10, 908. [Google Scholar] [CrossRef]
- Lutz, V.; Ès, C.F.; Bessis, D.; Cosnes, A.; Kluger, N.; Godet, J.A.; Sauleau, E.; Lipsker, D. High Frequency of Genital Lichen Sclerosus in a Prospective Series of 76 Patients with Morphea. Arch. Dermatol. 2012, 148, 24–28. [Google Scholar] [CrossRef]
- Prasad, S.; Black, S.M.; Zhu, J.L.; Sharma, S.; Jacobe, H. Morphea patients with mucocutaneous involvement: A cross-sectional study from the Morphea in Adults and Children (MAC) cohort. J. Am. Acad. Dermatol. 2021, 85, 114–120. [Google Scholar] [CrossRef]
- Chi, C.-C.; Kirtschig, G.; Baldo, M.; Brackenbury, F.; Lewis, F.; Wojnarowska, F. Topical interventions for genital lichen sclerosus. Cochrane Database Syst. Rev. 2011, 2011. [Google Scholar] [CrossRef]
- Lewis, F.; Tatnall, F.; Velangi, S.; Bunker, C.; Kumar, A.; Brackenbury, F.; Mustapa, M.M.; Exton, L.; McHenry, P.; Leslie, T.; et al. British Association of Dermatologists guidelines for the management of lichen sclerosus, 2018. Br. J. Dermatol. 2018, 178, 839–853. [Google Scholar] [CrossRef]
- Borghi, A.; Corazza, M. Novel Therapeutic Approaches and Targets for Treatment of Vulvar Lichen Sclerosus. Curr. Pharm. Biotechnol. 2020, 22, 99–114. [Google Scholar] [CrossRef]
- Carli, P.; Cattaneo, A.; Giannotti, B. Clobetasol propionate 0.05% cream in the treatment of vulvar lichen sclerosus: Effect on the immunohistological profile. Br. J. Dermatol. 1992, 127, 542–543. [Google Scholar] [CrossRef]
- Dalziel, K.L.; Wojnarowska, F. Long-term control of vulval lichen sclerosus after treatment with a potent topical steroid cream. J. Reprod. Med. 1993, 38, 25–27. [Google Scholar]
- Lorenz, B.; Kaufman, R.H.; Kutzner, S.K. Lichen sclerosus. Therapy with clobetasol propionate. J. Reprod. Med. 1998, 43, 790–794. [Google Scholar]
- Bracco, G.L.; Carli, P.; Sonni, L.; Maestrini, G.; De Marco, A.; Taddei, G.L.; Cattaneo, A. Clinical and histologic effects of topical treatments of vulval lichen sclerosus. A critical evaluation. J. Reprod. Med. 1993, 38, 37–40. [Google Scholar]
- Bornstein, J.; Heifetz, S.; Kellner, Y.; Stolar, Z.; Abramovici, H. Clobetasol dipropionate 0.05% versus testosterone propionate 2% topical application for severe vulvar lichen sclerosus. Am. J. Obstet. Gynecol. 1998, 178, 80–84. [Google Scholar] [CrossRef]
- Goldstein, A.T.; Creasey, A.; Pfau, R.; Phillips, D.; Burrows, L.J. A double-blind, randomized controlled trial of clobetasol versus pimecrolimus in patients with vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2011, 64, e99–e104. [Google Scholar] [CrossRef]
- Funaro, D.; Lovett, A.; Leroux, N.; Powell, J. A double-blind, randomized prospective study evaluating topical clobetasol propionate 0.05% versus topical tacrolimus 0.1% in patients with vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2014, 71, 84–91. [Google Scholar] [CrossRef]
- Terras, S.; Gambichler, T.; Moritz, R.K.C.; Stücker, M.; Kreuter, A. UV-A1 Phototherapy vs Clobetasol Propionate, 0.05%, in the Treatment of Vulvar Lichen Sclerosus. JAMA Dermatol. 2014, 150, 621. [Google Scholar] [CrossRef] [PubMed]
- Virgili, A.; Borghi, A.; Toni, G.; Minghetti, S.; Corazza, M. First randomized trial on clobetasol propionate and mometasone furoate in the treatment of vulvar lichen sclerosus: Results of efficacy and tolerability. Br. J. Dermatol. 2014, 171, 388–396. [Google Scholar] [CrossRef]
- Shi, L.; Miao, F.; Zhang, L.; Zhang, G.; Wang, P.; Ji, J.; Huang, Z.; Wang, H.; Wang, X. Comparison of 5-Aminolevulinic Acid Photodynamic Therapy and Clobetasol Propionate in Treatment of Vulvar Lichen Sclerosus. Acta Derm. Venereol. 2016, 96, 684–688. [Google Scholar] [CrossRef]
- Cattaneo, A.; De Magnis, A.; Botti, E.; Sonni, L.; Carli, P.; Taddei, G.L. Topical mometasone furoate for vulvar lichen sclerosus. J. Reprod. Med. 2003, 48, 444–448. [Google Scholar] [PubMed]
- Virgili, A.; Borghi, A.; Minghetti, S.; Corazza, M. Mometasone fuoroate 0.1% ointment in the treatment of vulvar lichen sclerosus: A study of efficacy and safety on a large cohort of patients. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Murina, F.; Rehman, S.; Di Francesco, S.; Mantegazza, V.; Felice, R.; Bianco, V. Vulvar Lichen Sclerosus. J. Low. Genit. Tract Dis. 2015, 19, 149–151. [Google Scholar] [CrossRef]
- Borghi, A.; Corazza, M.; Minghetti, S.; Toni, G.; Virgili, A. Continuous vs. tapering application of the potent topical corticosteroid mometasone furoate in the treatment of vulvar lichen sclerosus: Results of a randomized trial. Br. J. Dermatol. 2015, 173, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Corazza, M.; Virgili, A.; Toni, G.; Borghi, A. Mometasone furoate in the treatment of vulvar lichen sclerosus: Could its formulation influence efficacy, tolerability and adherence to treatment? J. Dermatol. Treat. 2017, 29, 305–309. [Google Scholar] [CrossRef]
- Stücker, M.; Grape, J.; Bechara, F.G.; Hoffmann, K.; Altmeyer, P. The Outcome after Cryosurgery and Intralesional Steroid Injection in Vulvar Lichen sclerosus Corresponds to Preoperative Histopathological Findings. Dermatology 2005, 210, 218–222. [Google Scholar] [CrossRef]
- Baggish, M.S.; Ventolini, G. Lichen Sclerosus: Subdermal Steroid Injection Therapy. A Large, Long-Term Follow-Up Study. J. Gynecol. Surg. 2006, 22, 137–141. [Google Scholar] [CrossRef]
- Diakomanolis, E.S.; Haidopoulos, D.; Syndos, M.; Rodolakis, A.; Stefanidis, K.; Chatzipapas, J.; Michalas, S. Vulvar lichen sclerosus in postmenopausal women: A comparative study for treating advanced disease with clobetasol propionate 0.05%. Eur. J. Gynaecol. Oncol. 2002, 23, 519–522. [Google Scholar]
- Bradford, J.; Fischer, G. Long-term management of vulval lichen sclerosus in adult women. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 148–152. [Google Scholar] [CrossRef]
- Lefevre, C.; Hoffstetter, S.; Meyer, S.; Gavard, J. Management of Lichen Sclerosus With Triamcinolone Ointment. J. Low. Genit. Tract Dis. 2011, 15, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Virgili, A.; Minghetti, S.; Borghi, A.; Corazza, M. Proactive maintenance therapy with a topical corticosteroid for vulvar lichen sclerosus: Preliminary results of a randomized study. Br. J. Dermatol. 2013, 168, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Corazza, M.; Borghi, A.; Minghetti, S.; Toni, G.; Virgili, A. Clobetasol propionate vs. mometasone furoate in 1-year proactive maintenance therapy of vulvar lichen sclerosus: Results from a comparative trial. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Hengge, U.; Krause, W.; Hofmann, H.; Stadler, R.; Gross, G.; Meurer, M.; Brinkmeier, T.; Frosch, P.; Moll, I.; Fritsch, P.; et al. Multicentre, phase II trial on the safety and efficacy of topical tacrolimus ointment for the treatment of lichen sclerosus. Br. J. Dermatol. 2006, 155, 1021–1028. [Google Scholar] [CrossRef]
- Virgili, A.; Lauriola, M.M.; Mantovani, L.; Corazza, M. Vulvar Lichen Sclerosus: 11 Women Treated with Tacrolimus 0.1% Ointment. Acta Derm. Venereol. 2007, 87, 69–72. [Google Scholar] [CrossRef]
- Sotiriou, E.; Apalla, Z.; Patsatsi, A.; Panagiotidou, D. Topical tacrolimus for recalcitrant vulvar lichen sclerosus. Eur. J. Dermatol. EJD 2009, 19, 515–516. [Google Scholar] [CrossRef]
- Kim, G.-W.; Park, H.-J.; Kim, H.-S.; Kim, S.-H.; Ko, H.-C.; Kim, B.-S.; Kim, M.-B. Topical tacrolimus ointment for the treatment of lichen sclerosus, comparing genital and extragenital involvement. J. Dermatol. 2011, 39, 145–150. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Wang, H.; Luo, X. Low-concentration Topical Tacrolimus for the Treatment of Anogenital Lichen Sclerosus in Childhood: Maintenance Treatment to Reduce Recurrence. J. Pediatr. Adolesc. Gynecol. 2013, 26, 239–242. [Google Scholar] [CrossRef]
- Mazzilli, S.; Diluvio, L.; Di Prete, M.; Rossi, P.; Orlandi, A.; Bianchi, L.; Campione, E. Tacrolimus 0.03% ointment for treatment of paediatric lichen sclerosus: A case series and literature review. J. Int. Med Res. 2018, 46, 3724–3728. [Google Scholar] [CrossRef] [PubMed]
- Oskay, T.; Sezer, H.K.; Genç, C.; Kutluay, L. Pimecrolimus 1% cream in the treatment of vulvar lichen sclerosus in postmenopausal women. Int. J. Dermatol. 2007, 46, 527–532. [Google Scholar] [CrossRef]
- Nissi, R.; Eriksen, H.; Risteli, J.; Niemimaa, M. Pimecrolimus Cream 1% in the Treatment of Lichen Sclerosus. Gynecol. Obstet. Investig. 2006, 63, 151–154. [Google Scholar] [CrossRef]
- Nissi, R.; Kotila, V.; Knuuti, E.; Väre, P.; Kauppila, S. Altered p53 and Bcl-2 expression in keratinocytes of vulvar lichen sclerosus during pimecrolimus treatment. Br. J. Dermatol. 2009, 161, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.; Gottlieb, A.; Pariser, D.; Caro, I.; Stewart, D.; Scott, G.; Abrams, K. A randomized study of the safety, absorption and efficacy of pimecrolimus cream 1% applied twice or four times daily in patients with atopic dermatitis. J. Dermatol. Treat. 2005, 16, 142–148. [Google Scholar] [CrossRef]
- Goldstein, A.; Marinoff, S.C.; Christopher, K. Pimecrolimus for the treatment of vulvar lichen sclerosus: A report of 4 cases. J. Reprod. Med. 2004, 49, 778–780. [Google Scholar] [PubMed]
- Boms, S.; Gambichler, T.; Freitag, M.; Altmeyer, P.; Kreuter, A. Pimecrolimus 1% cream for anogenital lichen sclerosus in childhood. BMC Dermatol. 2004, 4, 14. [Google Scholar] [CrossRef]
- Goldstein, A.T.; Marinoff, S.C.; Christopher, K. Pimecrolimus for the treatment of vulvar lichen sclerosus in a premenarchal girl. J. Pediatr. Adolesc. Gynecol. 2004, 17, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Frieder, J.; Kivelevitch, D.; Menter, A. Calcipotriene betamethasone dipropionate aerosol foam in the treatment of plaque psoriasis: A review of the literature. Ther. Deliv. 2017, 8, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Saraswat, A.; Kumar, B. Treatment of genital lichen sclerosus with topical calcipotriol. Int. J. STD AIDS 2005, 16, 772–774. [Google Scholar] [CrossRef]
- Origoni, M.; Ferrari, D.; Rossi, M.; Gandini, F.; Sideri, M.; Ferrari, A. Topical oxatomide: An alternative approach for the treatment of vulvar lichen sclerosus. Int. J. Gynecol. Obstet. 1996, 55, 259–264. [Google Scholar] [CrossRef]
- Goldstein, A.; Burrows, L.; Belkin, Z.; Pfau, R.; Bremmer, M.; Goldfinger, C.; Dreher, F. Safety and Efficacy of Human Fibroblast Lysate Cream for Vulvar Lichen Sclerosus: A Randomized Placebo-Controlled Trial. Acta Derm. Venereol. 2014, 95, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Başkan, E.B.; Turan, H.; Tunali, S.; Toker, S.C.; Saricaoglu, H. Open-label trial of cyclosporine for vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2007, 57, 276–278. [Google Scholar] [CrossRef]
- Nayeemuddin, F.; Yates, V.M. Lichen sclerosus et atrophicus responding to methotrexate. Clin. Exp. Dermatol. 2008, 33, 651–652. [Google Scholar] [CrossRef]
- Kreuter, A.; Tigges, C.; Gaifullina, R.; Kirschke, J.; Altmeyer, P.; Gambichler, T. Pulsed High-Dose Corticosteroids Combined With Low-Dose Methotrexate Treatment in Patients With Refractory Generalized Extragenital Lichen Sclerosus. Arch. Dermatol. 2009, 145, 1303–1308. [Google Scholar] [CrossRef]
- Li, J.; Zheng, W.; Tang, J.; Yang, B. Lichen sclerosus successfully treated with baricitinib plus psoralen and ultraviolet A. Dermatol. Ther. 2021, e14896. [Google Scholar] [CrossRef]
- Fritsch, P.O. Retinoids in psoriasis and disorders of keratinization. J. Am. Acad. Dermatol. 1992, 27, S8–S14. [Google Scholar] [CrossRef]
- Griffiths, C.; Russman, A.N.; Majmudar, G.; Singer, R.S.; Hamilton, T.A.; Voorhees, J.J. Restoration of Collagen Formation in Photodamaged Human Skin by Tretinoin (Retinoic Acid). N. Engl. J. Med. 1993, 329, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Bikowski, J.B. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J. Drugs DERMATOL. JDD 2005, 4, 41–47. [Google Scholar] [PubMed]
- DiGiovanna, J.J. Retinoid chemoprevention in the high-risk patient. J. Am. Acad. Dermatol. 1998, 39, S82–S85. [Google Scholar] [CrossRef]
- Virgili, A.; Corazza, M.; Bianchi, A.; Mollica, G.; Califano, A. Open study of topical 0.025% tretinoin in the treatment of vulvar lichen sclerosus. One year of therapy. J. Reprod. Med. 1995, 40, 614–618. [Google Scholar]
- Borghi, A.; Corazza, M.; Minghetti, S.; Virgili, A. Topical tretinoin in the treatment of vulvar lichen sclerosus: An advisable option? Eur. J. Dermatol. EJD 2015, 25, 404–409. [Google Scholar] [CrossRef]
- Markowska, J.; Wiese, E. Dystrophy of the vulva locally treated with 13-cis retinoic acid. Neoplasma 1992, 39, 133–135. [Google Scholar] [PubMed]
- Kaya, G.; Saurat, J.-H. Restored epidermal CD44 expression in lichen sclerosus et atrophicus and clinical improvement with topical application of retinaldehyde. Br. J. Dermatol. 2005, 152, 570–572. [Google Scholar] [CrossRef]
- Borghi, A.; Minghetti, S.; Toni, G.; Virgili, A.; Corazza, M. Combined therapy in vulvar lichen sclerosus: Does topical tretinoin improve the efficacy of mometasone furoate? J. Dermatol. Treat. 2017, 28, 559–563. [Google Scholar] [CrossRef]
- Corazza, M.; Maietti, E.; Toni, G.; Virgili, A.; Borghi, A. Combining topical tretinoin with mometasone furoate in the treatment of vulvar lichen sclerosus: Results of dermoscopic assessment. Dermatol. Ther. 2018, 31, e12735. [Google Scholar] [CrossRef]
- Ernst, E. Avocado?soybean unsaponifiables (ASU) for osteoarthritis ? a systematic review. Clin. Rheumatol. 2003, 22, 285–288. [Google Scholar] [CrossRef]
- Simental-Mendía, M.; Sánchez-García, A.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Osuna-Garate, J.; Peña-Martínez, V.M.; Simental-Mendía, L.E. Efficacy and safety of avocado-soybean unsaponifiables for the treatment of hip and knee osteoarthritis: A systematic review and meta-analysis of randomized placebo-controlled trials. Int. J. Rheum. Dis. 2019, 22, 1607–1615. [Google Scholar] [CrossRef]
- Lamaud, E.; Wepierre, J.; Robert, A.M. Biochemical effects of unsaponifiable lipidic components of avocado and soya bean administered percutaneously on the connective tissue components of hairless rat skin. Int. J. Cosmet. Sci. 1979, 1, 213–219. [Google Scholar] [CrossRef]
- Lamberton, J.N. Une thérapeutique “anti-sclérose” de la sclérodermie: L’insaponifiable des huiles d’avocat et de soja. Clin-quante applications cliniques du traitement de H. Thiers. Presse Med. 1970, 78, 1235–1236. Available online: https://europepmc.org/article/med/5463415 (accessed on 17 April 2021).
- Werman, M.J.; Mokady, S.; Ntmni, M.E.; Neeman, I. The Effect of Various Avocado Oils on Skin Collagen Metabolism. Connect. Tissue Res. 1991, 26, 1–10. [Google Scholar] [CrossRef]
- Salehi, B.; Rescigno, A.; Dettori, T.; Calina, D.; Docea, A.O.; Singh, L.; Cebeci, F.; Özçelik, B.; Bhia, M.; Beirami, A.D.; et al. Avocado–Soybean Unsaponifiables: A Panoply of Potentialities to Be Exploited. Biomolecules 2020, 10, 130. [Google Scholar] [CrossRef]
- Borghi, A.; Corazza, M.; Minghetti, S.; Toni, G.; Virgili, A. Avocado and soybean extracts as active principles in the treatment of mild-to-moderate vulvar lichen sclerosus: Results of efficacy and tolerability. J. Eur. Acad. Dermatol. Venereol. 2014, 29, 1225–1230. [Google Scholar] [CrossRef]
- Virgili, A.; Minghetti, S.; Borghi, A.; Corazza, M. Long-term maintenance therapy for vulvar lichen sclerosus: The results of a randomized study comparing topical vitamin E with an emollient. Eur. J. Dermatol. EJD 2013, 23, 189–194. [Google Scholar] [CrossRef]
- Simonart, T.; Lahaye, M.; Simonart, J.-M. Vulvar lichen sclerosus. Menopause 2008, 15, 74–77. [Google Scholar] [CrossRef]
- Gambichler, T.; Terras, S.; Kreuter, A. Treatment regimens, protocols, dosage, and indications for UVA1 phototherapy: Facts and controversies. Clin. Dermatol. 2013, 31, 438–454. [Google Scholar] [CrossRef]
- Kreuter, A.; Gambichler, T.; Avermaete, A.; Happe, M.; Bacharach-Buhles, M.; Hoffmann, K.; Jansen, T.; Altmeyer, P.; Von Kobyletzki, G. Low-dose ultraviolet A1 phototherapy for extragenital lichen sclerosus: Results of a preliminary study. J. Am. Acad. Dermatol. 2002, 46, 251–255. [Google Scholar] [CrossRef]
- Beattie, P.E.; Dawe, R.; Ferguson, J.; Ibbotson, S. UVA1 phototherapy for genital lichen sclerosus. Clin. Exp. Dermatol. 2006, 31, 343–347. [Google Scholar] [CrossRef]
- Reichrath, J.; Reinhold, U.; Tilgen, W. Treatment of Genito-Anal Lesions in Inflammatory Skin Diseases with PUVA Cream Photochemotherapy: An Open Pilot Study in 12 Patients. Dermatology 2002, 205, 245–248. [Google Scholar] [CrossRef]
- Garrido-Colmenero, C.; Martínez-Peinado, C.M.; Galán-Gutiérrez, M.; Barranco-Millán, V.; Ruiz-Villaverde, R. Successful response of vulvar lichen sclerosus with NB-UVB. Dermatol. Ther. 2021, 34, e14801. [Google Scholar] [CrossRef]
- Prodromidou, A.; Chatziioannou, E.; Daskalakis, G.; Stergios, K.; Pergialiotis, V. Photodynamic Therapy for Vulvar Lichen Sclerosus—A Systematic Review. J. Low. Genit. Tract Dis. 2018, 22, 58–65. [Google Scholar] [CrossRef]
- Hillemanns, P.; Untch, M.; Pröve, F.; Baumgartner, R.; Hillemanns, M.; Korell, M. Photodynamic therapy of vulvar lichen sclerosus with 5-aminolevulinic acid. Obstet. Gynecol. 1999, 93, 71–74. [Google Scholar] [CrossRef]
- Sotiriou, E.; Apalla, Z.; Patsatsi, A.; Panagiotidou, D. Recalcitrant vulvar lichen sclerosis treated with aminolevulinic acid-photodynamic therapy: A report of five cases. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1398–1399. [Google Scholar] [CrossRef]
- Olejek, A.; Stęplewska, K.; Gabriel, A.; Kozak-Darmas, I.; Jarek, A.; Kellas-Ślęczka, S.; Bydliński, F.; Sieroń-Stołtny, K.; Horak, S.; Chełmicki, A.; et al. Efficacy of Photodynamic Therapy in Vulvar Lichen Sclerosus Treatment Based on Immunohistochemical Analysis of CD34, CD44, Myelin Basic Protein, and Ki67 Antibodies. Int. J. Gynecol. Cancer 2010, 20, 879–887. [Google Scholar] [CrossRef]
- Imbernón-Moya, A.; Martínez-Pérez, M.; Churruca-Grijelmo, M.; Lobato-Berezo, A.; Vargas-Laguna, E.; Fernández-Cogolludo, E.; Aguilar-Martínez, A.; Gallego-Valdés, M. Ángel Photodynamic therapy as a therapeutic alternative in vulvar lichen sclerosus: Series of 8 cases. Photodermatol. Photoimmunol. Photomed. 2016, 32, 307–310. [Google Scholar] [CrossRef]
- Osiecka, B.; Jurczyszyn, K.; Nockowski, P.; Murawski, M.; Ziółkowski, P. Photodynamic therapy with green light for the treatment of vulvar lichen sclerosus–Preliminary results. Photodiagnosis Photodyn. Ther. 2017, 17, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Maździarz, A.; Osuch, B.; Kowalska, M.; Nalewczyńska, A.; Śpiewankiewicz, B. Photodynamic therapy in the treatment of vulvar lichen sclerosus. Photodiagnosis Photodyn. Ther. 2017, 19, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, D.; Shi, L.; Gu, Y.; Xu, Y. 5-ALA-photodynamic therapy in refractory vulvar lichen sclerosus et atrophicus. Int. J. Clin. Exp. Pathol. 2020, 13, 3100–3110. [Google Scholar] [PubMed]
- Li, Z.; Wang, Y.; Wang, J.; Li, S.; Xiao, Z.; Feng, Y.; Gu, J.; Li, J.; Peng, X.; Li, C.; et al. Evaluation of the efficacy of 5-aminolevulinic acid photodynamic therapy for the treatment of vulvar lichen sclerosus. Photodiagnosis Photodyn. Ther. 2020, 29, 101596. [Google Scholar] [CrossRef]
- Liu, J.; Hao, J.; Wang, Y.; Liu, Y.; Xu, T. Clinical and Dermoscopic Assessment of Vulvar Lichen Sclerosus After 5-Aminolevulinic Acid Photodynamic Therapy: A Prospective Study. Photodiagnosis Photodyn. Ther. 2021, 33, 102109. [Google Scholar] [CrossRef]
- Declercq, A.; Güvenç, C.; De Haes, P. Proposition of standardized protocol for photodynamic therapy for vulvar lichen sclerosus. J. Dermatol. Treat. 2020, 1–9. [Google Scholar] [CrossRef]
- Preti, M.; Vieira-Baptista, P.; Digesu, G.A.; Bretschneider, C.E.; Damaser, M.; Demirkesen, O.; Heller, D.S.; Mangir, N.; Marchitelli, C.; Mourad, S.; et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: An ICS/ISSVD best practice consensus document. Neurourol. Urodyn. 2019, 38, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Ogrinc, U.B.; Senčar, S.; Luzar, B.; Lukanović, A. Efficacy of Non-ablative Laser Therapy for Lichen Sclerosus: A Randomized Controlled Trial. J. Obstet. Gynaecol. Can. 2019, 41, 1717–1725. [Google Scholar] [CrossRef]
- Stuart, G.C.; Nation, J.G.; Malliah, V.S.; Robertson, D.I. Laser therapy of vulvar lichen sclerosus et atrophicus. Can. J. Surg. 1991, 34, 469–470. [Google Scholar]
- Kartamaa, M.; Reitamo, S. Treatment of lichen sclerosus with carbon dioxide laser vaporization. Br. J. Dermatol. 1997, 136, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.M.; Lane, J.E.; Ratz, J.L. Successful Carbon Dioxide Laser Therapy for Refractory Anogenital Lichen Sclerosus. Dermatol. Surg. 2004, 30, 1148–1151. [Google Scholar] [CrossRef]
- Lee, A.; Lim, A.; Fischer, G. Fractional carbon dioxide laser in recalcitrant vulval lichen sclerosus. Australas. J. Dermatol. 2015, 57, 39–43. [Google Scholar] [CrossRef]
- Balchander, D.; Nyirjesy, P. Fractionated CO2 Laser as Therapy in Recalcitrant Lichen Sclerosus. J. Low. Genit. Tract Dis. 2020, 24, 225–228. [Google Scholar] [CrossRef]
- Pagano, T.; Conforti, A.; Buonfantino, C.; Schettini, F.; Vallone, R.; Gallo, A.; Avino, L.; Alviggi, C.; De Placido, G.; Sopracordevole, F. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: A prospective longitudinal study. Menopause 2020, 27, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Kamilos, M.F.; Aguiar, L.M.; Batista, V.H.; Roa, C.L.; Aguiar, F.N.; Soares, J.M.; Baracat, E.C. Microablative fractional radiofrequency as a therapeutic option for vulvar lichen sclerosus: A pilot study. Clinics 2021, 76, e2567. [Google Scholar] [CrossRef] [PubMed]
- Li, H.O.-Y.; Bailey, A.M.J.; Tan, M.G.; Dover, J.S. Lasers as an adjuvant for vulvar lichen sclerosus: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef]
- Griffin, M.M.F.; Almadori, M.A.; Butler, P. Use of Lipotransfer in Scleroderma. Aesthetic Surg. J. 2017, 37, S33–S37. [Google Scholar] [CrossRef]
- Abu-Ghname, A.; Perdanasari, A.T.; Reece, E.M. Principles and Applications of Fat Grafting in Plastic Surgery. Semin. Plast. Surg. 2019, 33, 147–154. [Google Scholar] [CrossRef]
- Yotsu, R.R.; Hagiwara, S.; Okochi, H.; Tamaki, T. Case series of patients with chronic foot ulcers treated with autologous platelet-rich plasma. J. Dermatol. 2015, 42, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Casabona, F.; Priano, V.; Vallerino, V.; Cogliandro, A.; Lavagnino, G. New Surgical Approach to Lichen Sclerosus of the Vulva: The Role of Adipose-Derived Mesenchymal Cells and Platelet-Rich Plasma in Tissue Regeneration. Plast. Reconstr. Surg. 2010, 126, e210–e211. [Google Scholar] [CrossRef]
- Boero, V.; Brambilla, M.; Sipio, E.; Liverani, C.; Di Martino, M.; Agnoli, B.; Libutti, G.; Cribiù, F.; Del Gobbo, A.; Ragni, E.; et al. Vulvar lichen sclerosus: A new regenerative approach through fat grafting. Gynecol. Oncol. 2015, 139, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.T.; King, M.; Runels, C.; Gloth, M.; Pfau, R. Intradermal injection of autologous platelet-rich plasma for the treat-ment of vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2017, 76, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.; Pranteda, G.; Chichierchia, G.; Paolino, G.; Latini, A.; Orsini, D.; Cristaudo, A.; Foddai, M.; Migliano, E.; Morrone, A. The use of PRP (platelet-rich plasma) in patients affected by genital lichen sclerosus: Clinical analysis and results. J. Eur. Acad. Dermatol. Venereol. 2018, 33, e58–e59. [Google Scholar] [CrossRef]
- Eshtiaghi, P.; Sadownik, L.A. Fact or Fiction? Adipose-Derived Stem Cells and Platelet-Rich Plasma for the Treatment of Vulvar Lichen Sclerosus. J. Low. Genit. Tract Dis. 2019, 23, 65–70. [Google Scholar] [CrossRef]
- Tedesco, M.; Garelli, V.; Bellei, B.; Sperduti, I.; Chichierchia, G.; Latini, A.; Foddai, M.; Bertozzi, E.; Bonadies, A.; Pallara, T.; et al. Platelet-rich plasma for genital lichen sclerosus: Analysis and results of 94 patients. Are there gender-related differences in symptoms and therapeutic response to PRP? J. Dermatol. Treat. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.T.; Mitchell, L.; Govind, V.; Heller, D. A randomized double-blind placebo-controlled trial of autologous platelet-rich plasma intradermal injections for the treatment of vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2019, 80, 1788–1789. [Google Scholar] [CrossRef]
- Tedesco, M.; Bellei, B.; Garelli, V.; Caputo, S.; Latini, A.; Giuliani, M.; Cota, C.; Chichierchia, G.; Romani, C.; Foddai, M.L.; et al. Adipose tissue stromal vascular fraction and adipose tissue stromal vascular fraction plus platelet-rich plasma grafting: New regenerative perspectives in genital lichen sclerosus. Dermatol. Ther. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Gkouvi, A.; Gregoriou, S. Vulvar Lichen Sclerosus. Dermatol. Surg. 2020, 46. [Google Scholar] [CrossRef]
- Ohara, H.; Ichikawa, S.; Matsumoto, H.; Akiyama, M.; Fujimoto, N.; Kobayashi, T.; Tajima, S. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol. 2010, 37, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Bousema, M.; Romppanen, U.; Geiger, J.-M.; Baudin, M.; Vähä-Eskeli, K.; Vartiainen, J.; Vuopala, S. Acitretin in the treatment of severe lichen sclerosus et atrophicus of the vulva: A double-blind, placebo-controlled study. J. Am. Acad. Dermatol. 1994, 30, 225–231. [Google Scholar] [CrossRef]
- Mørk, N.J.; Jensen, P.; Hoel, P.S. Vulval lichen sclerosus et atrophicus treated with etretinate (Tigason). Acta Derm Venereol 1986, 66, 363–365. [Google Scholar]
- Romppanen, U.; Tuimala, R.; Ellmen, J.; Lauslahti, K. Treatment of dystrophic changes of the vulva with etretinate or placebo. Curr. Ther. Res. 1987, 42, 211–218. [Google Scholar]
- Buxton, P.K.; Priestley, G.C. Para-aminobenzoate in lichen sclerosus et atrophicus. J. Dermatol. Treat. 1990, 1, 255–256. [Google Scholar] [CrossRef]
- Van Cranenburgh, O.; Nijland, S.; Lindeboom, R.; De Korte, J.; De Rie, M.; Ter Stege, J.; Prinsen, C. Patients with lichen sclerosus experience moderate satisfaction with treatment and impairment of quality of life: Results of a cross-sectional study. Br. J. Dermatol. 2017, 176, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Borghi, A.; Virgili, A.; Minghetti, S.; Toni, G.; Corazza, M. Clearance in vulvar lichen sclerosus: A realistic treatment endpoint or a chimera? J. Eur. Acad. Dermatol. Venereol. 2017, 32, 96–101. [Google Scholar] [CrossRef]
- Corazza, M.; Toni, G.; Valpiani, G.; Morotti, C.; Borghi, A. Does longer duration of corticosteroid treatment improve clearance in vulvar lichen sclerosus? Results from a single centre, comparative, open label study. Dermatol. Ther. 2021, 9. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corazza, M.; Schettini, N.; Zedde, P.; Borghi, A. Vulvar Lichen Sclerosus from Pathophysiology to Therapeutic Approaches: Evidence and Prospects. Biomedicines 2021, 9, 950. https://doi.org/10.3390/biomedicines9080950
Corazza M, Schettini N, Zedde P, Borghi A. Vulvar Lichen Sclerosus from Pathophysiology to Therapeutic Approaches: Evidence and Prospects. Biomedicines. 2021; 9(8):950. https://doi.org/10.3390/biomedicines9080950
Chicago/Turabian StyleCorazza, Monica, Natale Schettini, Pierantonia Zedde, and Alessandro Borghi. 2021. "Vulvar Lichen Sclerosus from Pathophysiology to Therapeutic Approaches: Evidence and Prospects" Biomedicines 9, no. 8: 950. https://doi.org/10.3390/biomedicines9080950
APA StyleCorazza, M., Schettini, N., Zedde, P., & Borghi, A. (2021). Vulvar Lichen Sclerosus from Pathophysiology to Therapeutic Approaches: Evidence and Prospects. Biomedicines, 9(8), 950. https://doi.org/10.3390/biomedicines9080950






