Adipose Tissue Hypoxia Correlates with Adipokine Hypomethylation and Vascular Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Participants
2.2. Body Composition and Cardiometabolic Measurements
2.3. Brachial Artery Flow-Mediated Dilation (FMD)
2.4. Serum Measurements of Inflammatory Biomarkers
2.5. Sample Acquisition
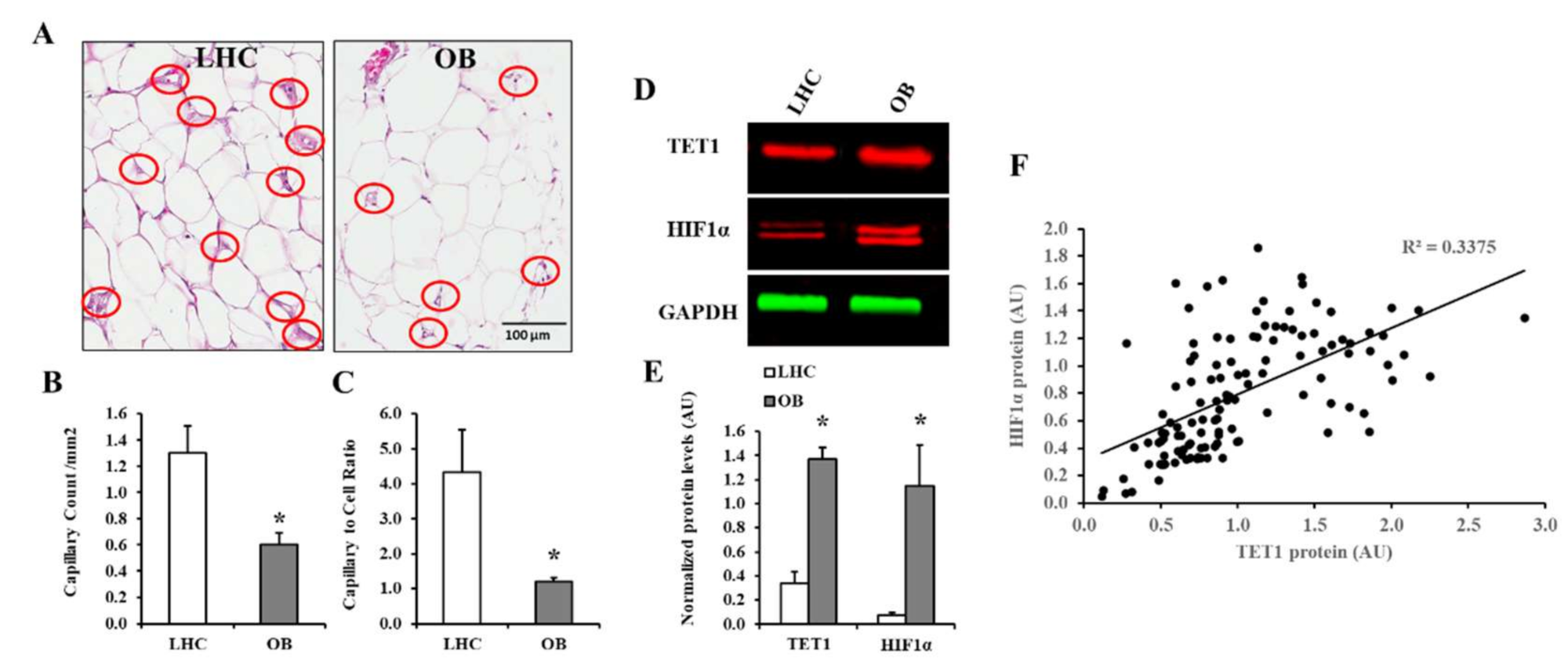
2.6. Adipose Tissue Capillary Density Measurements
2.7. Microvascular Preparation and Flow-Induced Dilation Measurements
2.8. Arteriolar Nitric Oxide (NO) Measurements
2.9. Real-Time PCR (Polymerase Chain Reaction)
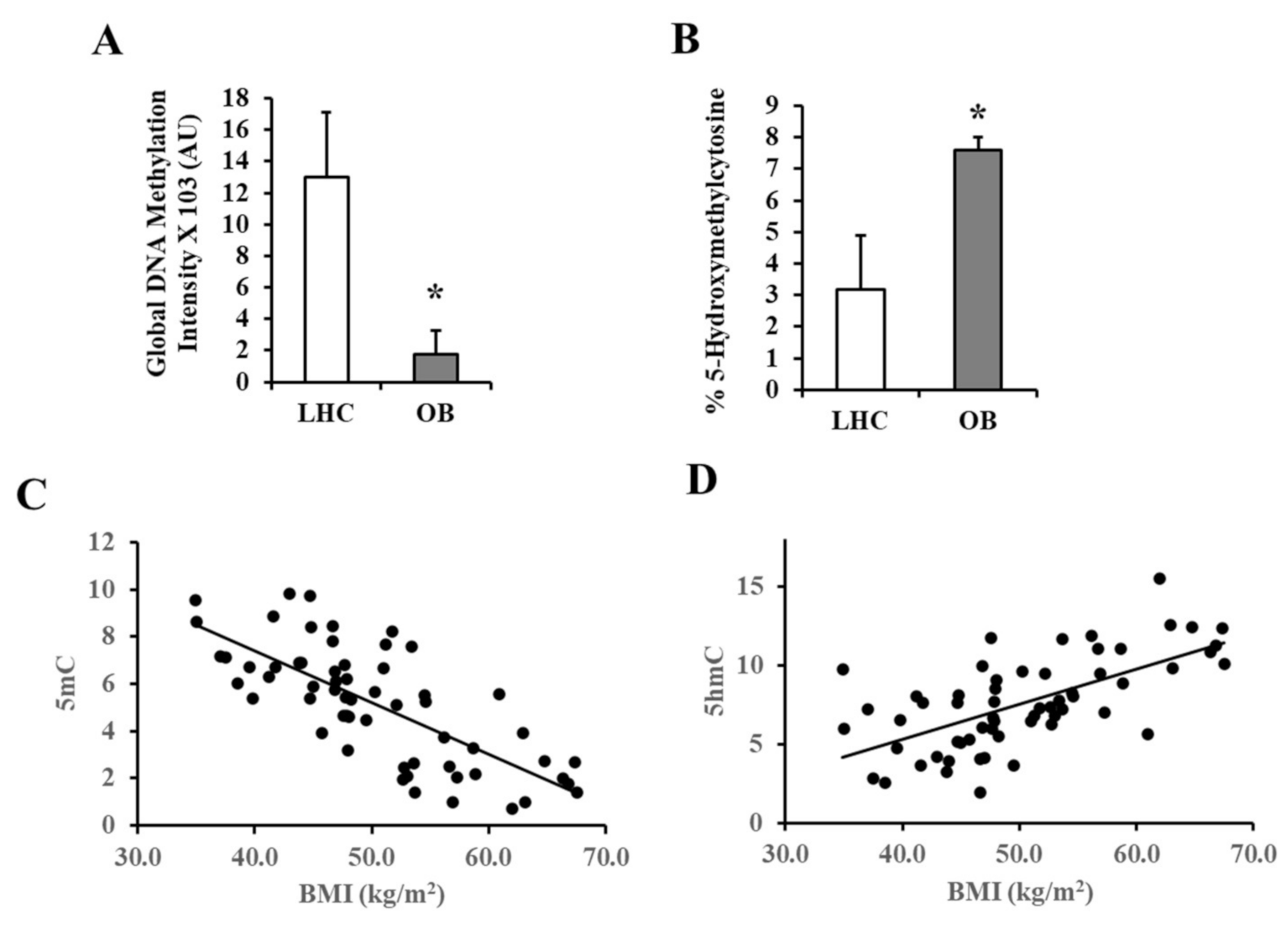
2.10. Global DNA Methylation Analysis
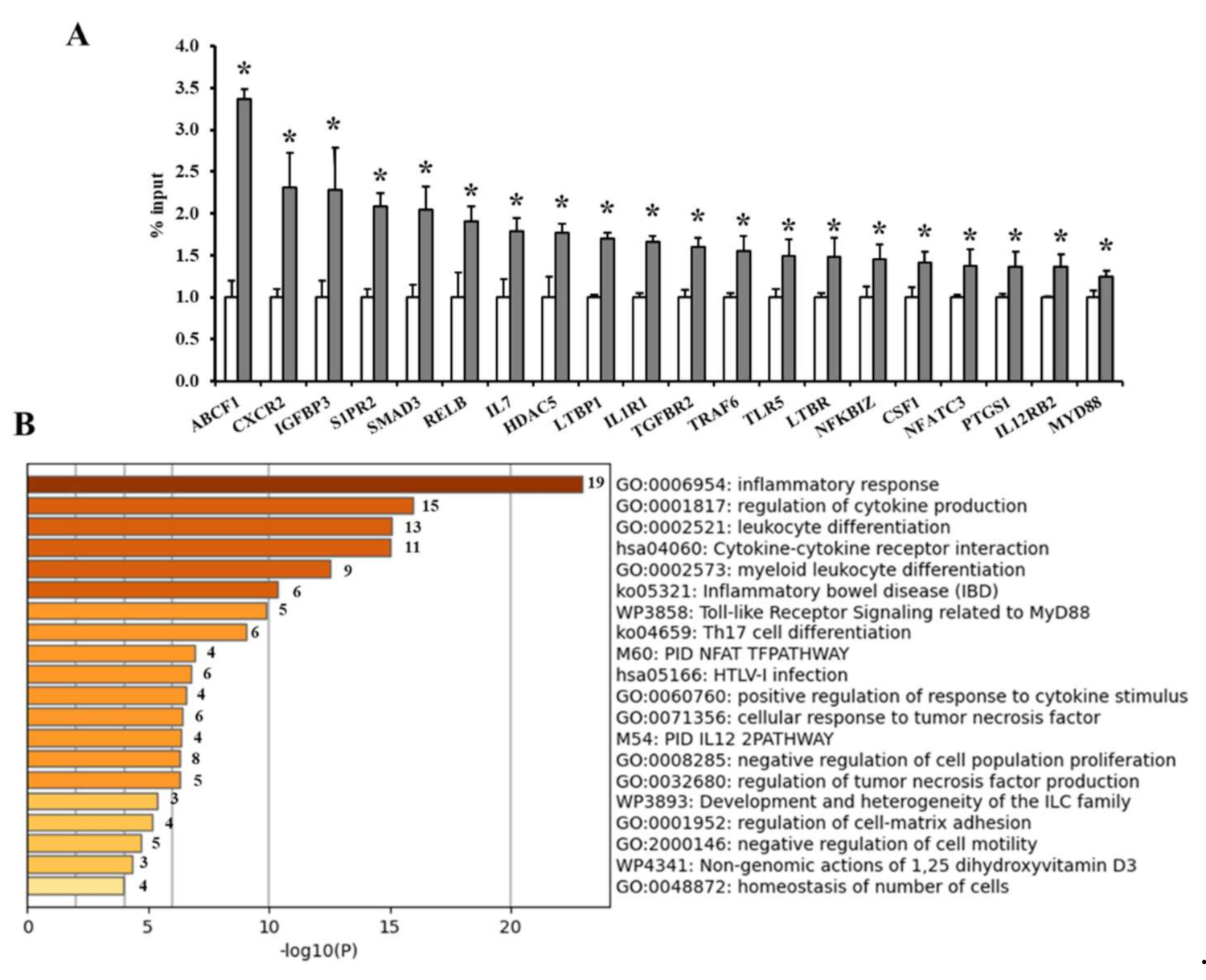
2.11. Methylation PCR Analysis
2.12. Western Blotting
2.13. Chromatin Immunoprecipitation Assay (ChIP)
2.14. Statistical Analyses
3. Results
3.1. Cardiometabolic Risk Factors
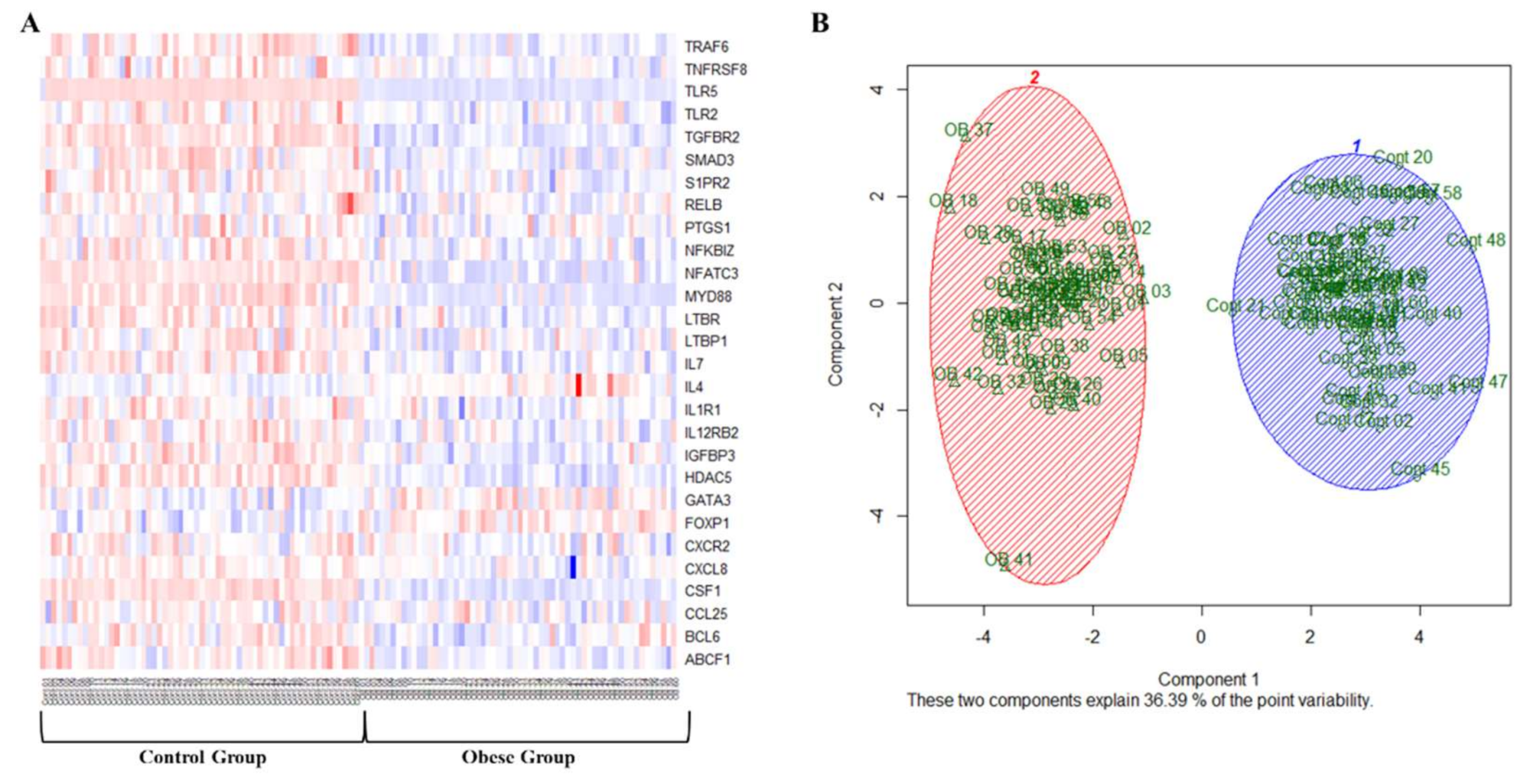
3.2. Analysis of VAT and Circulating Cytokines
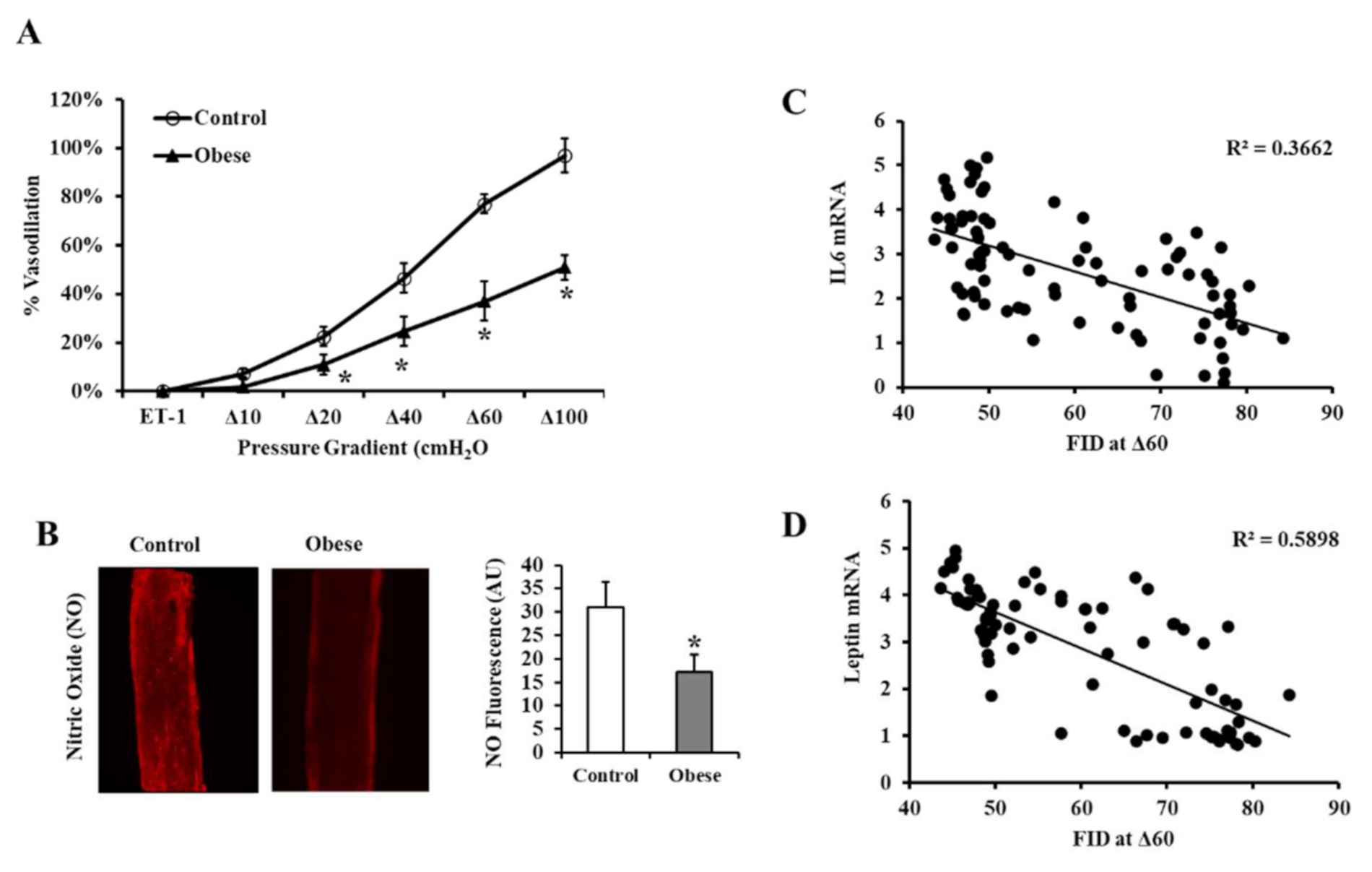
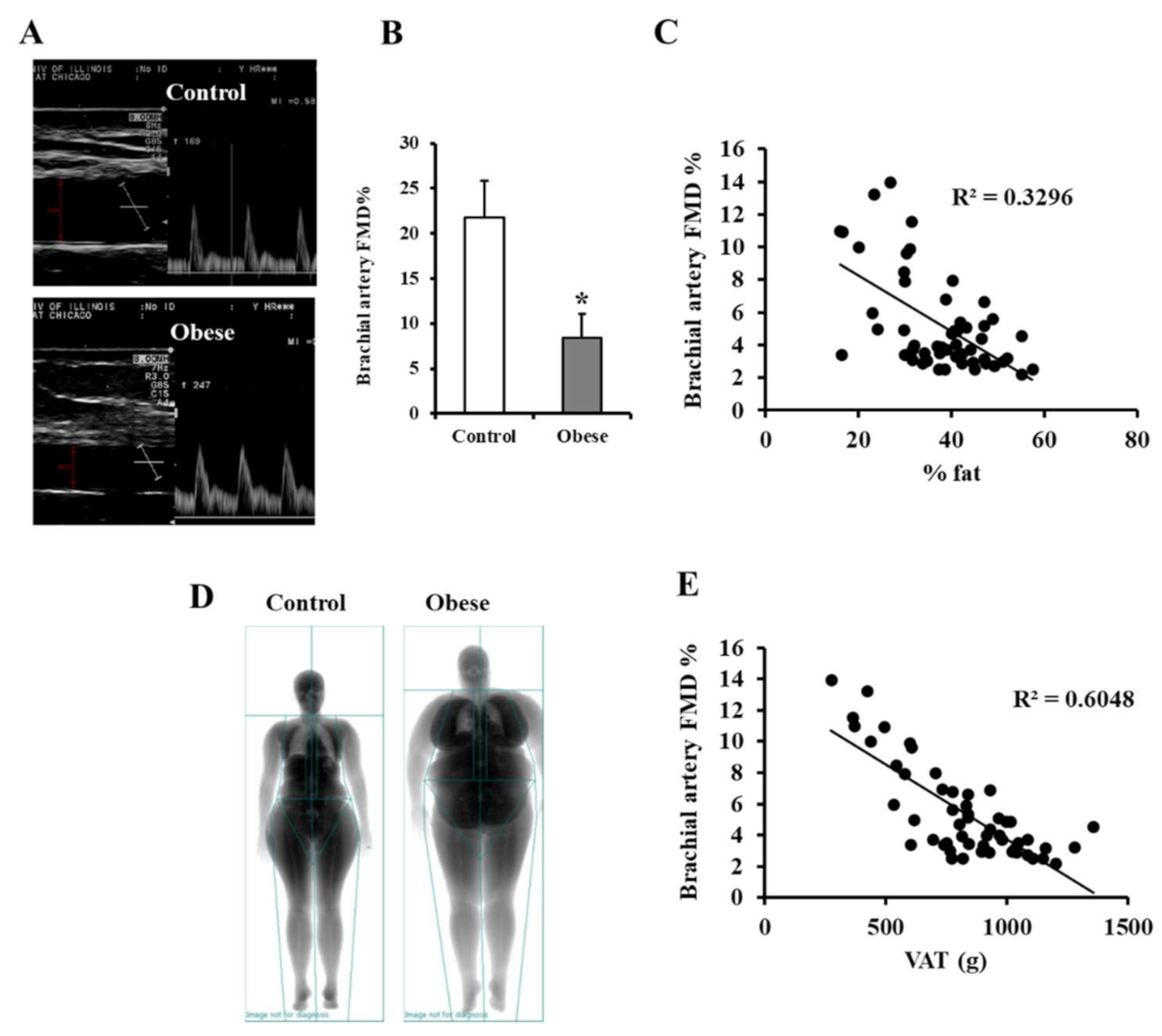
3.3. Vascular Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Current State of Obesity Solutions in the United States: Workshop Summary; The National Academies Press: Washington, DC, USA, 2014; p. 94.
- Khaodhiar, L.; McCowen, K.C.; Blackburn, G.L. Obesity and its comorbid conditions. Clin. Cornerstone 1999, 2, 17–31. [Google Scholar] [CrossRef]
- Lian, X.; Gollasch, M. A Clinical Perspective: Contribution of Dysfunctional Perivascular Adipose Tissue (PVAT) to Cardiovascular Risk. Curr. Hypertens. Rep. 2016, 18, 82. [Google Scholar] [CrossRef]
- Trayhurn, P. Hypoxia and Adipose Tissue Function and Dysfunction in Obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafeev, I.S.; Michurina, S.S.; Podkuychenko, N.V.; Menshikov, M.Y.; Parfyonova, Y.V.; Vorotnikov, A.V. Chemical Inducers of Obesity-Associated Metabolic Stress Activate Inflammation and Reduce Insulin Sensitivity in 3T3-L1 Adipocytes. Biochemistry (Moscow) 2019, 84, 553–561. [Google Scholar] [CrossRef]
- Lawler, H.M.; Underkofler, C.M.; Kern, P.A.; Erickson, C.; Bredbeck, B.; Rasouli, N. Adipose Tissue Hypoxia, Inflammation, and Fibrosis in Obese Insulin-Sensitive and Obese Insulin-Resistant Subjects. J. Clin. Endocrinol. Metab. 2016, 101, 1422–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int. J. Obes. (Lond.) 2009, 33, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Nanduri, J.; Semenza, G.L.; Prabhakar, N.R. Epigenetic changes by DNA methylation in chronic and intermittent hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L1096–L1100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhang, Y.; Jiang, S.; Liu, Y.; Huang, L.; Zhang, T.; Lu, G.; Gong, K.; Ji, X.; Shao, G. The Effect of Hypoxia Preconditioning on DNA Methyltransferase and PP1gamm in Hippocampus of Hypoxia Preconditioned Mice. High Alt. Med. Biol. 2014, 15, 483–490. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Chen, M.; Dasgupta, C.; Xiao, D.; Huang, X.; Yang, S.; Zhang, L. Chronic hypoxia upregulates DNA methyltransferase and represses large conductance Ca2+-activated K+ channel function in ovine uterine arteries. Biol. Reprod. 2017, 96, 424–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Liu, L.; Zhao, Y.; Zhang, J.; Wang, D.; Chen, J.; He, Y.; Wu, J.; Zhang, Z.; Liu, Z. Hypoxia Induces Genomic DNA Demethylation through the Activation of HIF-1alpha and Transcriptional Upregulation of MAT2A in Hepatoma Cells. Mol. Cancer Ther. 2011, 10, 1113–1123. [Google Scholar] [CrossRef] [Green Version]
- Alivand, M.R.; Soheili, Z.-S.; Pornour, M.; Solali, S.; Sabouni, F. Novel Epigenetic Controlling of Hypoxia Pathway Related to Overexpression and Promoter Hypomethylation of TET1 and TET2 in RPE Cells. J. Cell. Biochem. 2017, 118, 3193–3204. [Google Scholar] [CrossRef]
- Wu, S.C.; Zhang, Y. Active DNA demethylation: Many roads lead to Rome. Nat. Rev. Mol. Cell Biol. 2010, 11, 607–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; D’Alessio, A.C.; Taranova, O.V.; Hong, K.; Sowers, L.C.; Zhang, Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010, 466, 1129–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, K.D.; Helin, K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016, 30, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Phillips, S.A.; Mahmoud, A.M. HIF1alpha/TET1 Pathway Mediates Hypoxia-Induced Adipocytokine Promoter Hypomethylation in Human Adipocytes. Cells 2020, 9, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haloul, M.; Vinjamuri, S.J.; Naquiallah, D.; Mirza, M.I.; Qureshi, M.; Hassan, C.; Masrur, M.; Bianco, F.M.; Frederick, P.; Cristoforo, G.P.; et al. Hyperhomocysteinemia and Low Folate and Vitamin B12 Are Associated with Vascular Dysfunction and Impaired Nitric Oxide Sensitivity in Morbidly Obese Patients. Nutrients 2020, 12, 2014. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; Szczurek, M.R.; Blackburn, B.K.; Mey, J.T.; Chen, Z.; Robinson, A.T.; Bian, J.-T.; Unterman, T.G.; Minshall, R.D.; Brown, M.D.; et al. Hyperinsulinemia augments endothelin-1 protein expression and impairs vasodilation of human skeletal muscle arterioles. Physiol. Rep. 2016, 4, e12895. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.M.; Naquiallah, D.; Hassan, C.; Masrur, M.; Bianco, F.M.; Frederick, P.; Cristoforo, G.; Gangemi, A.; Phillips, S.A.; Mahmoud, A.M. Obesity-associated Hypoxia Contributes to Aberrant Methylation of Genes Implicated in Inflammation and vascular Function. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Szczurek, M.; Hassan, C.; Masrur, M.; Gangemi, A.; Phillips, S.A. Vitamin D Improves Nitric Oxide-Dependent Vasodilation in Adipose Tissue Arterioles from Bariatric Surgery Patients. Nutrients 2019, 11, 2521. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; Hwang, C.-L.; Szczurek, M.R.; Bian, J.-T.; Ranieri, C.; Gutterman, D.D.; Phillips, S.A. Low-Fat Diet Designed for Weight Loss But Not Weight Maintenance Improves Nitric Oxide-Dependent Arteriolar Vasodilation in Obese Adults. Nutrients 2019, 11, 1339. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.M.; Naquiallah, D.; Qureshi, M.; Mirza, M.I.; Hassan, C.; Masrur, M.; Bianco, F.M.; Frederick, P.; Cristoforo, G.P.; Gangemi, A.; et al. DNA methylation profile of genes involved in inflammation and autoimmunity correlates with vascular function in morbidly obese adults. Epigenetics 2021, 1–17. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Solomon, T.P.J.; Phillips, S.A.; Kirwan, J.P.; Haus, J.M. Aerobic Exercise Reduces NOX2 in Skeletal Muscle of Obese Insulin-resistant Adults Via Interfering with RAGE/p-IkB-α Axis. FASEB J. 2016, 30, lb762. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Selhub, J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J. Nutr. Health Aging 2002, 6, 39–42. [Google Scholar] [PubMed]
- Wierzbicki, A.S. Homocysteine and cardiovascular disease: A review of the evidence. Diabetes Vasc. Dis. Res. 2007, 4, 143–150. [Google Scholar] [CrossRef]
- Humphrey, L.L.; Fu, R.; Rogers, K.; Freeman, M.; Helfand, M. Homocysteine Level and Coronary Heart Disease Incidence: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2008, 83, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Gibson, A.; Woodside, J.V.; Young, I.S.; Sharpe, P.C.; Mercer, C.; Patterson, C.C.; McKinley, M.C.; Kluijtmans, L.A.; Whitehead, A.S.; Evans, A. Alcohol increases homocysteine and reduces B vitamin concentration in healthy male volunteers—A randomized, crossover intervention study. QJM Int. J. Med. 2008, 101, 881–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alegría-Torres, J.A.; Baccarelli, A.; Bollati, V. Epigenetics and lifestyle. Epigenomics 2011, 3, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Ho, S.-M. Epigenetics meets endocrinology. J. Mol. Endocrinol. 2011, 46, R11–R32. [Google Scholar] [CrossRef] [Green Version]
- Stoger, R. Epigenetics and obesity. Pharmacogenomics 2008, 9, 1851–1860. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, A.K.; Damgaard, A.; Mackey, A.L.; Schjerling, P.; Magnusson, P.; Olesen, A.T.; Kjaer, M.; Scheele, C. An anti-inflammatory phenotype in visceral adipose tissue of old lean mice, augmented by exercise. Sci. Rep. 2019, 9, 12069. [Google Scholar] [CrossRef] [Green Version]
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2016, 138, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; De La Higuera, M.; Fruhbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef] [Green Version]
- Bullo, M.; Casas-Agustench, P.; Amigo-Correig, P.; Aranceta, J.; Salas-Salvado, J. Inflammation, obesity and comorbidities: The role of diet. Public Health Nutr. 2007, 10, 1164–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unamuno, X.; Gomez-Ambrosi, J.; Rodriguez, A.; Becerril, S.; Fruhbeck, G.; Catalan, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef] [Green Version]
- Fujisaka, S.; Usui, I.; Ikutani, M.; Aminuddin, A.; Takikawa, A.; Tsuneyama, K.; Mahmood, A.; Goda, N.; Nagai, Y.; Takatsu, K.; et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1alpha-dependent and HIF-1alpha-independent manner in obese mice. Diabetologia 2013, 56, 1403–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Q.; Yun, Z. The Hypoxia-Inducible Factor Pathway in Adipocytes: The Role of HIF-2 in Adipose Inflammation and Hypertrophic Cardiomyopathy. Front. Endocrinol. (Lausanne) 2015, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Sun, W.; Yang, Z.; Guo, J.; Liu, H.; Liang, J. Hypoxia induces the expression of TET enzymes in HepG2 cells. Oncol. Lett. 2017, 14, 6457–6462. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-Z.; Chen, S.-F.; Nieh, S.; Benner, C.; Ger, L.-P.; Jan, C.-I.; Ma, L.; Chen, C.-H.; Hishida, T.; Chang, H.-T.; et al. Hypoxia Drives Breast Tumor Malignancy through a TET–TNFalpha-–p38–MAPK Signaling Axis. Cancer Res. 2015, 75, 3912–3924. [Google Scholar] [CrossRef] [Green Version]
- Shahrzad, S.; Bertrand, K.; Minhas, K.; Coomber, B.L. Induction of DNA Hypomethylation by Tumor Hypoxia. Epigenetics 2007, 2, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.-P.; Chen, H.-F.; Chen, S.-Y.; Cheng, W.-C.; Wang, H.-W.; Shen, Z.-J.; Song, C.; Teng, S.-C.; He, C.; Wu, K.-J. TET1 regulates hypoxia-induced epithelial-mesenchymal transition by acting as a co-activator. Genome Biol. 2014, 15, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judd, S.E.; Tangpricha, V. Vitamin D Deficiency and Risk for Cardiovascular Disease. Am. J. Med Sci. 2009, 338, 40–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahl, S.; Drong, A.; Lehne, B.; Loh, M.; Scott, W.R.; Kunze, S.; Tsai, P.-C.; Ried, J.S.; Zhang, W.; Yang, Y.; et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 2017, 541, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Campanella, G.; Gunter, M.J.; Polidoro, S.; Krogh, V.; Palli, D.; Panico, S.; Sacerdote, C.; Tumino, R.; Fiorito, G.; Guarrera, S.; et al. Epigenome-wide association study of adiposity and future risk of obesity-related diseases. Int. J. Obes. (Lond.) 2018, 42, 2022–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayols-Baixeras, S.; Subirana, I.; Fernández-Sanlés, A.; Senti, M.; Lluís-Ganella, C.; Marrugat, J.; Elosua, R. DNA methylation and obesity traits: An epigenome-wide association study. The REGICOR study. Epigenetics 2017, 12, 909–916. [Google Scholar] [CrossRef]
- Fradin, D.; Boelle, P.-Y.; Belot, M.-P.; Lachaux, F.; Tost, J.; Besse, C.; Deleuze, J.-F.; De Filippo, G.; Bougneres, P. Genome-Wide Methylation Analysis Identifies Specific Epigenetic Marks In Severely Obese Children. Sci. Rep. 2017, 7, 46311. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Zheng, D.; Fan, C.; Liu, Z.; Dong, H.; Lu, Y.; Qi, K. Genome-wide screen of DNA methylation identifies novel markers in childhood obesity. Gene 2015, 566, 74–83. [Google Scholar] [CrossRef]
- Huang, R.C.; Garratt, E.S.; Pan, H.; Wu, Y.; Davis, E.A.; Barton, S.J.; Burdge, G.C.; Godfrey, K.M.; Holbrook, J.D.; Lillycrop, K.A. Genome-wide methylation analysis identifies differentially methylated CpG loci associated with severe obesity in childhood. Epigenetics 2015, 10, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Castellano-Castillo, D.; Moreno-Indias, I.; Sanchez-Alcoholado, L.; Ramos-Molina, B.; Alcaide-Torres, J.; Morcillo, S.; Ocana-Wilhelmi, L.; Tinahones, F.; Queipo-Ortuno, M.I.; Cardona, F. Altered Adipose Tissue DNA Methylation Status in Metabolic Syndrome: Relationships Between Global DNA Methylation and Specific Methylation at Adipogenic, Lipid Metabolism and Inflammatory Candidate Genes and Metabolic Variables. J. Clin. Med. 2019, 8, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunstan, J.; Bressler, J.P.; Moran, T.H.; Pollak, J.S.; Hirsch, A.G.; Bailey-Davis, L.; Glass, T.A.; Schwartz, B.S. Associations of LEP, CRH, ICAM-1, and LINE-1 methylation, measured in saliva, with waist circumference, body mass index, and percent body fat in mid-childhood. Clin. Epigenet. 2017, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.Y.; Park, Y.J.; Pan, X.; Shin, K.C.; Kwak, S.-H.; Bassas, A.F.; Sallam, R.M.; Park, K.S.; Alfadda, A.A.; Xu, A.; et al. Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance. Nat. Commun. 2015, 6, 7585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Gu, H.F.; Frystyk, J.; Efendic, S.; Brismar, K.; Thorell, A. Analyses of IGFBP2 DNA methylation and mRNA expression in visceral and subcutaneous adipose tissues of obese subjects. Growth Horm. IGF Res. 2019, 45, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Liu, S.; Su, Z.; Cheng, R.; Bai, X.; Li, X. LINE-1 Hypomethylation is Associated with the Risk of Coronary Heart Disease in Chinese Population. Arq. Bras. Cardiol. 2014, 102, 481–488. [Google Scholar] [CrossRef]
- Lin, R.-T.; Hsi, E.; Lin, H.-F.; Liao, Y.-C.; Wang, Y.-S.; Juo, S.H. LINE-1 Methylation is Associated with an Increased Risk of Ischemic Stroke in Men. Curr. Neurovasc. Res. 2014, 11, 4–9. [Google Scholar] [CrossRef]
- Baccarelli, A.; Wright, R.; Bollati, V.; Litonjua, A.; Zanobetti, A.; Tarantini, L.; Sparrow, D.; Vokonas, P.; Schwartz, J. Ischemic Heart Disease and Stroke in Relation to Blood DNA Methylation. Epidemiology 2010, 21, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Poli, V.F.S.; Sanches, R.B.; Moraes, A.D.S.; Fidalgo, J.P.N.; Nascimento, M.A.; Bresciani, P.; Andrade-Silva, S.G.; Cipullo, M.A.T.; Clemente, J.C.; Caranti, D.A. The excessive caloric intake and micronutrient deficiencies related to obesity after a long-term interdisciplinary therapy. Nutrition 2017, 38, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I. Folate and DNA methylation: A mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol. Biomark. Prev. 2004, 13, 511–519. [Google Scholar]
- Kulis, M.; Esteller, M. DNA Methylation and Cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar] [CrossRef]
- Wang, X.; Guan, Z.; Chen, Y.; Dong, Y.; Niu, Y.; Wang, J.; Zhang, T.; Niu, B. Genomic DNA Hypomethylation Is Associated with Neural Tube Defects Induced by Methotrexate Inhibition of Folate Metabolism. PLoS ONE 2015, 10, e0121869. [Google Scholar] [CrossRef] [PubMed]

| Variable | Non-Obese Controls | Obese Bariatric Patients | p-Value |
|---|---|---|---|
| n | 60 (18 ♂) | 60 (12 ♁) | |
| Age, y | 35.8 ± 1.8 | 36.4 ± 1.8 | 0.541 |
| Weight, kg | 75.8 ± 2.2 | 143.2 ± 3.4 * | <0.0001 |
| BMI, kg/m2 | 24.9 ± 0.6 | 51.1 ± 1.3 * | <0.0001 |
| Waist circumference, cm | 89.2 ± 1.9 | 133.5 ± 3.8 * | <0.0001 |
| Body fat, % | 32.2 ± 2.5 | 52.5 ± 1.1 * | <0.0001 |
| Body lean, % | 65.3 ± 2.4 | 46.8 ± 1.2 * | <0.0001 |
| VAT mass, kg | 0.7 ± 0.1 | 2.2 ± 0.2 * | <0.0001 |
| Heart rate, bpm | 75 ± 4 | 86 ± 3 * | 0.001 |
| Systolic BP, mmHg | 118 ± 2 | 135 ± 6 * | 0.005 |
| Diastolic BP, mmHg | 74 ± 1 | 82 ± 3 * | 0.008 |
| FPG, mg/dL | 92 ± 1 | 99 ± 6 | 0.211 |
| FPI, µU/mL | 8.3 ± 0.3 | 16.1 ± 2.2 * | <0.001 |
| HOMA-IR | 1.9 ± 0.1 | 5.2 ± 0.5 * | <0.001 |
| HbA1c, % | 5.4 ± 0.2 | 5.8 ± 0.2 | 0.148 |
| Total cholesterol, mg/dL | 156 ± 10 | 165 ± 10 | 0.327 |
| HDL, mg/dL | 56 ± 6 | 45 ± 2 * | <0.001 |
| LDL, mg/dL | 87 ± 8 | 96 ± 6 | 0.221 |
| Triglycerides, mg/dL | 92 ± 11 | 110 ± 7 | 0.160 |
| C-reactive protein, mg/dL | 0.7 ± 0.1 | 4.0 ± 0.4 * | <0.0001 |
| Plasma NO, µmol/L | 4.5 ± 0.6 | 3.2 ± 0.4 * | 0.002 |
| Folate, ng/mL | 18.7 ± 0.7 | 14.8 ± 0.8 * | 0.0004 |
| Vitamin B12, pg/mL | 562 ± 18 | 398 ± 26 * | <0.0001 |
| Homocysteine, µmol/L | 8.7 ± 0.2 | 11.4 ± 0.3 * | 0.003 |
| Vitamin D, ng/mL | 37.2 ± 4.7 | 16.9 ± 1.1 * | <0.0001 |
| Alcohol Consumption Category | Non-Obese (n = 60) | Obese (n = 60) | p-Value |
|---|---|---|---|
| Abstainers | 38 (63%) | 22 (37%) | 0.002 |
| Light drinkers | 12 (20%) | 9 (15%) | |
| Moderate drinkers | 10 (17%) | 18 (30%) | |
| Heavy drinkers | 0 (0%) | 11 (18%) |
| HIF1α Protein in VAT | TET1 Protein in VAT | |||
|---|---|---|---|---|
| Pearson Correlation | Sig (2-Tailed) | Pearson Correlation | Sig (2-Tailed) | |
| Body weight | 0.925 ** | 7.5 × 10−37 | 0.884 ** | 1.9 × 10−29 |
| Waist circumference | 0.767 ** | 6.1 × 10−9 | 0.748 ** | 2.2 × 10−8 |
| BMI | 0.960 ** | 5.9 × 10−48 | 0.929 ** | 6.1 × 10−38 |
| Total fat % | 0.782 ** | 7.3 × 10−11 | 0.763 ** | 3.6 × 10−10 |
| Total lean % | −0.775 ** | 1.3 × 10−10 | −0.755 ** | 6.7 × 10−10 |
| VAT mass | 0.620 ** | 2.1 × 10−4 | 0.0591 ** | 4.6 × 10−4 |
| Heart rate | 0.157 | 7.5 × 10−2 | 0.186 * | 4.4 × 10−2 |
| Systolic BP | 0.393 ** | 9.9 × 10−5 | 0.383 ** | 1.5 × 10−4 |
| Diastolic BP | 0.156 | 7.7 × 10−2 | 0.170 | 5.9 × 10−2 |
| FPI | 0.414 ** | 1.2 × 10−4 | 0.480 ** | 7.5 × 10−6 |
| FPG | 0.078 | 2.5 × 10−1 | 0.127 | 1.4 × 10−1 |
| HOMA-IR | 0.208 * | 3.8 × 10−2 | 0.269 * | 1.1 × 10−2 |
| HbA1c | 0.146 | 1.5 × 10−1 | 0.145 | 1.5 × 10−1 |
| Total cholesterol | 0.082 | 2.7 × 10−1 | 0.079 | 2.8 × 10−1 |
| LDL | 0.199 | 7.3 × 10−2 | 0.177 | 9.8 × 10−2 |
| HDL | −0.295 * | 1.4 × 10−2 | −0.313 ** | 9.4 × 10−3 |
| Triglycerides | 0.097 | 2.4 × 10−1 | 0.176 | 9.9 × 10−2 |
| Plasma Hcy | 0.437 ** | 2.7 × 10−3 | 0.438 ** | 2.7 × 10−3 |
| Plasma NO | −0.414 ** | 4.1 × 10−5 | −0.423 ** | 2.7 × 10−5 |
| Plasma Folate | −0.368 ** | 1.1 × 10−3 | −0.353 ** | 1.6 × 10−3 |
| Plasma Vitamin B12 | −0.384 ** | 6.1 × 10−4 | −0.393 ** | 4.6 × 10−4 |
| Plasma Vitamin D | −0.622 ** | 5.9 × 10−9 | −0.595 ** | 3.4 × 10−8 |
| Alcohol consumption | 0.516 ** | 2.2 × 10−7 | 0.457 ** | 5.6 × 10−6 |
| Genes | Control | Obese | p-Value |
|---|---|---|---|
| % of Methylated Fraction (Mean ± SE) | |||
| ABCF1 | 72% ± 7% | 63% ± 7% | 2.3 × 10−9 |
| BCL6 | 66% ± 5% | 61% ± 1% | 1.5 × 10−4 |
| CCL25 | 54% ± 3% | 52% ± 3% | 1.3 × 10−2 |
| CSF1 | 92% ± 17% | 19% ± 9% | 2.7 × 10−32 |
| CXCL8 | 44% ± 3% | 39% ±6% | 4.1 × 10−5 |
| CXCR2 | 67% ± 5% | 63% ± 5% | 1.2 × 10−3 |
| FOXP1 | 72% ± 6% | 78% ± 6% | 1.9 × 10−8 |
| GATA3 | 68% ± 6% | 73% ± 6% | 1.1 × 10−4 |
| HDAC5 | 86% ± 5% | 78% ± 5% | 2.4 × 10−16 |
| IGFBP3 | 71% ± 4% | 65% ± 4% | 1.6 × 10−10 |
| IL12RB2 | 86% ± 5% | 83% ± 5% | 9.2 × 10−6 |
| IL1R1 | 74% ± 4% | 70% ± 4% | 1.9 × 10−5 |
| IL4 | 54% ± 4% | 61% ± 9% | 7.5 × 10−5 |
| IL7 | 77% ± 5% | 71% ± 4% | 9.2 × 10−10 |
| LTBP1 | 74% ± 4% | 70% ± 5% | 1.3 × 10−5 |
| LTBR | 84% ± 5% | 79% ± 4% | 1.2 × 10−9 |
| MYD88 | 86% ± 12% | 29% ± 8% | 7.2 × 10−21 |
| NFATC3 | 83% ± 8% | 49% ± 16% | 5.4 × 10−20 |
| NFκB | 84% ± 6% | 80% ± 5% | 3.2 × 10−6 |
| PTGS1 | 82% ± 6% | 79% ± 6% | 5.1 × 10−3 |
| RELB | 72% ± 8% | 68% ± 6% | 5.8 × 10−4 |
| S1PR2 | 73% ± 5% | 67% ± 3% | 1.4 × 10−7 |
| SMAD3 | 77% ± 6% | 70% ± 5% | 2.6 × 10−10 |
| TGFBR2 | 77% ± 1% | 73% ± 1% | 4.8 × 10−23 |
| TLR2 | 32% ± 5% | 26% ± 4% | 6.7 × 10−7 |
| TLR5 | 80% ±6% | 33% ± 4% | 1.7 × 10−43 |
| TNFRSF8 | 50% ±5% | 45% ± 3% | 1.2 × 10−5 |
| TRAF6 | 82% ±4% | 77% ± 3% | 2.0 × 10−12 |
| Variable | Adipokine Methylation Score | |
|---|---|---|
| Correlation | Significance (2-Tailed) | |
| Body weight | −0.797 ** | 5.07 × 10−23 |
| Waist circumference | −0.717 ** | 2.04 × 10−7 |
| BMI | −0.841 ** | 1.05 × 10−27 |
| Total fat % | −0.776 ** | 8.09 × 10−10 |
| Total lean % | 0.767 ** | 1.52 × 10−9 |
| VAT mass | −0.680 ** | 6.66 × 10−5 |
| Heart rate | −0.259 ** | 4.94 × 10−3 |
| Systolic BP | −0.380 ** | 5.60 × 10−5 |
| Diastolic BP | −0.229 * | 1.15 × 10−2 |
| FPI | −0.570 ** | 2.04 × 10−8 |
| FPG | −0.157 | 8.33 × 10−2 |
| HOMA−IR | −0.331 ** | 1.45 × 10−3 |
| HbA1c | −0.140 | 1.57 × 10−1 |
| Total cholesterol | −0.039 | 3.85 × 10−1 |
| LDL | −0.184 | 8.35 × 10−2 |
| HDL | 0.387 ** | 1.22 × 10−3 |
| Triglycerides | −0.165 | 1.08 × 10−1 |
| Plasma Hcy | −0.452 ** | 2.81 × 10−3 |
| Plasma NO | 0.459 ** | 1.62 × 10−6 |
| Plasma Folate | 0.375 ** | 4.92 × 10−4 |
| Plasma Vitamin B12 | 0.459 ** | 1.75 × 10−5 |
| Plasma Vitamin D | 0.623 ** | 7.41 × 10−10 |
| Brachial FMD | 0.632 ** | 6.93 × 10−12 |
| Arteriolar FID Δ60 | 0.952 ** | 1.25 × 10−40 |
| Arteriolar NO | 0.767 ** | 6.02 × 10−9 |
| Alcohol consumption | −0.374 ** | 7.53 × 10−5 |
| VAT vascularity | −0.875 ** | 1.29 × 10−25 |
| VAT HIF1α | −0.854 ** | 2.65 × 10−23 |
| VAT TET−1 | −0.608 ** | 2.19 × 10−9 |
| Plasma IL6 | −0.482 ** | 1.69 × 10−3 |
| Plasma IL1β | −0.501 ** | 3.31 × 10−9 |
| Plasma TNFα | −0.434 ** | 1.79 × 10−5 |
| Plasma IFNγ | −0.489 ** | 2.06 × 10−7 |
| Plasma VEGF | −0.453 ** | 1.00 × 10−5 |
| Plasma CRP | −0.710 ** | 1.48 × 10−10 |
| Brachial Artery FMD | Arteriolar FID at Δ60 | |||
|---|---|---|---|---|
| Pearson Correlation | Sig (2-Tailed) | Pearson Correlation | Sig (2-Tailed) | |
| Body weight | −0.462 ** | 5.82 × 10−7 | −0.873 ** | 6.23 × 10−28 |
| Waist circumference | −0.332 * | 1.69 × 10−2 | −0.761 ** | 9.63 × 10−9 |
| BMI | −0.518 ** | 1.42 × 10−8 | −0.937 ** | 5.81 × 10−40 |
| Total fat % | −0.350 ** | 7.45 × 10−3 | −0.843 ** | 9.44 × 10−14 |
| Total lean % | 0.349 ** | 7.52 × 10−3 | 0.837 ** | 2.08 × 10−13 |
| VAT mass | −0.236 | 1.09 × 10−1 | −0.649 ** | 9.34 × 10−5 |
| Heart rate | 0.020 | 4.22 × 10−1 | −0.187 * | 4.34 × 10−2 |
| Systolic BP | −0.232 ** | 9.68 × 10−3 | −0.355 ** | 4.25 × 10−4 |
| Diastolic BP | −0.105 | 1.49 × 10−1 | −0.172 | 5.73 × 10−2 |
| FPI | −0.304 ** | 2.11 × 10−3 | −0.500 ** | 2.89 × 10−6 |
| FPG | −0.098 | 1.83 × 10−1 | −0.130 | 1.35 × 10−1 |
| HOMA−IR | −0.190 * | 3.93 × 10−2 | −0.289 ** | 6.18 × 10−3 |
| HbA1c | −0.136 | 1.50 × 10−1 | −0.150 | 1.44 × 10−1 |
| Total cholesterol | −0.139 | 1.35 × 10−1 | 0.003 | 4.91 × 10−1 |
| LDL | −0.212 * | 4.65 × 10−2 | −0.139 | 1.56 × 10−1 |
| HDL | 0.155 | 1.09 × 10−1 | 0.373 ** | 2.33 × 10−3 |
| Triglycerides | −0.006 | 4.83 × 10−1 | −0.101 | 2.31 × 10−1 |
| Plasma Hcy | −0.209 | 9.80 × 10−2 | −0.420 ** | 3.89 × 10−3 |
| Plasma NO | 0.287 ** | 1.79 × 10−3 | 0.433 ** | 1.72 × 10−5 |
| Plasma Folate | 0.303 ** | 3.14 × 10−3 | 0.341 ** | 2.21 × 10−3 |
| Plasma Vitamin B12 | 0.241 * | 1.52 × 10−2 | 0.421 ** | 1.73 × 10−4 |
| Plasma Vitamin D | 0.367 ** | 3.16 × 10−4 | 0.633 ** | 2.70 × 10−9 |
| Alcohol consumption | −0.239 ** | 8.00 × 10−3 | −0.400 ** | 7.43 × 10−5 |
| VAT HIF1α | −0.620 ** | 1.22 × 10−10 | −0.895 ** | 4.36 × 10−31 |
| VAT TET−1 | −0.381 ** | 1.61 × 10−4 | −0.653 ** | 6.49 × 10−12 |
| Plasma IL6 | −0.285 * | 3.94 × 10−2 | −0.453 ** | 2.13 × 10−3 |
| Plasma CRP | −0.463 ** | 5.18 × 10−5 | −0.798 ** | 2.43 × 10−15 |
| Methylation Score | 0.632 ** | 6.93 × 10−12 | 0.952 ** | 1.25 × 10−40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.M.; Hassan, C.; Masrur, M.; Bianco, F.M.; Naquiallah, D.; Mirza, I.; Frederick, P.; Fernandes, E.T.; Giulianotti, C.P.; Gangemi, A.; et al. Adipose Tissue Hypoxia Correlates with Adipokine Hypomethylation and Vascular Dysfunction. Biomedicines 2021, 9, 1034. https://doi.org/10.3390/biomedicines9081034
Ali MM, Hassan C, Masrur M, Bianco FM, Naquiallah D, Mirza I, Frederick P, Fernandes ET, Giulianotti CP, Gangemi A, et al. Adipose Tissue Hypoxia Correlates with Adipokine Hypomethylation and Vascular Dysfunction. Biomedicines. 2021; 9(8):1034. https://doi.org/10.3390/biomedicines9081034
Chicago/Turabian StyleAli, Mohamed M., Chandra Hassan, Mario Masrur, Francesco M. Bianco, Dina Naquiallah, Imaduddin Mirza, Patrice Frederick, Eduardo T. Fernandes, Cristoforo P. Giulianotti, Antonio Gangemi, and et al. 2021. "Adipose Tissue Hypoxia Correlates with Adipokine Hypomethylation and Vascular Dysfunction" Biomedicines 9, no. 8: 1034. https://doi.org/10.3390/biomedicines9081034
APA StyleAli, M. M., Hassan, C., Masrur, M., Bianco, F. M., Naquiallah, D., Mirza, I., Frederick, P., Fernandes, E. T., Giulianotti, C. P., Gangemi, A., Phillips, S. A., & Mahmoud, A. M. (2021). Adipose Tissue Hypoxia Correlates with Adipokine Hypomethylation and Vascular Dysfunction. Biomedicines, 9(8), 1034. https://doi.org/10.3390/biomedicines9081034







