Pathophysiological and Pharmacological Characteristics of KCNJ5 157-159delITE Somatic Mutation in Aldosterone-Producing Adenomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Unilateral PA (uPA) Identification
2.2. Nucleic Acid Extraction
2.3. Sequencing of the KCNJ5 Gene
2.4. Tissue Immunohistochemistry
2.5. Cell Culture
2.6. Mutant KCNJ5 Transfection and Drugs Treatment
2.7. Patch-Clamp Recording
2.8. Western Blot Analysis
2.9. Aldosterone Secretion
2.10. Docking Analysis of Roxithromycin with KCNJ5
2.11. Molecular Dynamics Simulation of Roxithromycin with KCNJ5
2.12. Statistical Analysis
3. Results
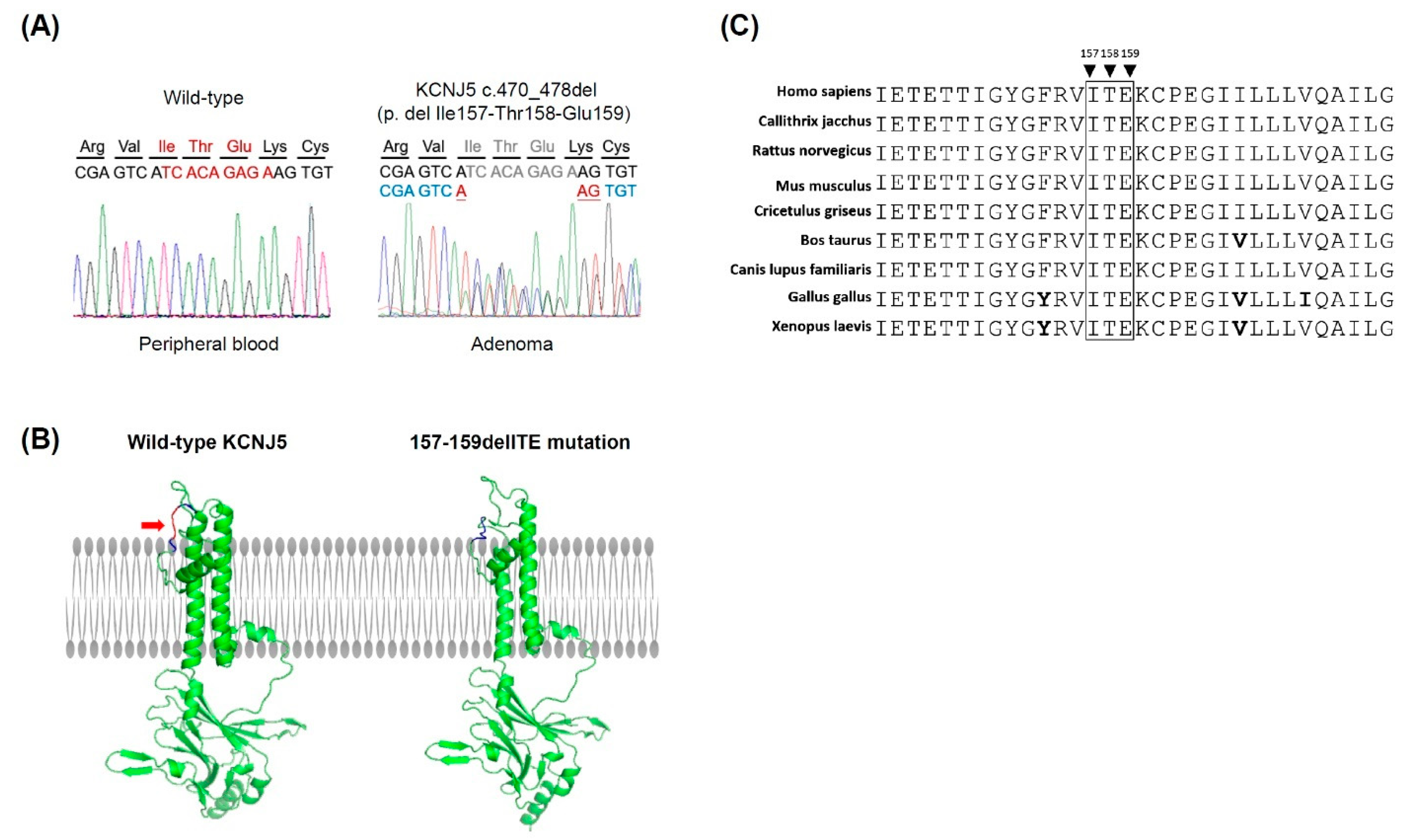
3.1. Identification of a KCNJ5 157-159delITE Mutation and Characteristics of the Identified Patient
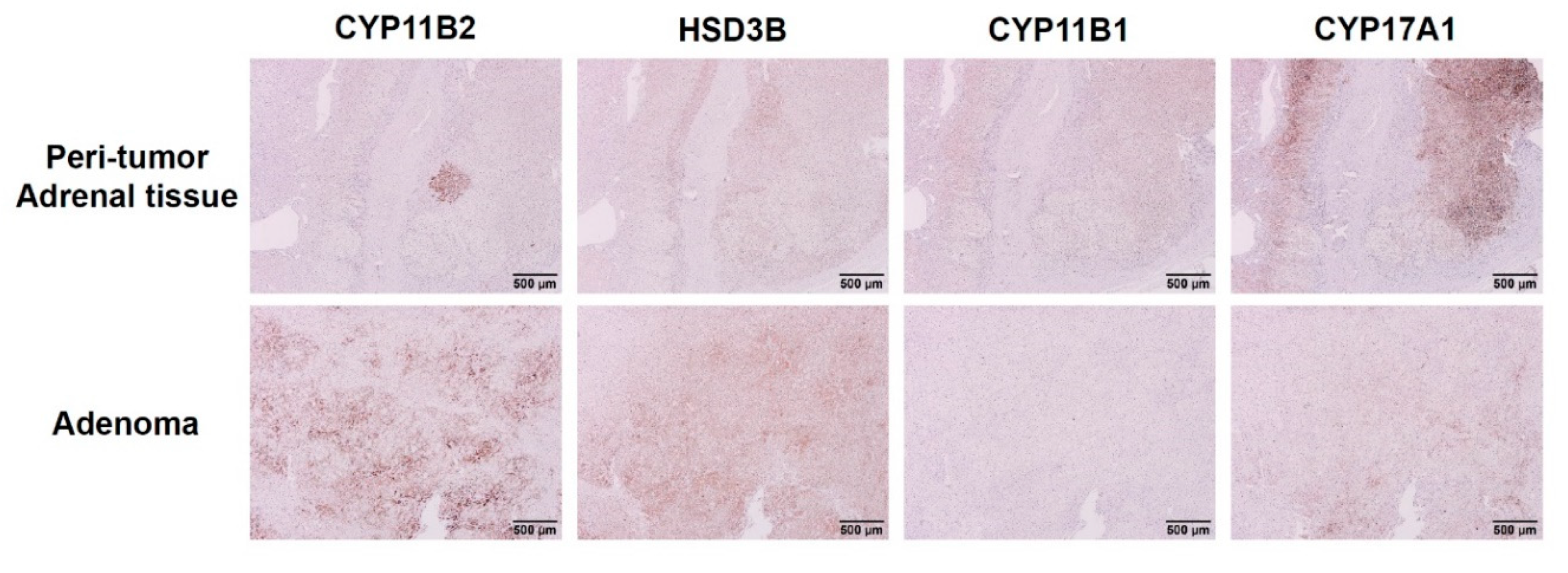
3.2. Expression of Steroidogenic Enzymes in Human APA Tissues Harboring KCNJ5 157-159delITE Mutation
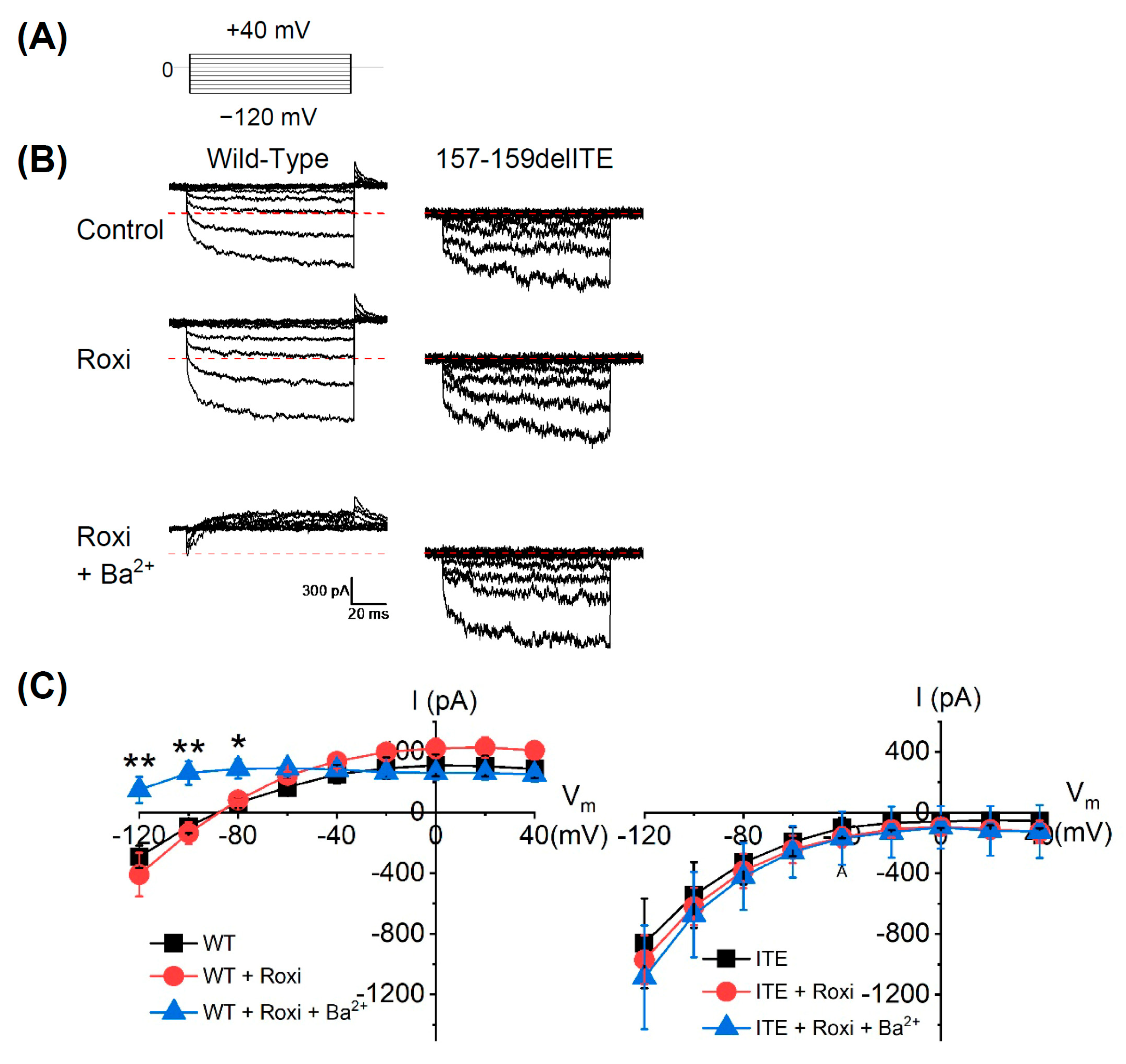
3.3. The Electrophysiological Characteristics of the KCNJ5 157-159delITE Mutation
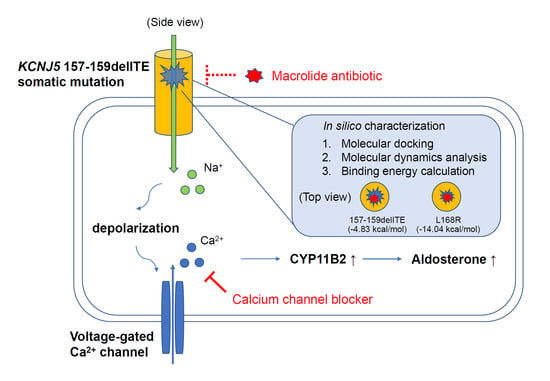
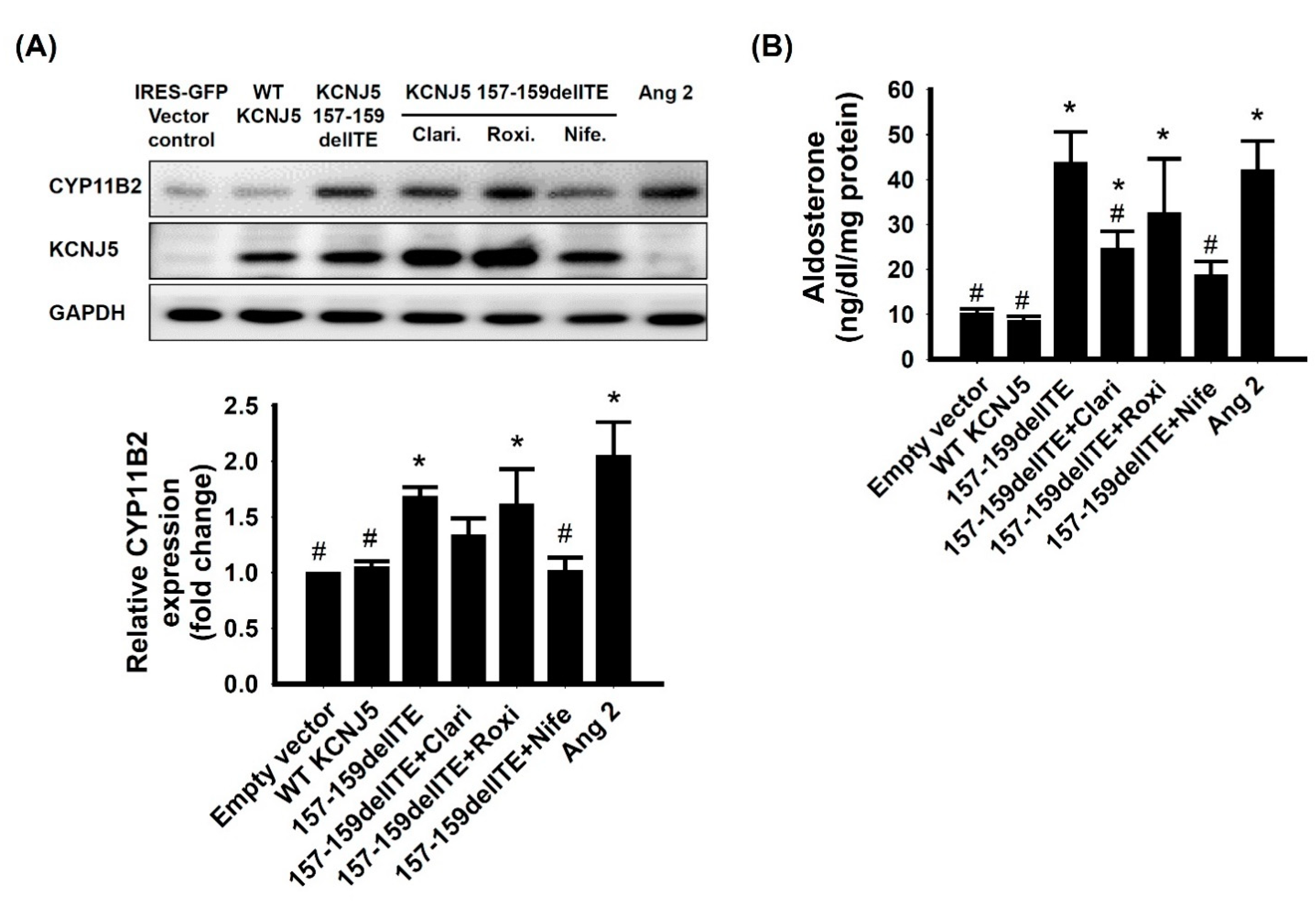
3.4. Effect of KCNJ5 157-159delITE on CYP11B2 Expression and Aldosterone Secretion
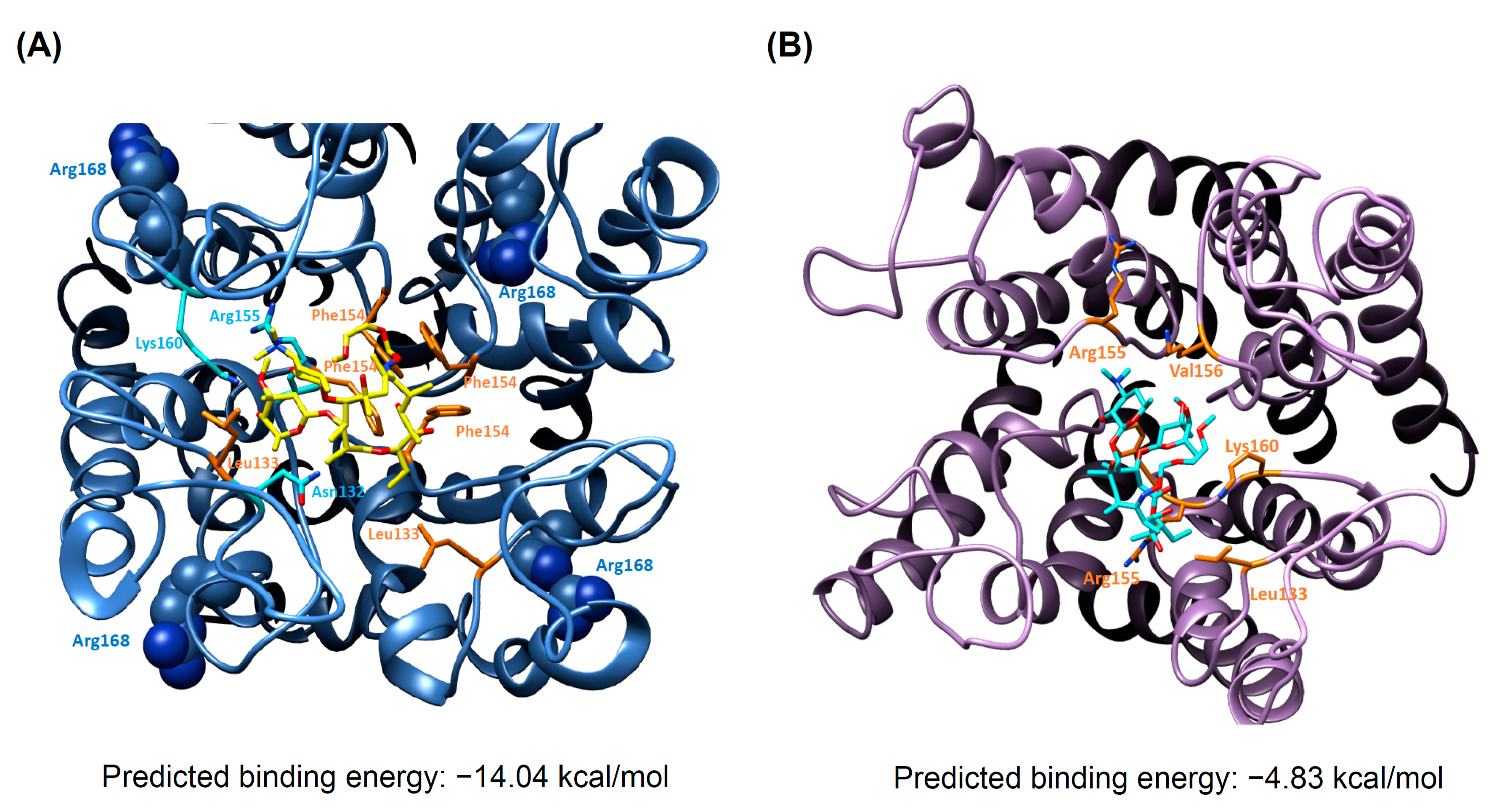
3.5. Docking Analysis and Molecular Dynamics Simulation of Roxithromycin with KCNJ5
4. Discussion
4.1. Electrophysiology of Mutant KCNJ5 157-159delITE
4.2. Expression of Steroidogenic Enzymes in Immunohistochemistry
4.3. Pharmacology and KCNJ5 Mutation
4.4. Limitation and Strength
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, B.M.; Schmieder, R.E. Aldosterone-induced cardiac damage: Focus on blood pressure independent effects. Am. J. Hypertens. 2003, 16, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Lenzini, L.; Rossi, G.P. The molecular basis of primary aldosteronism: From chimeric gene to channelopathy. Curr. Opin. Pharmacol. 2015, 21, 35–42. [Google Scholar] [CrossRef]
- Boulkroun, S.; Fernandes-Rosa, F.L.; Zennaro, M.C. Molecular and Cellular Mechanisms of Aldosterone Producing Adenoma Development. Front. Endocrinol. (Lausanne) 2015, 6, 95. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.; Scholl, U.I.; Yue, P.; Bjorklund, P.; Zhao, B.; Nelson-Williams, C.; Ji, W.; Cho, Y.; Patel, A.; Men, C.J.; et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011, 331, 768–772. [Google Scholar] [CrossRef] [Green Version]
- Lenzini, L.; Rossitto, G.; Maiolino, G.; Letizia, C.; Funder, J.W.; Rossi, G.P. A Meta-Analysis of Somatic KCNJ5 K(+) Channel Mutations in 1636 Patients With an Aldosterone-Producing Adenoma. J. Clin. Endocrinol. Metab. 2015, 100, E1089–E1095. [Google Scholar] [CrossRef] [Green Version]
- Nanba, K.; Yamazaki, Y.; Bick, N.; Onodera, K.; Tezuka, Y.; Omata, K.; Ono, Y.; Blinder, A.R.; Tomlins, S.A.; Rainey, W.E.; et al. Prevalence of Somatic Mutations in Aldosterone-Producing Adenomas in Japanese Patients. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Itcho, K.; Oki, K.; Ohno, H.; Yoneda, M. Update on Genetics of Primary Aldosteronism. Biomedicines 2021, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Beuschlein, F.; Boulkroun, S.; Osswald, A.; Wieland, T.; Nielsen, H.N.; Lichtenauer, U.D.; Penton, D.; Schack, V.R.; Amar, L.; Fischer, E.; et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 2013, 45. [Google Scholar] [CrossRef]
- Azizan, E.A.; Poulsen, H.; Tuluc, P.; Zhou, J.; Clausen, M.V.; Lieb, A.; Maniero, C.; Garg, S.; Bochukova, E.G.; Zhao, W.; et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 2013, 45, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Scholl, U.I.; Goh, G.; Stolting, G.; de Oliveira, R.C.; Choi, M.; Overton, J.D.; Fonseca, A.L.; Korah, R.; Starker, L.F.; Kunstman, J.W.; et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 2013, 45, 1050–1054. [Google Scholar] [CrossRef]
- Ishihara, K.; Yamamoto, T.; Kubo, Y. Heteromeric assembly of inward rectifier channel subunit Kir2.1 with Kir3.1 and with Kir3.4. Biochem. Biophys. Res. Commun. 2009, 380, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Scholl, U.I.; Lifton, R.P. New insights into aldosterone-producing adenomas and hereditary aldosteronism: Mutations in the K+ channel KCNJ5. Curr. Opin. Nephrol. Hypertens. 2013, 22, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Azizan, E.A.; Murthy, M.; Stowasser, M.; Gordon, R.; Kowalski, B.; Xu, S.; Brown, M.J.; O’Shaughnessy, K.M. Somatic Mutations Affecting the Selectivity Filter of KCNJ5 Are Frequent in 2 Large Unselected Collections of Adrenal Aldosteronomas. Hypertension 2012, 59, 587–591. [Google Scholar] [CrossRef]
- Dibb, K.M.; Rose, T.; Makary, S.Y.; Claydon, T.W.; Enkvetchakul, D.; Leach, R.; Nichols, C.G.; Boyett, M.R. Molecular basis of ion selectivity, block, and rectification of the inward rectifier Kir3.1/Kir3.4 K(+) channel. J. Biol. Chem. 2003, 278, 49537–49548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condon, J.C.; Pezzi, V.; Drummond, B.M.; Yin, S.; Rainey, W.E. Calmodulin-dependent kinase I regulates adrenal cell expression of aldosterone synthase. Endocrinology 2002, 143, 3651–3657. [Google Scholar] [CrossRef] [Green Version]
- Scholl, U.I.; Abriola, L.; Zhang, C.; Reimer, E.N.; Plummer, M.; Kazmierczak, B.I.; Zhang, J.; Hoyer, D.; Merkel, J.S.; Wang, W.; et al. Macrolides selectively inhibit mutant KCNJ5 potassium channels that cause aldosterone-producing adenoma. J. Clin. Investig. 2017, 127, 2739–2750. [Google Scholar] [CrossRef] [Green Version]
- Maiolino, G.; Ceolotto, G.; Battistel, M.; Barbiero, G.; Cesari, M.; Amar, L.; Caroccia, B.; Padrini, R.; Azizi, M.; Rossi, G.P. Macrolides for KCNJ5-mutated aldosterone-producing adenoma (MAPA): Design of a study for personalized diagnosis of primary aldosteronism. Blood Press. 2018, 27, 200–205. [Google Scholar] [CrossRef]
- Wu, V.C.; Hu, Y.H.; Er, L.K.; Yen, R.F.; Chang, C.H.; Chang, Y.L.; Lu, C.C.; Chang, C.C.; Lin, J.H.; Lin, Y.H.; et al. Case detection and diagnosis of primary aldosteronism—The consensus of Taiwan Society of Aldosteronism. J. Formos. Med. Assoc. 2017, 116, 993–1005. [Google Scholar] [CrossRef]
- Chan, C.K.; Kim, J.H.; Chueh, E.; Chang, C.C.; Lin, Y.F.; Lai, T.S.; Huang, K.H.; Lin, Y.H.; Wu, V.C. Aldosterone level after saline infusion test could predict clinical outcome in primary aldosteronism after adrenalectomy. Surgery 2019, 166, 362–368. [Google Scholar] [CrossRef]
- Wu, V.C.; Huang, K.H.; Peng, K.Y.; Tsai, Y.C.; Wu, C.H.; Wang, S.M.; Yang, S.Y.; Lin, L.Y.; Chang, C.C.; Lin, Y.H.; et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci. Rep. 2015, 5, 11396. [Google Scholar] [CrossRef]
- Gomez-Sanchez, C.E.; Qi, X.; Velarde-Miranda, C.; Plonczynski, M.W.; Parker, C.R.; Rainey, W.; Satoh, F.; Maekawa, T.; Nakamura, Y.; Sasano, H.; et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol. Cell Endocrinol. 2014, 383, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Bird, I.M.; Hanley, N.A.; Word, R.A.; Mathis, J.M.; McCarthy, J.L.; Mason, J.I.; Rainey, W.E. Human NCI-H295 adrenocortical carcinoma cells: A model for angiotensin-II-responsive aldosterone secretion. Endocrinology 1993, 133, 1555–1561. [Google Scholar] [CrossRef]
- Chang, H.W.; Chu, T.S.; Huang, H.Y.; Chueh, S.C.; Wu, V.C.; Chen, Y.M.; Hsieh, B.S.; Wu, K.D. Down-regulation of D2 dopamine receptor and increased protein kinase Cmu phosphorylation in aldosterone-producing adenoma play roles in aldosterone overproduction. J. Clin. Endocrinol. Metab. 2007, 92, 1863–1870. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.W.; Wu, V.C.; Huang, C.Y.; Huang, H.Y.; Chen, Y.M.; Chu, T.S.; Wu, K.D.; Hsieh, B.S. D4 dopamine receptor enhances angiotensin II-stimulated aldosterone secretion through PKC-epsilon and calcium signaling. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E622–E629. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Kopf, P.G.; Campbell, W.B.; Luis Lam, M.; Yamazaki, T.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P. Angiotensin II and III metabolism and effects on steroid production in the HAC15 human adrenocortical cell line. Endocrinology 2013, 154, 214–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.Q.; Xie, D.; Choudhary, V.; Seremwe, M.; Tsai, Y.Y.; Olala, L.; Chen, X.; Bollag, W.B. The effect of pioglitazone on aldosterone and cortisol production in HAC15 human adrenocortical carcinoma cells. Mol. Cell. Endocrinol. 2014, 394, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, X.; Avalos, J.L.; Chen, J.; MacKinnon, R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 A resolution. Science 2009, 326, 1668–1674. [Google Scholar] [CrossRef] [Green Version]
- Jirouskova, Z.; Varekova, R.S.; Vanek, J.; Koca, J. Electronegativity equalization method: Parameterization and validation for organic molecules using the Merz-Kollman-Singh charge distribution scheme. J. Comput. Chem. 2009, 30, 1174–1178. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Vanderspoel, D.; Vandrunen, R. Gromacs—A Message-Passing Parallel Molecular-Dynamics Implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Schuttelkopf, A.W.; van Aalten, D.M. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta. Crystallogr. D Biol. Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef] [Green Version]
- Berger, O.; Edholm, O.; Jahnig, F. Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure, and constant temperature. Biophys. J. 1997, 72, 2002–2013. [Google Scholar] [CrossRef] [Green Version]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004, 25, 1656–1676. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Bekker, H.; Berendsen, H.J.; Fraaije, J.G. LINCS: A linear constraint solver for molecular simulations. J. Comp. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF. Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Charmandari, E.; Sertedaki, A.; Kino, T.; Merakou, C.; Hoffman, D.A.; Hatch, M.M.; Hurt, D.E.; Lin, L.; Xekouki, P.; Stratakis, C.A.; et al. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J. Clin. Endocrinol. Metab. 2012, 97, E1532–E1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes-Rosa, F.L.; Boulkroun, S.; Zennaro, M.C. Somatic and inherited mutations in primary aldosteronism. J. Mol. Endocrinol. 2017, 59, R47–R63. [Google Scholar] [CrossRef] [Green Version]
- Bassett, M.H.; White, P.C.; Rainey, W.E. The regulation of aldosterone synthase expression. Mol. Cell. Endocrinol. 2004, 217, 67–74. [Google Scholar] [CrossRef]
- Nanba, K.; Tsuiki, M.; Sawai, K.; Mukai, K.; Nishimoto, K.; Usui, T.; Tagami, T.; Okuno, H.; Yamamoto, T.; Shimatsu, A.; et al. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J. Clin. Endocrinol. Metab. 2013, 98, 1567–1574. [Google Scholar] [CrossRef] [Green Version]
- Meyer, L.S.; Handgriff, L.; Lim, J.S.; Udager, A.M.; Kinker, I.S.; Ladurner, R.; Wildgruber, M.; Knosel, T.; Bidlingmaier, M.; Rainey, W.E.; et al. Single-Center Prospective Cohort Study on the Histopathology, Genotype, and Postsurgical Outcomes of Patients with Primary Aldosteronism. Hypertension 2021, 78, 738–746. [Google Scholar] [CrossRef]
- Williams, T.A.; Gomez-Sanchez, C.E.; Rainey, W.E.; Giordano, T.J.; Lam, A.K.; Marker, A.; Mete, O.; Yamazaki, Y.; Zerbini, M.C.N.; Beuschlein, F.; et al. International Histopathology Consensus for Unilateral Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2021, 106, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Lenders, J.W.M.; Mulatero, P.; Burrello, J.; Rottenkolber, M.; Adolf, C.; Satoh, F.; Amar, L.; Quinkler, M.; Deinum, J.; et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: An international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017, 5, 689–699. [Google Scholar] [CrossRef] [Green Version]
- Sasano, H.; Mason, J.L.; Sasano, N.; Nagura, H. Immunolocalization of 3beta-hydroxysteroid dehydrogenase in human adrenal cortex and in its disorders. Endocr. Pathol. 1990, 1, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, X.; Wang, B.; Ma, X.; Li, H.; Gao, Y.; Gu, L.; Nie, W.; Zhang, X. Clinical Characteristics of Aldosterone- and Cortisol-Coproducing Adrenal Adenoma in Primary Aldosteronism. Int. J. Endocrinol. 2018, 2018, 4920841. [Google Scholar] [CrossRef]
- Inoue, K.; Yamazaki, Y.; Tsurutani, Y.; Suematsu, S.; Sugisawa, C.; Saito, J.; Omura, M.; Sasano, H.; Nishikawa, T. Evaluation of Cortisol Production in Aldosterone-Producing Adenoma. Horm. Metab. Res. 2017, 49, 847–853. [Google Scholar] [CrossRef]
- Arnesen, T.; Glomnes, N.; Stromsoy, S.; Knappskog, S.; Heie, A.; Akslen, L.A.; Grytaas, M.; Varhaug, J.E.; Gimm, O.; Brauckhoff, M. Outcome after surgery for primary hyperaldosteronism may depend on KCNJ5 tumor mutation status: A population-based study from Western Norway. Langenbeck’s Arch. Surg. 2013, 398, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, L.; Jiang, L.; Su, T.; Zhou, W.; Zhong, X.; Xie, J.; Sun, F.; Zhu, Y.; Jiang, Y.; et al. KCNJ5 Mutation Contributes to Complete Clinical Success in Aldosterone-Producing Adenoma: A Study from a Single Center. Endocr. Pract. 2021. [Google Scholar] [CrossRef]
- Kitamoto, T.; Omura, M.; Suematsu, S.; Saito, J.; Nishikawa, T. KCNJ5 mutation as a predictor for resolution of hypertension after surgical treatment of aldosterone-producing adenoma. J. Hypertens. 2018, 36, 619–627. [Google Scholar] [CrossRef]
- Caroccia, B.; Prisco, S.; Seccia, T.M.; Piazza, M.; Maiolino, G.; Rossi, G.P. Macrolides Blunt Aldosterone Biosynthesis: A Proof-of-Concept Study in KCNJ5 Mutated Adenoma Cells Ex Vivo. Hypertension 2017, 70, 1238–1242. [Google Scholar] [CrossRef]
- Hattangady, N.G.; Karashima, S.; Yuan, L.; Ponce-Balbuena, D.; Jalife, J.; Gomez-Sanchez, C.E.; Auchus, R.J.; Rainey, W.E.; Else, T. Mutated KCNJ5 activates the acute and chronic regulatory steps in aldosterone production. J. Mol. Endocrinol. 2016, 57, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauber, P.; Penton, D.; Stindl, J.; Humberg, E.; Tegtmeier, I.; Sterner, C.; Beuschlein, F.; Reincke, M.; Barhanin, J.; Bandulik, S.; et al. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology 2014, 155, 1353–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Ma, X.; Tong, A.; Zhang, Y.; Wen, J.; Li, Y. The Effects of Different Calcium Channel Blockers on Aldosterone-Producing Adenoma Cells. Front. Endocrinol. (Lausanne) 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, K.-Y.; Liao, H.-W.; Chueh, J.S.; Pan, C.-Y.; Lin, Y.-H.; Chen, Y.-M.; Chen, P.-Y.; Huang, C.-L.; Wu, V.-C., on behalf of the Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group. Pathophysiological and Pharmacological Characteristics of KCNJ5 157-159delITE Somatic Mutation in Aldosterone-Producing Adenomas. Biomedicines 2021, 9, 1026. https://doi.org/10.3390/biomedicines9081026
Peng K-Y, Liao H-W, Chueh JS, Pan C-Y, Lin Y-H, Chen Y-M, Chen P-Y, Huang C-L, Wu V-C on behalf of the Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group. Pathophysiological and Pharmacological Characteristics of KCNJ5 157-159delITE Somatic Mutation in Aldosterone-Producing Adenomas. Biomedicines. 2021; 9(8):1026. https://doi.org/10.3390/biomedicines9081026
Chicago/Turabian StylePeng, Kang-Yung, Hung-Wei Liao, Jeff S. Chueh, Chien-Yuan Pan, Yen-Hung Lin, Yung-Ming Chen, Peng-Ying Chen, Chun-Lin Huang, and Vin-Cent Wu on behalf of the Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group. 2021. "Pathophysiological and Pharmacological Characteristics of KCNJ5 157-159delITE Somatic Mutation in Aldosterone-Producing Adenomas" Biomedicines 9, no. 8: 1026. https://doi.org/10.3390/biomedicines9081026
APA StylePeng, K.-Y., Liao, H.-W., Chueh, J. S., Pan, C.-Y., Lin, Y.-H., Chen, Y.-M., Chen, P.-Y., Huang, C.-L., & Wu, V.-C., on behalf of the Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group. (2021). Pathophysiological and Pharmacological Characteristics of KCNJ5 157-159delITE Somatic Mutation in Aldosterone-Producing Adenomas. Biomedicines, 9(8), 1026. https://doi.org/10.3390/biomedicines9081026