Biologic Therapy and Severe Asthma in Children
Abstract
1. Introduction
2. Severe Asthma
- -
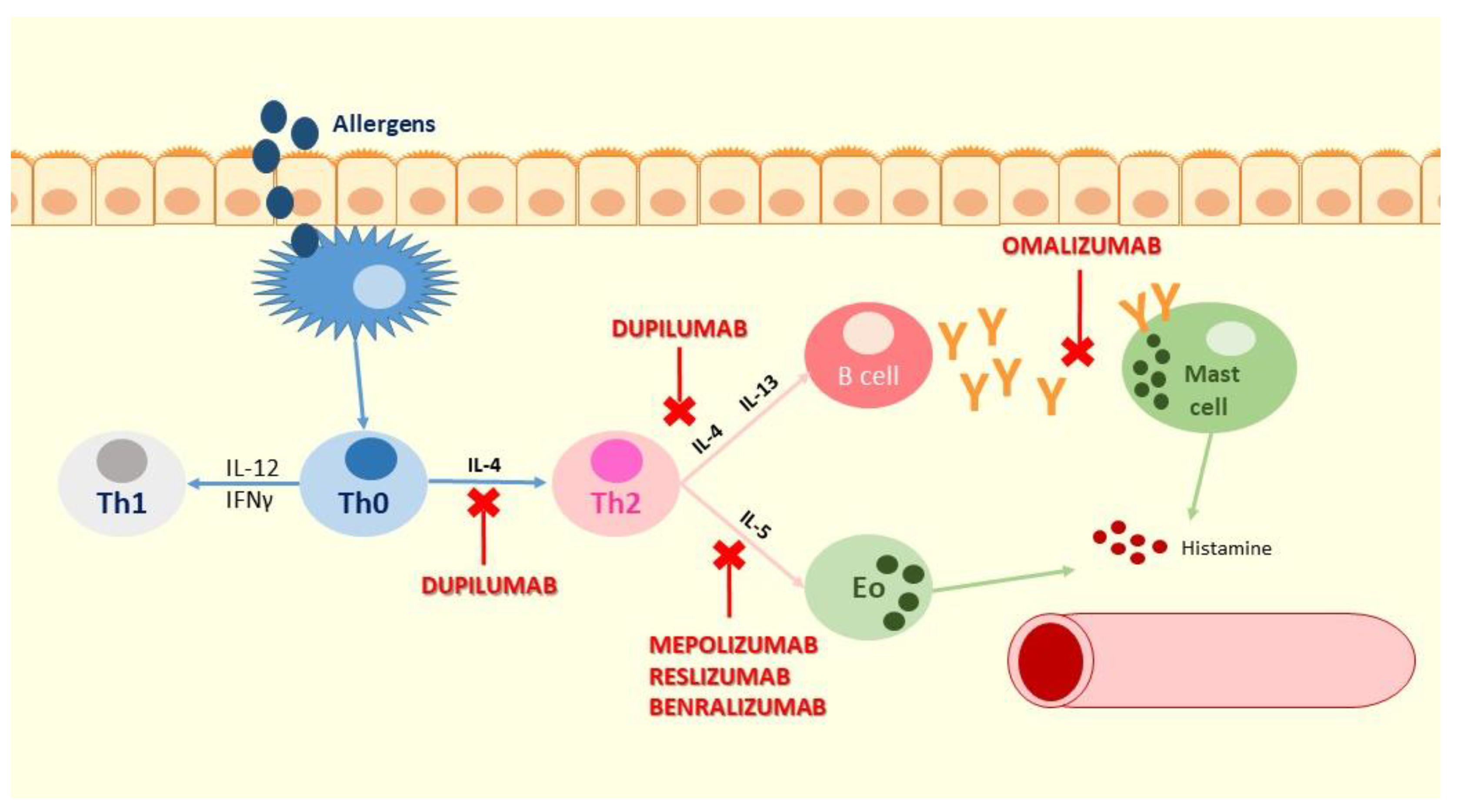
- IL-5 is released by mast cells, T2 and innate lymphoid 2 cells (ILC2s); IL-5 binds to the IL-5 receptor (IL-5R) on eosinophils and basophils, inducing eosinophil proliferation, activation, recruitment and release of cytokines that lead to airway hyperresponsiveness and remodeling [38]
- -
- IL–4 and IL-13 are released by mast cells, ILC2s and Th2 cells; they bind to the type 2 receptor complex (IL-4Rα/IL-13Rα1) on airway epithelial and smooth muscle cells, eosinophils, and mast cells. IL-4 also binds to the type 1 receptor complex, consisting of IL-4Rα and a γc chain, which leads to upregulation of T2 responses, downregulation of T1 responses and accumulation of IgE. IL-13 directly affects airway contraction and increases airway mucous production. It also stimulates periostin release from airway epithelial cells, contributing to tissue remodeling [38].
3. Biologic Drugs in Severe Asthma
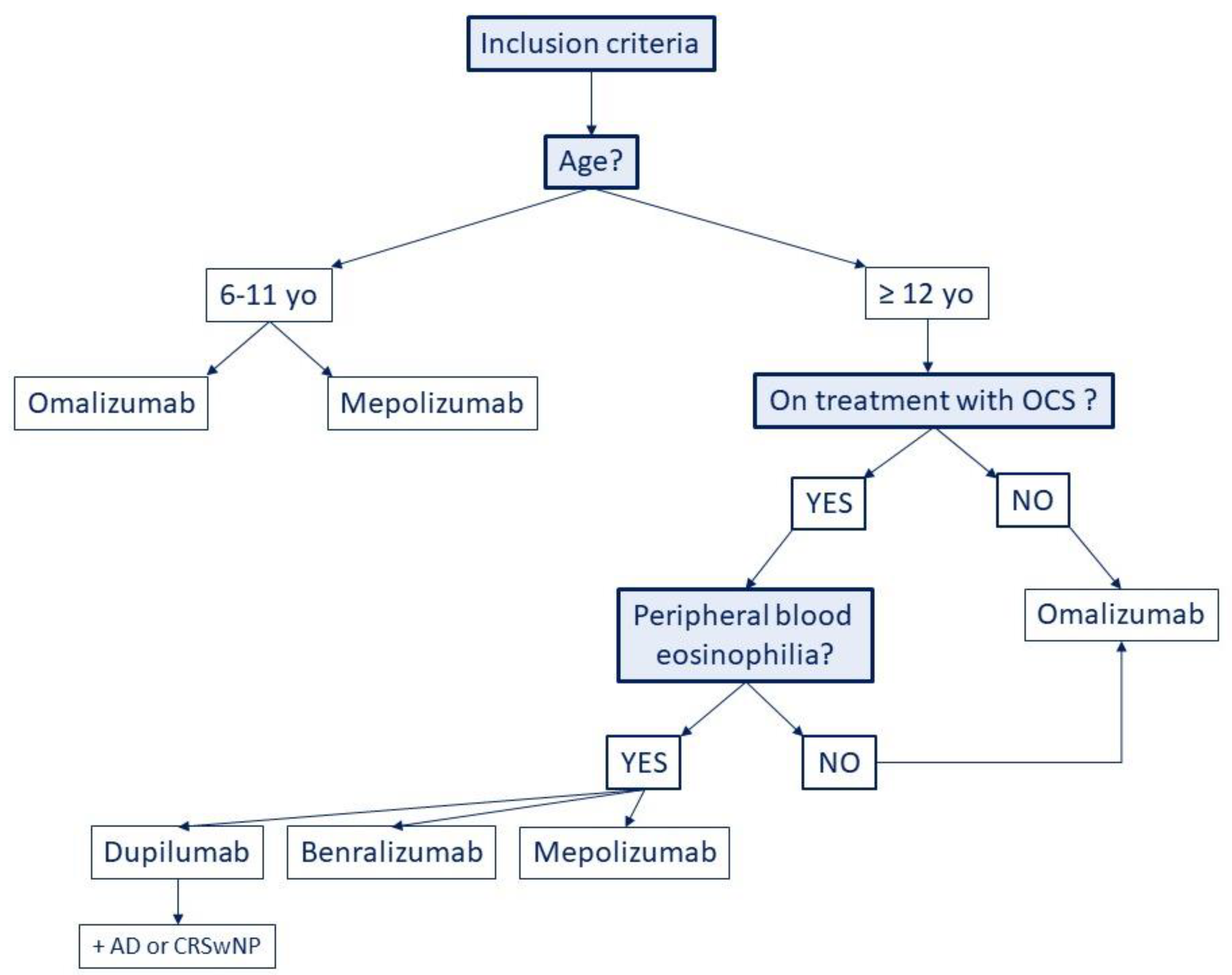
3.1. Omalizumab
3.2. Mepolizumab
3.3. Reslizumab
3.4. Benralizumab
3.5. Dupilumab
3.6. Tezepelumab
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Little, M.; Kipriyanov, S.; Le Gall, F.; Moldenhauer, G. Of mice and men: Hybridoma and recombinant antibodies. Immunol. Today 2000, 21, 364–370. [Google Scholar] [CrossRef]
- Goulet, D.R.; Atkins, W.M. Considerations for the design of antibody-based therapeutics. J. Pharm. Sci. 2020, 109, 74–103. [Google Scholar] [CrossRef]
- Shepard, H.M.; Phillips, G.L.; Thanos, C.D.; Feldmann, M. Developments in therapy with monoclonal antibodies and related proteins. Clin. Med. 2017, 17, 220–232. [Google Scholar] [CrossRef]
- Rita Costa, A.; Elisa Rodrigues, M.; Henriques, M.; Azeredo, J.; Oliveira, R. Guidelines to cell engineering for monoclonal antibody production. Eur. J. Pharm. Biopharm. 2010, 74, 127–138. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Leavy, O. Therapeutic antibodies: Past, present and future. Nat. Rev. Immunol. 2010, 10, 297. [Google Scholar] [CrossRef]
- Morrison, S.L.; Johnson, M.J.; Herzenberg, L.A.; Oi, V.T. Chimeric human antibody molecules: Mouse antigen-binding domains with human constant region domains. Proc. Natl. Acad. Sci. USA 1984, 81, 6851–6855. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.H.; Wiseman, L.R. Abciximab. Drugs 1998, 56, 629–665. [Google Scholar] [CrossRef] [PubMed]
- Maloney, D.G.; Grillo-López, A.J.; White, C.A.; Bodkin, D.; Schilder, R.J.; Neidhart, J.A.; Janakiraman, N.; Foon, K.A.; Liles, T.-M.; Dallaire, B.K.; et al. IDEC-C2B8 (Rituximab) Anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood 1997, 90, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Maloney, D.G.; Grillo-López, A.J.; Bodkin, D.J.; White, C.A.; Liles, T.M.; Royston, I.; Varns, C.; Rosenberg, J.; Levy, R. IDEC-C2B8: Results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J. Clin. Oncol. 1997, 15, 3266–3274. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.H. The history of monoclonal antibody development—progress, remaining challenges and future innovations. Ann. Med. Surg. 2014, 3, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.T.; Dear, P.H.; Foote, J.; Neuberger, M.S.; Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 1986, 321, 522–525. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, J.; Griffiths, A.D.; Winter, G.; Chiswell, D.J. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature 1990, 348, 552–554. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Buss, N.A.; Henderson, S.J.; McFarlane, M.; Shenton, J.M.; de Haan, L. Monoclonal antibody therapeutics: History and future. Curr. Opin. Pharmacol. 2012, 12, 615–622. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of asthma in children and adults. Front. Pediatr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.S.; Bailey, C.M.; Damon, S.A.; Garbe, P.L.; Breysse, P.N. Vital signs: Asthma in children—United States, 2001–2016. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 149–155. [Google Scholar] [CrossRef]
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- British Thoracic Society Scottish intercollegiate guidelines network. British guideline on the management of asthma. Thorax 2014, 69 (Suppl. 1), 1–121. [Google Scholar]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2021. Available online: www.ginasthma.org (accessed on 10 April 2021).
- Pijnenburg, M.W.; Fleming, L. Advances in understanding and reducing the burden of severe asthma in children. Lancet Respir. Med. 2020, 8, 1032–1044. [Google Scholar] [CrossRef]
- Fleming, L.; Murray, C.; Bansal, A.T.; Hashimoto, S.; Bisgaard, H.; Bush, A.; Frey, U.; Hedlin, G.; Singer, F.; van Aalderen, W.M.; et al. The burden of severe asthma in childhood and adolescence: Results from the paediatric U-BIOPRED cohorts. Eur. Respir. J. 2015, 46, 1322–1333. [Google Scholar] [CrossRef]
- British Thoracic Society/Scottish intercollegiate guideline network, British guideline on the management of asthma. 2016. Available online: https://www.brit-thoracic.org.uk/document-library/guidelines/asthma/btssign-asthma-guideline-2016 (accessed on 15 April 2021).
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef]
- Hedlin, G.; Bush, A.; Lodrup Carlsen, K.; Wennergren, G.; De Benedictis, F.M.; Melen, E.; Paton, J.; Wilson, N.; Carlsen, K.-H. Problematic severe asthma in children, not one problem but many: A GA2LEN initiative. Eur. Respir. J. 2010, 36, 196–201. [Google Scholar] [CrossRef]
- Dinakar, C.; Chipps, B.E. Clinical tools to assess asthma control in children. Pediatrics 2017, 139, e20163438. [Google Scholar] [CrossRef] [PubMed]
- Teague, W.G.; Phillips, B.R.; Fahy, J.V.; Wenzel, S.E.; Fitzpatrick, A.M.; Moore, W.C.; Hastie, A.T.; Bleecker, E.R.; Meyers, D.A.; Peters, S.P.; et al. Baseline features of the severe asthma research program (SARP III) cohort: Differences with age. J. Allergy Clin. Immunol. Pract. 2018, 6, 545–554.e4. [Google Scholar] [CrossRef]
- Fleming, M.; Fitton, C.A.; Steiner, M.F.C.; McLay, J.S.; Clark, D.; King, A.; Mackay, D.F.; Pell, J.P. Educational and health outcomes of children treated for asthma: Scotland-wide record linkage study of 683 716 children. Eur. Respir. J. 2019, 54, 1802309. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, L.; Ferrante, G.; Montella, S.; Cilluffo, G.; Di Marco, A.; Bozzetto, S.; Di Palmo, E.; Licari, A.; Leonardi, L.; Caldarelli, V.; et al. Relationship between quality of life and behavioural disorders in children with persistent asthma: A multiple indicators multiple causes (MIMIC) model. Sci. Rep. 2020, 10, 6957. [Google Scholar] [CrossRef] [PubMed]
- Porsbjerg, C.; Menzies-Gow, A. Co-morbidities in severe asthma: Clinical impact and management. Respirology 2017, 22, 651–661. [Google Scholar] [CrossRef]
- Lötvall, J.; Akdis, C.A.; Bacharier, L.B.; Bjermer, L.; Casale, T.B.; Custovic, A.; Lemanske, R.F., Jr.; Wardlaw, A.J.; Wenzel, S.E.; Greenberger, P.A. Asthma endotypes: A new approach to classification of disease entities within the asthma syndrome. J. Allergy Clin. Immunol. 2011, 127, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Licari, A.; Castagnoli, R.; Brambilla, I.; Marseglia, A.; Tosca, M.A.; Marseglia, G.L.; Ciprandi, G. Asthma endotyping and biomarkers in childhood asthma. Pediatr. Allergy. Immunol. Pulmonol. 2018, 31, 44–55. [Google Scholar] [CrossRef]
- Schoettler, N.; Strek, M.E. Recent advances in severe asthma. Chest 2020, 157, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Licari, A.; Manti, S.; Marseglia, A.; De Filippo, M.; De Sando, E.; Foiadelli, T.; Marseglia, G.L. Biologics in children with allergic diseases. Curr. Pediatr. Rev. 2020, 16, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Casale, T.B. Biologics and biomarkers for asthma, urticaria, and nasal polyposis. J. Allergy Clin. Immunol. 2017, 139, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.N.; Renz, H.; Casale, T.B.; Garn, H. Biologic therapy and novel molecular targets of severe asthma. J. Allergy Clin. Immunol. Pract. 2017, 5, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.R.; Casale, T.B. Characterization of asthma endotypes: Implications for therapy. Ann. Allergy Asthma Immunol. 2016, 117, 121–125. [Google Scholar] [CrossRef]
- Saco, T.V.; Pepper, A.; Casale, T.B. Uses of biologics in allergic diseases. Ann. Allergy Asthma Immunol. 2018, 120, 357–366. [Google Scholar] [CrossRef]
- Walsh, G.M. An update on biologic-based therapy in asthma. Immunotherapy 2013, 5, 1255–1264. [Google Scholar] [CrossRef]
- XOLAIR (Omalizumab) prescribing information. Available online: http://www.xolair.com/ (accessed on 5 October 2017).
- Milgrom, H.; Berger, W.; Nayak, A.; Gupta, N.; Pollard, S.; McAlary, M.; Taylor, A.F.; Rohane, P. Treatment of childhood asthma with anti-immunoglobulin e antibody (omalizumab). Pediatrics 2001, 108, e36. [Google Scholar] [CrossRef] [PubMed]
- Chipps, B.E.; Lanier, B.; Milgrom, H.; Deschildre, A.; Hedlin, G.; Szefler, S.J.; Kattan, M.; Kianifard, F.; Ortiz, B.; Haselkorn, T.; et al. Omalizumab in children with uncontrolled allergic asthma: Review of clinical trial and real-world experience. J. Allergy Clin. Immunol. 2017, 139, 1431–1444. [Google Scholar] [CrossRef]
- Brodlie, M.; McKean, M.C.; Moss, S.; Spencer, D.A. The oral corticosteroid-sparing effect of omalizumab in children with severe asthma. Arch. Dis. Child. 2012, 97, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Mumm, J.; Mahr, T.A. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. Pediatrics 2011, 128, 1005–1015. [Google Scholar] [CrossRef]
- Teach, S.J.; Gill, M.A.; Togias, A.; Sorkness, C.A.; Arbes, S.J.; Calatroni, A.; Wildfire, J.J.; Gergen, P.J.; Cohen, R.T.; Pongracic, J.A.; et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J. Allergy Clin. Immunol. 2015, 136, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Deschildre, A.; Marguet, C.; Salleron, J.; Pin, I.; Rittié, J.L.; Derelle, J.; Taam, R.A.; Fayon, M.; Brouard, J.; Dubus, J.C.; et al. Add-on omalizumab in children with severe allergic asthma: A 1-year real life survey. Eur. Respir. J. 2013, 42, 1224–1233. [Google Scholar] [CrossRef]
- Deschildre, A.; Marguet, C.; Langlois, C.; Pin, I.; Rittié, J.L.; Derelle, J.; Taam, R.A.; Fayon, M.; Brouard, J.; Dubus, J.C.; et al. Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur. Respir. J. 2015, 46, 856–859. [Google Scholar] [CrossRef]
- Licari, A.; Castagnoli, R.; Denicolo, C.; Rossini, L.; Seminara, M.; Sacchi, L.; Testa, G.; De Amici, M.; Marseglia, G.L. Omalizumab in children with severe allergic asthma: The Italian real- life experience. Curr. Respir. Med. Rev. 2017, 13, 36–42. [Google Scholar] [CrossRef]
- Pitrez, P.M.; de Souza, R.G.; Roncada, C.; Heinzmann-Filho, J.P.; Santos, G.; Pinto, L.A.; Jones, M.H.; Stein, R.T. Impact of omalizumab in children from a middle-income country with severe therapy-resistant asthma: A real-life study. Pediatr. Pulmonol. 2017, 52, 1408–1413. [Google Scholar] [CrossRef]
- NUCALA® (Mepolizumab) EMA Approval. Available online: https://gskprocom/content/ (accessed on 20 April 2021).
- GlaxoSmithKline. Nucala (Mepolizumab) for Injection; Prescribing Information; GlaxoSmithKline: Zebulon, NC, USA, 2015. [Google Scholar]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Drick, N.; Seeliger, B.; Welte, T.; Fuge, J.; Suhling, H. Anti-IL-5 therapy in patients with severe eosinophilic asthma—clinical efficacy and possible criteria for treatment response. BMC Pulm. Med. 2018, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Haldar, P. Patient profiles and clinical utility of mepolizumab in severe eosinophilic asthma. Biol. Targets Ther. 2017, 11, 81–95. [Google Scholar] [CrossRef][Green Version]
- Mepolizumab for Treating Severe Refractory Eosinophilic Asthma. NICE Technology Appraisal Guidance [TA431]. Available online: https://www.nice.org.uk/guidance/ta431 (accessed on 20 April 2021).
- Farne, H.A.; Wilson, A.; Powell, C.; Bax, L.; Milan, S.J. Anti-IL5 therapies for asthma. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Flood-Page, P.; Swenson, C.; Faiferman, I.; Matthews, J.; Williams, M.; Brannick, L.; Robinson, D.; Wenzel, S.; Busse, W.; Hansel, T.T.; et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am. J. Respir. Crit. Care Med. 2007, 176, 1062–1071. [Google Scholar] [CrossRef]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Lugogo, N.; Domingo, C.; Chanez, P.; Leigh, R.; Gilson, M.J.; Price, R.G.; Yancey, S.W.; Ortega, H.G. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: A multi-center, open-label, phase IIIb study. Clin. Ther. 2016, 38, 2058–2070.e1. [Google Scholar] [CrossRef]
- Khatri, S.; Moore, W.; Gibson, P.G.; Leigh, R.; Bourdin, A.; Maspero, J.; Barros, M.; Buhl, R.; Howarth, P.; Albers, F.C.; et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J. Allergy Clin. Immunol. 2019, 143, 1742–1751.e7. [Google Scholar] [CrossRef]
- Corren, J.; Weinstein, S.; Janka, L.; Zangrilli, J.; Garin, M. Phase 3 study of reslizumab in patients with poorly controlled asthma. Chest 2016, 150, 799–810. [Google Scholar] [CrossRef]
- Bjermer, L.; Lemiere, C.; Maspero, J.; Weiss, S.; Zangrilli, J.; Germinaro, M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels. Chest 2016, 150, 789–798. [Google Scholar] [CrossRef]
- Castro, M.; Zangrilli, J.; Wechsler, M.E.; Bateman, E.D.; Brusselle, G.G.; Bardin, P.; Murphy, K.; Maspero, J.F.; O’Brien, C.; Korn, S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: Results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015, 3, 355–366. [Google Scholar] [CrossRef]
- Mukherjee, M.; Aleman Paramo, F.; Kjarsgaard, M.; Salter, B.; Nair, G.; LaVigne, N.; Radford, K.; Sehmi, R.; Nair, P. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am. J. Respir. Crit. Care Med. 2018, 197, 38–46. [Google Scholar] [CrossRef]
- Murphy, K.; Jacobs, J.; Bjermer, L.; Fahrenholz, J.M.; Shalit, Y.; Garin, M.; Zangrilli, J.; Castro, M. Long-term safety and efficacy of reslizumab in patients with eosinophilic asthma. J. Allergy Clin. Immunol. Pract. 2017, 5, 1572.e3–1581.e3. [Google Scholar] [CrossRef]
- FASENRA® (Benralizumab) Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000l-bl.pdf (accessed on 23 April 2021).
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef]
- Busse, W.W.; Bleecker, E.R.; FitzGerald, J.M.; Ferguson, G.T.; Barker, P.; Sproule, S.; Olsson, R.F.; Martin, U.J.; Goldman, M.; Yañez, A.; et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir. Med. 2019, 7, 46–59. [Google Scholar] [CrossRef]
- LaPorte, S.L.; Juo, Z.S.; Vaclavikova, J.; Colf, L.A.; Qi, X.; Heller, N.M.; Keegan, A.D.; Garcia, K.C. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell 2008, 132, 259–272. [Google Scholar] [CrossRef]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef]
- Huang, S.C.-C.; Smith, A.M.; Everts, B.; Colonna, M.; Pearce, E.L.; Schilling, J.D.; Pearce, E.J. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity 2016, 45, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, T.R.; Pesce, J.T.; Sheikh, F.; Cheever, A.W.; Mentink-Kane, M.M.; Wilson, M.S.; Stevens, S.; Valenzuela, D.M.; Murphy, A.J.; Yancopoulos, G.D.; et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat. Immunol. 2008, 9, 25–33. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Mannent, L.; Naclerio, R.M.; Mullol, J.; Ferguson, B.J.; Gevaert, P.; Hellings, P.; Jiao, L.; Wang, L.; Evans, R.R.; et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis. JAMA 2016, 315, 469. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in adults with uncontrolled asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
| Biological Drug | Structure | Action | Dosage | Age (Years) | References |
|---|---|---|---|---|---|
| Omalizumab | Humanized IgG1 | Anti-IgE | In moderate to severe allergic asthma:
| ≥6 (AIFA) ≥6 (EMA) ≥6 (FDA) ≥18 in CRSwNP | [39,40] |
| Mepolizumab | Humanized IgG1 | Anti-IL-5 | In severe eosinophilic asthma:
| ≥6 (AIFA) ≥6 (EMA) ≥6 (FDA) | [50,51] |
| Reslizumab | Humanized IgG4 | Anti-IL-5 | In severe eosinophilic asthma:
| ≥18 (AIFA) ≥18 (EMA) ≥18 (FDA) | [62,63] |
| Benralizumab | Humanized IgG1 | Anti-IL-5Rα | In severe eosinophilic asthma:
| ≥18 (AIFA) ≥18 (EMA) ≥12 (FDA) | [67] |
| Dupilumab | Human IgG4 | Anti-IL-4Rα | In moderate-to-severe eosinophilic asthma, CRSwNP & moderate-to-severe atopic dermatitis
| ≥12 (AIFA) ≥12 (EMA) ≥12 in asthma ≥6 DA (FDA) | [72,73] |
| Tezepelumab | Human IgG2 | Anti-TSLP | In severe asthma, especially with:
| Phase 3 RCTs (NAVIGATOR, SOURCE) ongoing ≥ 12 | [80,81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, D.; Di Filippo, P.; Attanasi, M.; Lizzi, M.; Di Pillo, S.; Chiarelli, F. Biologic Therapy and Severe Asthma in Children. Biomedicines 2021, 9, 760. https://doi.org/10.3390/biomedicines9070760
Russo D, Di Filippo P, Attanasi M, Lizzi M, Di Pillo S, Chiarelli F. Biologic Therapy and Severe Asthma in Children. Biomedicines. 2021; 9(7):760. https://doi.org/10.3390/biomedicines9070760
Chicago/Turabian StyleRusso, Daniele, Paola Di Filippo, Marina Attanasi, Mauro Lizzi, Sabrina Di Pillo, and Francesco Chiarelli. 2021. "Biologic Therapy and Severe Asthma in Children" Biomedicines 9, no. 7: 760. https://doi.org/10.3390/biomedicines9070760
APA StyleRusso, D., Di Filippo, P., Attanasi, M., Lizzi, M., Di Pillo, S., & Chiarelli, F. (2021). Biologic Therapy and Severe Asthma in Children. Biomedicines, 9(7), 760. https://doi.org/10.3390/biomedicines9070760








