Leukotriene B4 Receptors Are Necessary for the Stimulation of NLRP3 Inflammasome and IL-1β Synthesis in Neutrophil-Dominant Asthmatic Airway Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Model for HDM-Driven Neutrophil-Dominant Airway Inflammation
2.3. Preparation of Lung Tissue Lysates and Immunoblot Analysis
2.4. Measurement of LTB4, IL-1β and Myeloperoxidase (MPO)
2.5. Bronchoalveolar Lavage (BAL) Cell Counting
2.6. Histological Analysis of Lung Tissues Including Immunofluorescence (IF) Staining
2.7. Statistical Analysis
3. Results
3.1. Stimulation of the NLRP3 Inflammasome Is Necessary for HDM/LPS-Induced Neutrophilic Airway Inflammation
3.2. IL-1β Is Produced via NLRP3 Stimulation and Is Critical for the HDM/LPS-Induced Neutrophilic Airway Inflammation
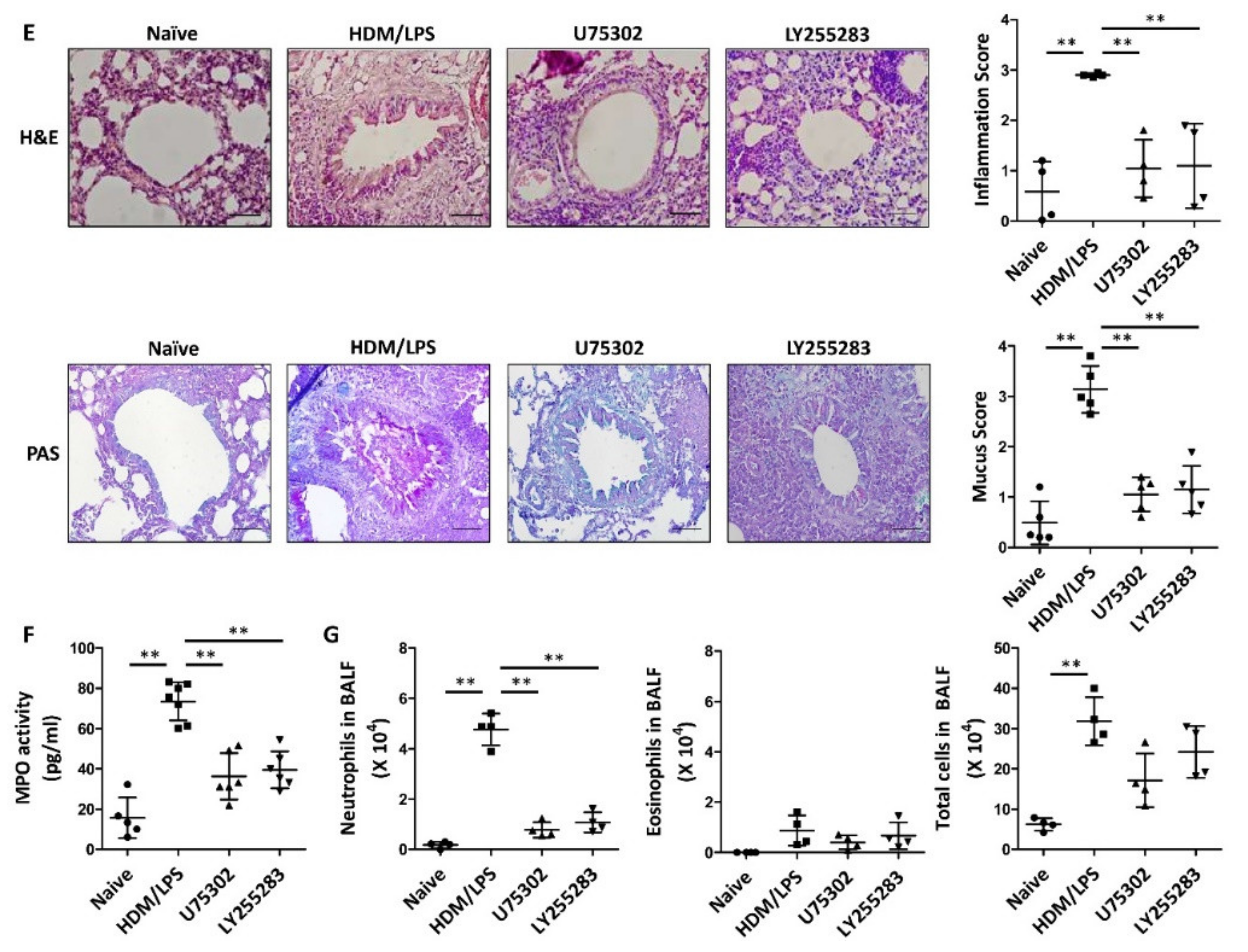
3.3. LTB4 Receptors Are Necessary for the Stimulation of the NLRP3 Inflammasome and IL-1β Synthesis in HDM/LPS-Induced Neutrophilic Airway Inflammation
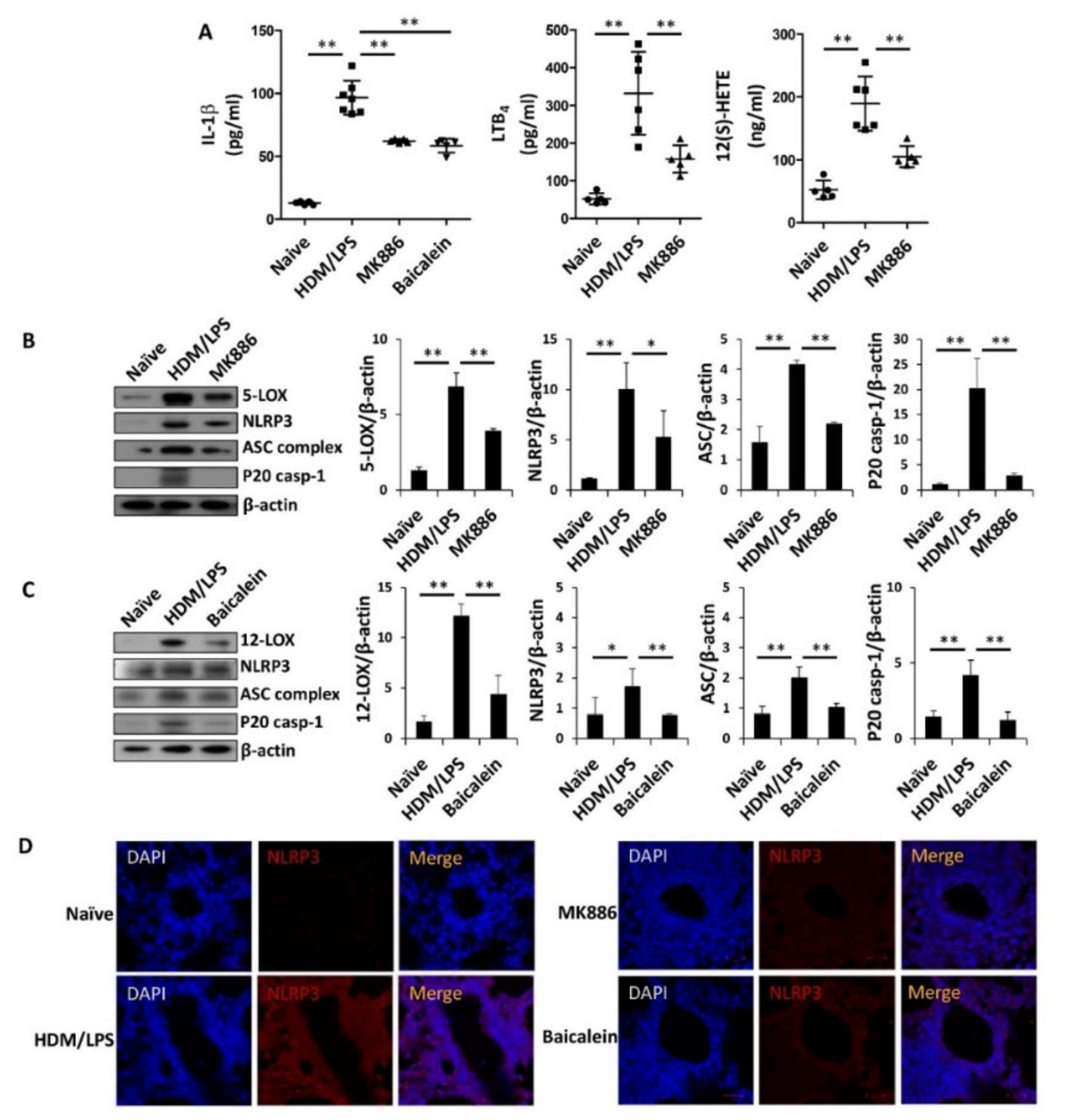
3.4. 5-/12-Lipoxygenase Are also Necessary for the Stimulation of the NLRP3 Inflammasome and IL-1β Production in HDM/LPS-Driven Neutrophilic Airway Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fajt, M.L.; Wenzel, S.E. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: The next steps toward personalized care. J. Allergy Clin. Immunol. 2015, 135, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Scott, R.; Boyle, M.J.; Gibson, P.G. Inflammatory subtypes in asthma: Assessment and identification using induced sputum. Respirology 2006, 11, 54–61. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.J.; Foster, P.S.; Hansbro, P.M.; Yang, M. Potential therapeutic targets for steroid-resistant asthma. Curr. Drug Targets 2010, 11, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.; Bracke, K. Targeting immune pathways for therapy in asthma and chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2014, 11, 322–328. [Google Scholar] [CrossRef]
- Bullens, D.M.A.; Truyen, E.; Coteur, L.; Dilissen, E.; Hellings, P.W.; Dupont, L.J.; Ceuppens, J.L. IL-17 mRNA in sputum of asthmatic patients: Linking T cell driven inflammation and granulocytic influx? Respir. Res. 2006, 7, 135. [Google Scholar] [CrossRef]
- Kwak, D.-W.; Park, D.; Kim, J.-H. Leukotriene B4 receptors play critical roles in house dust mites-induced neutrophilic airway inflammation and IL-17 production. Biochem. Biophys. Res. Commun. 2021, 534, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Adcock, I.M. Glucocorticoid resistance in inflammatory diseases. Lancet 2009, 373, 1905–1917. [Google Scholar] [CrossRef]
- Chung, K.F.; Godard, P.; Adelroth, E.; Ayres, J.; Barnes, N.; Barnes, P.; Bel, E.; Burney, P.; Chanez, P.; Connett, G.; et al. Difficult/therapy-resistant asthma: The need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma. European Respiratory Society. Eur. Respir J. 1999, 13, 1198–1208. [Google Scholar]
- Simpson, J.L.; Phipps, S.; Baines, K.J.; Oreo, K.M.; Gunawardhana, L.; Gibson, P.G. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur. Respir. J. 2013, 43, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.Y.; Pinkerton, J.W.; Essilfie, A.T.; Robertson, A.A.B.; Baines, K.J.; Brown, A.C.; Mayall, J.R.; Ali, M.K.; Starkey, M.R.; Hansbro, N.G.; et al. Role for NLRP3 Inflammasome-mediated, IL-1beta-dependent responses in severe, steroid-resistant asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 283–297. [Google Scholar] [CrossRef]
- Afonso, P.V.; Janka-Junttila, M.; Lee, Y.J.; McCann, C.P.; Oliver, C.M.; Aamer, K.A.; Losert, W.; Cicerone, M.T.; Parent, C.A. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell 2012, 22, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Sze, E.; Bhalla, A.; Nair, P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy 2020, 75, 311–325. [Google Scholar] [CrossRef]
- Gelfand, E.W. Importance of the leukotriene B4-BLT1 and LTB4-BLT2 pathways in asthma. Semin. Immunol. 2017, 33, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Vachier, I.; Bonnans, C.; Chavis, C.; Farce, M.; Godard, P.; Bousquet, J.; Chanez, P. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J. Allergy Clin. Immunol. 2005, 115, 55–60. [Google Scholar] [CrossRef]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Leishangthem, G.D.; Singh, S.; Dinda, A.K.; Biswal, S.; Agrawal, A.; Ghosh, B. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013, 3, srep01540. [Google Scholar] [CrossRef] [PubMed]
- Kujur, W.; Gurram, R.K.; Haleem, N.; Maurya, S.K.; Agrewala, J.N. Caerulomycin A inhibits Th2 cell activity: A possible role in the management of asthma. Sci. Rep. 2015, 5, 15396. [Google Scholar] [CrossRef]
- Israel, E.; Reddel, H.K. Severe and difficult-to-treat asthma in adults. N. Engl. J. Med. 2017, 377, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Eder, W.; Ege, M.J.; Von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Hansbro, P.M.; Scott, G.V.; Essilfie, A.-T.; Kim, R.Y.; Starkey, M.R.; Nguyen, D.H.; Allen, P.D.; Kaiko, G.E.; Yang, M.; Horvat, J.C.; et al. Th2 cytokine antagonists: Potential treatments for severe asthma. Expert Opin. Investig. Drugs 2012, 22, 49–69. [Google Scholar] [CrossRef]
- Kim, H.Y.; DeKruyff, R.H.; Umetsu, D.T. The many paths to asthma: Phenotype shaped by innate and adaptive immunity. Nat. Immunol. 2010, 11, 577–584. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Hansbro, P.M. Harnessing regulatory T cells to suppress asthma: From potential to therapy. Am. J. Respir. Cell Mol. Biol. 2010, 43, 511–519. [Google Scholar] [CrossRef]
- Park, D.; Kwak, D.-W.; Kim, J.-H. Leukotriene B4 receptors contribute to house dust mite-induced eosinophilic airway inflammation via TH2 cytokine production. BMB Rep. 2021, 54, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Grissell, T.V.; Douwes, J.; Scott, R.J.; Boyle, M.J.; Gibson, P.G. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax 2007, 62, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ro, M.; Kwon, S.; Kim, J. Leukotriene B4 receptors mediate the production of IL-17, thus contributing to neutrophil-dominant asthmatic airway inflammation. Allergy 2019, 74, 1797–1799. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, H.; Wang, S.; Li, Y. Different doses of lipopolysaccharides regulate the lung inflammation of asthmatic mice via TLR4 pathway in alveolar macrophages. J. Asthma 2009, 46, 229–233. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, D.I.; Kang, M.R.; Lee, K.S.; Park, S.Y.; Jeong, J.S.; Lee, Y.C. Endoplasmic reticulum stress influences bronchial asthma pathogenesis by modulating nuclear factor kappaB activation. J. Allergy Clin. Immunol. 2013, 132, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Oh, S.-Y.; Jeon, S.G.; Park, H.-W.; Lee, S.-Y.; Chun, E.-Y.; Bang, B.; Lee, H.-S.; Oh, M.-H.; Kim, Y.-Y.; et al. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J. Immunol. 2007, 178, 5375–5382. [Google Scholar] [CrossRef]
- Alam, R.; Good, J.; Rollins, D.; Verma, M.; Chu, H.; Pham, T.-H.; Martin, R.J. Airway and serum biochemical correlates of refractory neutrophilic asthma. J. Allergy Clin. Immunol. 2017, 140, 1004–1014.e13. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Douda, D.N.; Brüggemann, T.R.; Ricklefs, I.; Duvall, M.G.; Abdulnour, R.-E.E.; Martinod, K.; Tavares, L.; Wang, X.; Cernadas, M.; et al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci. Immunol. 2018, 3, eaao4747. [Google Scholar] [CrossRef]
- Daan de Boer, J.; Roelofs, J.J.; de Vos, A.F.; de Beer, R.; Schouten, M.; Hommes, T.J.; Hoogendijk, A.J.; de Boer, O.J.; Stroo, I.; van der Zee, J.S.; et al. Lipopolysaccharide inhibits Th2 lung inflammation induced by house dust mite allergens in mice. Am. J. Respir. Cell Mol. Biol. 2013, 48, 382–389. [Google Scholar] [CrossRef]
- Ather, J.L.; Ckless, K.; Martin, R.; Foley, K.L.; Suratt, B.T.; Boyson, J.E.; Fitzgerald, K.A.; Flavell, R.A.; Eisenbarth, S.C.; Poynter, M.E. Serum amyloid a activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J. Immunol. 2011, 187, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Van De Veerdonk, F.L.; Joosten, L.A.B.; Shaw, P.J.; Smeekens, S.P.; Malireddi, R.K.S.; Van Der Meer, J.W.M.; Kullberg, B.-J.; Netea, M.G.; Kanneganti, T.-D. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur. J. Immunol. 2011, 41, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.A.; Costa, V.V.; Tavares, L.D.; Sachs, D.; Coelho, F.M.; Fagundes, C.T.; Soriani, F.M.; Silveira, T.N.; Cunha, L.D.; Zamboni, D.S.; et al. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B4 in a murine model of gout. Arthritis Rheum. 2012, 64, 474–484. [Google Scholar] [CrossRef]
- Chaves, M.M.; Sinflorio, D.A.; Thorstenberg, M.L.; Martins, M.D.A.; Moreira-Souza, A.C.A.; Rangel, T.P.; Silva, C.L.M.; Bellio, M.; Canetti, C.; Coutinho-Silva, R. Non-canonical NLRP3 inflammasome activation and IL-1beta signaling are necessary to L. amazonensis control mediated by P2X7 receptor and leukotriene B4. PLoS Pathog. 2019, 15, e1007887. [Google Scholar] [CrossRef] [PubMed]
- Salina, A.C.G.; Brandt, S.L.; Klopfenstein, N.; Blackman, A.; Bazzano, J.M.R.; Sá-Nunes, A.; Byers-Glosson, N.; Brodskyn, C.; Tavares, N.M.; Da Silva, I.B.S.; et al. Leukotriene B4licenses inflammasome activation to enhance skin host defense. Proc. Natl. Acad. Sci. USA 2020, 117, 30619–30627. [Google Scholar] [CrossRef]
- Kim, R.Y.; Pinkerton, J.W.; Gibson, P.G.; Cooper, M.A.; Horvat, J.C.; Hansbro, P.M. Inflammasomes in COPD and neutrophilic asthma. Thorax 2015, 70, 1199–1201. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Sharif, H.; Wang, L.; Wang, W.L.; Magupalli, V.G.; Andreeva, L.; Qiao, Q.; Hauenstein, A.V.; Wu, Z.; Núñez, G.; Mao, Y.; et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nat. Cell Biol. 2019, 570, 338–343. [Google Scholar] [CrossRef]
- Próchnicki, T.; Latz, E. Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab. 2017, 26, 71–93. [Google Scholar] [CrossRef]
- Pellegrini, C.; Antonioli, L.; Lopez-Castejon, G.; Blandizzi, C.; Fornai, M. Canonical and non-canonical activation of NLRP3 inflammasome at the crossroad between immune tolerance and intestinal inflammation. Front. Immunol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Henao-Mejia, J.; A Flavell, R. Integrative inflammasome activity in the regulation of intestinal mucosal immune responses. Mucosal Immunol. 2012, 6, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 inflammasome and inflammatory diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Martin, R.A.; Ather, J.L.; Lundblad, L.K.A.; Suratt, B.T.; Boyson, J.E.; Budd, R.C.; Alcorn, J.F.; Flavell, R.A.; Eisenbarth, S.C.; Poynter, M.E. Interleukin-1 receptor and caspase-1 are required for the Th17 response in nitrogen dioxide–Promoted allergic airway disease. Am. J. Respir. Cell Mol. Biol. 2013, 48, 655–664. [Google Scholar] [CrossRef]
- Gao, P.; Gibson, P.G.; Baines, K.J.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; et al. Anti-inflammatory deficiencies in neutrophilic asthma: Reduced galectin-3 and IL-1RA/IL-1beta. Respir. Res. 2015, 16, 5. [Google Scholar] [CrossRef]
- Prince, L.R.; Allen, L.; Jones, E.C.; Hellewell, P.G.; Dower, S.K.; Whyte, M.K.; Sabroe, I. The role of interleukin-1beta in direct and toll-like receptor 4-mediated neutrophil activation and survival. Am. J. Pathol. 2004, 165, 1819–1826. [Google Scholar] [CrossRef]
- Biondo, C.; Mancuso, G.; Midiri, A.; Signorino, G.; Domina, M.; Lanza Cariccio, V.; Mohammadi, N.; Venza, M.; Venza, I.; Teti, G.; et al. The interleukin-1beta/CXCL1/2/neutrophil axis mediates host protection against group B streptococcal infection. Infect Immun. 2014, 82, 4508–4517. [Google Scholar] [CrossRef]
- Mahmutovic Persson, I.; Menzel, M.; Ramu, S.; Cerps, S.; Akbarshahi, H.; Uller, L. IL-1beta mediates lung neutrophilia and IL-33 expression in a mouse model of viral-induced asthma exacerbation. Respir. Res. 2018, 19, 16. [Google Scholar] [CrossRef]
- Saiwai, H.; Ohkawa, Y.; Yamada, H.; Kumamaru, H.; Harada, A.; Okano, H.; Yokomizo, T.; Iwamoto, Y.; Okada, S. The LTB4-BLT1 axis mediates neutrophil infiltration and secondary injury in experimental spinal cord injury. Am. J. Pathol. 2010, 176, 2352–2366. [Google Scholar] [CrossRef]
- Oyoshi, M.K.; He, R.; Li, Y.; Mondal, S.; Yoon, J.; Afshar, R.; Chen, M.; Lee, D.M.; Luo, H.R.; Luster, A.D.; et al. Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity 2012, 37, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Ferraro, M.; Di Vincenzo, S.; Bruno, A.; Giarratano, A.; Scafidi, V.; Lipari, L.; Di Benedetto, D.V.; Sciarrino, S.M.; Gjomarkaj, M. Cigarette smoke increases BLT2 receptor functions in bronchial epithelial cells: In vitro and ex vivo evidence. Immunology 2013, 139, 245–255. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, D.-W.; Park, D.; Kim, J.-H. Leukotriene B4 Receptors Are Necessary for the Stimulation of NLRP3 Inflammasome and IL-1β Synthesis in Neutrophil-Dominant Asthmatic Airway Inflammation. Biomedicines 2021, 9, 535. https://doi.org/10.3390/biomedicines9050535
Kwak D-W, Park D, Kim J-H. Leukotriene B4 Receptors Are Necessary for the Stimulation of NLRP3 Inflammasome and IL-1β Synthesis in Neutrophil-Dominant Asthmatic Airway Inflammation. Biomedicines. 2021; 9(5):535. https://doi.org/10.3390/biomedicines9050535
Chicago/Turabian StyleKwak, Dong-Wook, Donghwan Park, and Jae-Hong Kim. 2021. "Leukotriene B4 Receptors Are Necessary for the Stimulation of NLRP3 Inflammasome and IL-1β Synthesis in Neutrophil-Dominant Asthmatic Airway Inflammation" Biomedicines 9, no. 5: 535. https://doi.org/10.3390/biomedicines9050535
APA StyleKwak, D.-W., Park, D., & Kim, J.-H. (2021). Leukotriene B4 Receptors Are Necessary for the Stimulation of NLRP3 Inflammasome and IL-1β Synthesis in Neutrophil-Dominant Asthmatic Airway Inflammation. Biomedicines, 9(5), 535. https://doi.org/10.3390/biomedicines9050535






