Mandible Biomechanics and Continuously Erupting Teeth: A New Defect Model for Studying Load-Bearing Biomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rat
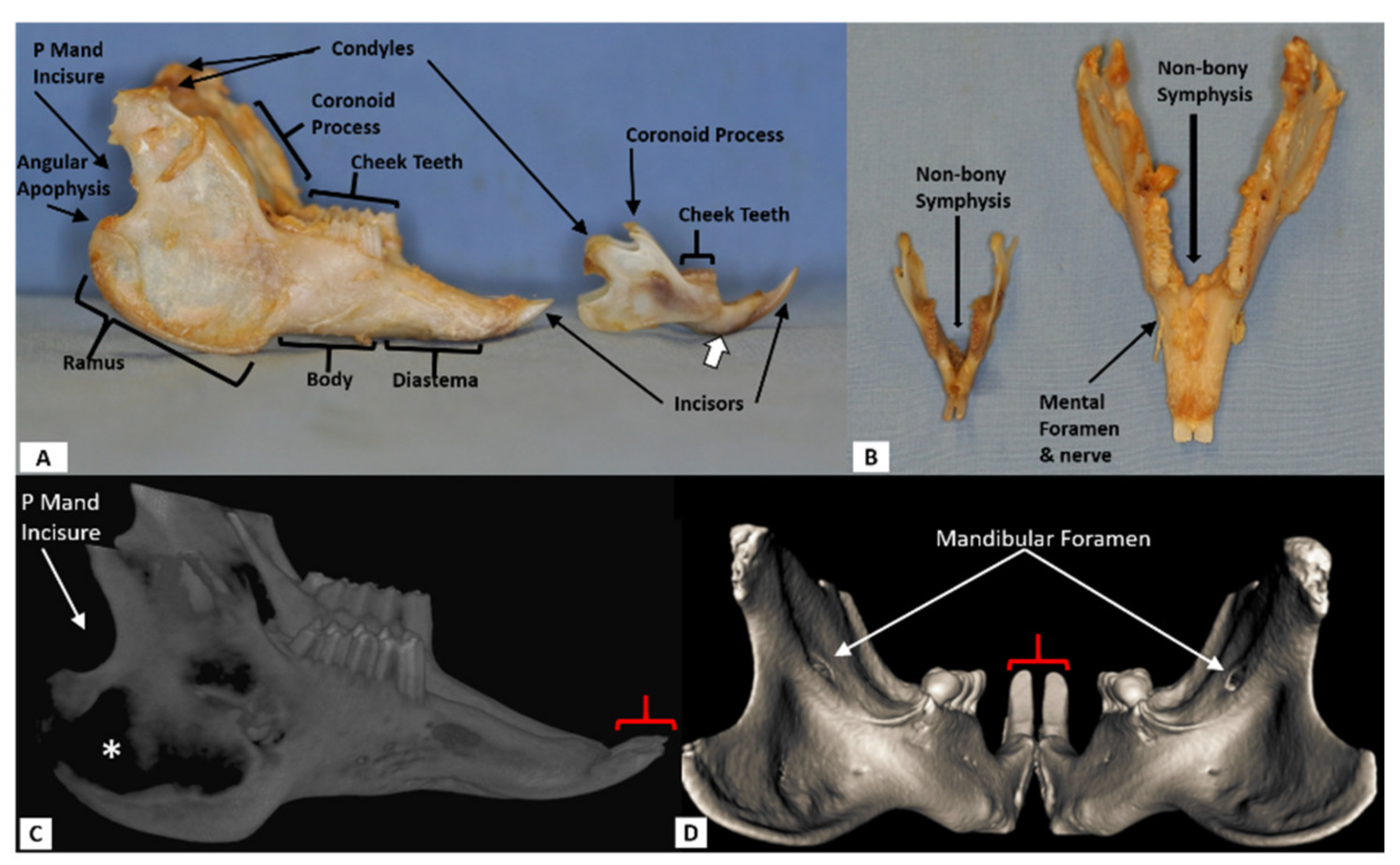
2.1.1. CT Scanning
2.1.2. CT Image Processing
2.1.3. Surgical Defect
2.1.4. Postoperative procedures
2.2. Rabbit
2.2.1. CT Scanning
2.2.2. CT Image Processing
2.2.3. Surgical Defect
2.2.4. Postoperative Procedures
2.3. Statistical analysis
3. Results
3.1. Rat Model
3.2. Rabbit Model
4. Discussion
4.1. Are Morphological Tooth Measures as Assessed by High Resolution CT Reproducible?
4.2. Does Left/Right Symmetry Exist in Normal Rat and Rabbit Tooth Morphology?
4.3. What Is the Ideal Location of a Mandible Defect?
4.4. Relationship between Defect Volume/Type and Ipsilateral Dental Morphology
4.5. Is the Rat or the Rabbit a Better Model?
4.6. Clinical Translational Issues
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rafferty, K.L.; Sun, Z.; Egbert, M.A.; Baird, E.E.; Herring, S.W. Mandibular Mechanics Following Osteotomy and Appliance Placement II: Bone Strain on the Body and Condylar Neck. J. Oral Maxillofac. Surg. 2006, 64, 620–627. [Google Scholar] [CrossRef] [Green Version]
- Law, K.-T.; Lee, C.-K.; King, N.M.; Rabie, A.-B.M. The relationship between eruption and length of mandibular incisors in young rats. Med. Sci. Monit. 2003, 9, BR47–BR53. [Google Scholar]
- Müller, J.; Clauss, M.; Codron, D.; Schulz, E.; Hummel, J.; Fortelius, M.; Kircher, P.; Hatt, J.-M. Growth and wear of incisor and cheek teeth in domestic rabbits (Oryctolagus cuniculus) fed diets of different abrasiveness. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2014, 321, 283–298. [Google Scholar] [CrossRef]
- Weijs, W.A. Mandibular movements of the albino rat during feeding. J. Morphol. 1975, 145, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.Z.; Soenjaya, Y.; McMasters, J.; Ko, A.; Vasanji, A.; Morris, N.; Eppell, S.J. Nanophase bone substitute for craniofacial load bearing application: Pilot study in the rodent. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Kleinschmidt, J.C. The critical size defect as an experimental model to test bone repair materials. J. Craniofac. Surg. 1990, 1, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Takayama, T.; Yamamoto, T.; Ozawa, Y.; Nagao, M.; Tanabe, N.; Nakajima, A.; Suzuki, N.; Maeno, M.; Yamano, S.; et al. A collagen membrane containing osteogenic protein-1 facilitates bone regeneration in a rat mandibular bone defect. Arch. Oral Biol. 2017, 84, 19–28. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Eppell, S.J.; Tong, W.; McMasters, J.; Soenjaya, Y.; Barbu, A.M.; Ko, A.; Baskin, J.Z. Minor Review: An Overview of a Synthetic Nanophase Bone Substitute. Materials 2018, 11, 1556. [Google Scholar] [CrossRef] [Green Version]
- O’Loughlin, P.F.; Morr, S.; Bogunovic, L.; Kim, A.D.; Park, B.; Lane, J.M. Selection and development of preclinical models in fracture-healing research. J. Bone Jt. Surg. Am. 2008, 90 (Suppl. 1), 79–84. [Google Scholar] [CrossRef]
- Carlisle, P.L.; Guda, T.; Silliman, D.T.; Hale, R.G.; Brown Baer, P.R. Are critical size bone notch defects possible in the rabbit mandible? J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Meng, Z.; Chen, G.; Xie, D.; Chen, Y.; Wang, H.; Tang, W.; Liu, L.; Jing, W.; Long, J.; et al. Restoration of critical-size defects in the rabbit mandible using porous nanohydroxyapatite-polyamide scaffolds. Tissue Eng. Part A 2012, 18, 1239–1252. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Zuo, Y.; Li, J.; Ma, S.; Cheng, L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials 2007, 28, 3338–3348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Lu, H.Y.; Lv, G.Y.; Mo, A.C.; Yan, Y.G.; Huang, C. The repair of critical-size defects with porous hydroxyapatite/polyamide nanocomposite: An experimental study in rabbit mandibles. Int. J. Oral Maxillofac. Surg. 2010, 39, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lin, H.; Wang, J.; Zhao, Y.; Wang, B.; Zhao, W.; Sun, W.; Dai, J. Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials 2007, 28, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E.; Hogue, A.S.; Ravosa, M.J. The adaptive significance of mandibular symphyseal fusion in mammals. J. Evol. Biol. 2012, 25, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.E.; Crompton, A.W. Why fuse the mandibular symphysis? A comparative analysis. Am. J. Phys. Anthropol. 2000, 112, 517–540. [Google Scholar] [CrossRef]
- Jheon, A.H.; Seidel, K.; Biehs, B.; Klein, O.D. From molecules to mastication: The development and evolution of teeth. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 165–182. [Google Scholar] [CrossRef] [Green Version]
- Mubarak, R. Histomorphological Study of Dentine Pulp Complex of Continuously Growing Teeth in the Rabbits. Life Sci. J.-Acta Zhengzhou Univ. Overseas Ed. 2012, 9, 1554–1564. [Google Scholar]
- Trejo-Iriarte, C.G.; Serrano-Bello, J.; Gutierrez-Escalona, R.; Mercado-Marques, C.; Garcia-Honduvilla, N.; Bujan-Varela, J.; Medina, L.A. Evaluation of bone regeneration in a critical size cortical bone defect in rat mandible using microCT and histological analysis. Arch. Oral. Biol. 2019, 101, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Seol, Y.J.; Gruber, R.; Giannobile, W.V. Pre-clinical models for oral and periodontal reconstructive therapies. J. Dent. Res. 2009, 88, 1065–1076. [Google Scholar] [CrossRef]
- Legendre, L. Rodent and Lagomorph Tooth Extractions. J. Vet. Dent. 2012, 29, 204–209. [Google Scholar] [CrossRef]
- Hedgecock, N.L.; Hadi, T.; Chen, A.A.; Curtiss, S.B.; Martin, R.B.; Hazelwood, S.J. Quantitative regional associations between remodeling, modeling, and osteocyte apoptosis and density in rabbit tibial midshafts. Bone 2007, 40, 627–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebschner, M.A.K. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials 2004, 25, 1697–1714. [Google Scholar] [CrossRef]
- Weijs, W.A. Biomechanical models and the analysis of form: A study of the mammalian masticatory apparatus. Am. Zool. 1980, 20, 707–719. [Google Scholar] [CrossRef]
- Widmer, C.G.; English, A.W.; Carrasco, D.I.; Malick, C.L. Modeling Rabbit Temporomandibular Joint Torques During a Power Stroke. Angle Orthod. 2002, 72, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Campillo, V.-E.; Langonnet, S.; Pierrefeu, A.; Chaux-Bodard, A.-G. Anatomic and histological study of the rabbit mandible as an experimental model for wound healing and surgical therapies. Lab. Anim. 2014, 48, 273–277. [Google Scholar] [CrossRef]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies differences in bone composition, density, and quality: Potential implications for in vivo bone research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef]
- Ma, J.L.; Pan, J.L.; Tan, B.S.; Cui, F.Z. Determination of critical size defect of minipig mandible. J. Tissue Eng. Regen. Med. 2009, 3, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Barausse, C.; Pistilli, R.; Checchi, V.; Diazzi, M.; Gatto, M.R.; Felice, P. Posterior jaws rehabilitated with partial prostheses supported by 4.0 × 4.0 mm or by longer implants: Four-month post-loading data from a randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 221–230. [Google Scholar]
- Liverani, C.; De Vita, A.; Minardi, S.; Kang, Y.B.; Mercatali, L.; Amadori, D.; Bongiovanni, A.; La Manna, F.; Ibrahim, T.; Tasciotti, E. A biomimetic 3D model of hypoxia-driven cancer progression. Sci. Rep. 2019, 9, 12263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liverani, C.; Mercatali, L.; Cristofolini, L.; Giordano, E.; Minardi, S.; Della Porta, G.; De Vita, A.; Miserocchi, G.; Spadazzi, C.; Tasciotti, E.; et al. Investigating the Mechanobiology of Cancer Cell-ECM Interaction Through Collagen-Based 3D Scaffolds. Cell. Mol. Bioeng. 2017, 10, 223–234. [Google Scholar] [CrossRef] [PubMed]










| Analysis of Cheek Teeth Height (mm) in Rabbit with Mandible Body Defect | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time | #2 | #3 | #4 | #5 | ||||
| L | R | L | R | L | R | L | R | |
| Baseline (predefect) | 5.3 | 4.8 | 3.4 | 3.6 | 4.1 | 3.9 | 3.0 | 3.6 |
| One week | 5.4 | 5.2 | 3.7 | 3.6 | 3.5 | 3.8 | 3.8 | 3.9 |
| 10 weeks | 3.8 | 5.3 | 2.1 | 3.8 | 3.0 | 3.8 | 3.2 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baskin, J.Z.; White, B.M.; Vasanji, A.; Love, T.E.; Eppell, S.J. Mandible Biomechanics and Continuously Erupting Teeth: A New Defect Model for Studying Load-Bearing Biomaterials. Biomedicines 2021, 9, 730. https://doi.org/10.3390/biomedicines9070730
Baskin JZ, White BM, Vasanji A, Love TE, Eppell SJ. Mandible Biomechanics and Continuously Erupting Teeth: A New Defect Model for Studying Load-Bearing Biomaterials. Biomedicines. 2021; 9(7):730. https://doi.org/10.3390/biomedicines9070730
Chicago/Turabian StyleBaskin, Jonathan Z., Brandon M. White, Amit Vasanji, Thomas E. Love, and Steven J. Eppell. 2021. "Mandible Biomechanics and Continuously Erupting Teeth: A New Defect Model for Studying Load-Bearing Biomaterials" Biomedicines 9, no. 7: 730. https://doi.org/10.3390/biomedicines9070730






