1. Introduction
Natural killer (NK) cells constitute the first line of defense against viruses and tumors. They recognize altered and infected cells with a reduced surface expression of MHC I, and cells with increased expression of stress molecules and pathogen-associated molecular patterns via a set of activating and inhibiting receptors [
1]. Due to their high cytotoxic potential and natural ability to recognize tumors through a wide spectrum of receptors, NK cells can be considered as a promising candidate cell type for cancer immunotherapy. Genetically modified NK cells can provide several key advantages over engineered T cells as therapeutic agents. Specifically, NK cells are unable to cause graft versus host disease [
2], and their antitumor activity may be further enhanced by combining with therapeutic antibodies, chemotherapeutic agents, and radiation [
3,
4].
Numerous studies targeted enhancing NK cell expansion and engineering them genetically, striving to improve their desired functional characteristics. Based on recent advances in understanding the regulation of NK cell activity, genetic engineering of NK cells is expected to help unlock their full potential for immunotherapy. Despite the advantageous safety profile, the efficacy of NK cells in human trials remains poor, especially for solid tumor indications. Several inherent properties of NK cells restrict their use in cancer immunotherapy [
5]. In particular, NK cells have rather limited capability to expand both in vitro and in vivo [
6,
7,
8], with an estimated half-life of major NK subsets in humans to be less than ten days [
9]. The inability to expand beyond naturally modest total cell numbers curtails the maximum-achievable dose of the cells during ex vivo engineering and expansion while decreasing the longevity of adoptively transferred engineered NK cells in patients.
The activation of NK cells followed by their long-term in vitro expansion leads to replicative senescence, which results from a loss of integrity and length of telomeres. Telomere loss negatively affects the proliferative capacity of the cells via genome-wide epigenetic changes in the chromatin and associated modulation of transcriptional activity in key regulatory circuits. Endogenous telomerase activity in NK cells is typically insufficient to support the continuous restoration of telomeres, which are shortened with each cell division [
10]. One way to overcome this problem would be to ectopically express the catalytic subunit of human telomerase reverse transcriptase (hTERT) in NK cells. Overexpression of hTERT, a rate-limiting component of the telomerase complex, could be expected to compensate for an insufficient amount of endogenous enzyme, thus protecting the cells from replicative senescence [
11]. Healthy donor-derived NK cells transduced with a retroviral vector expressing hTERT had a longer lifespan in cell culture compared to the control cells [
12]. However, retroviral transduction of NK cells is typically difficult and less efficient in comparison to the other cells of hematopoietic lineage. This is attributed to the fact that NK cells as the first responders to viral infections [
13] are designed and evolutionarily selected to be highly resistant to viral penetration [
14] during natural infections. Accordingly, NK cells cannot be efficiently transduced with viral vectors. To increase the efficiency of transduction, it is typically necessary to carry out some additional manipulations with NK cells, such as in vitro pre-stimulation of the cells [
15].
IL-2 is one of the key growth factors supporting the expansion of NK cells and increasing the transduction efficiency. However, IL-2 alone is insufficient, and for achieving significant activation and expansion of these cells in culture, additional cofactors (such as IL-21) or stimuli (IL-21-engineered feeder cells) are normally required. In comparison with soluble IL-21, K562 cells engineered with membrane-bound IL-21 (K562-mbIL21) were shown to induce a substantial increase in the number of NK cells in co-culture in the presence of IL-2 [
16]. These observations clearly suggest that the expansion of NK cells and the efficiency of viral vector-mediated gene delivery to them could be increased by including membrane-bound cytokines such as IL-21 in the ex vivo culture conditions.
In this study, we compared the efficiency of gene delivery for various subsets of freshly isolated NK cells, as well as for bulk and clonal cultures that were activated in vitro in the presence of IL-2 and K562-mbIL21 feeder cells before transduction. We evaluated the enzymatic activity of ectopically expressed hTERT and studied the effect of transduction on the phenotypic and functional properties of NK cells. Our results demonstrate a substantial increase in proliferative activity and expansion of NK cells caused by hTERT overexpression, which prompted us to suggest that hTERT-engineered NK cells could become a valuable tool in the cancer cell therapy toolbox.
2. Materials and Methods
2.1. Cell Lines
The erythroblastic leukemia cell line K562 was obtained from ATCC (Manassass, VA, USA). The genetically modified K562 clone expressing membrane IL-21 (K562-mbIL21) was kindly provided by Dean Lee (MD Anderson Cancer Center, Houston, TX, USA). In addition to mbIL-21, this clone also expresses CD64, CD86, CD137L, and the fragment of the CD19 receptor [
17]. GP2-293 retroviral packaging cell line expressing gag and pol genes, a derivative of human embryonic kidney HEK293T cells (Clontech/Takara, Terra Bella Ave. Mountain View, CA, USA), was used for vector packaging and preparation of high titer retroviral vector stocks. GP2-293 cells were cultured in a DMEM medium (PanEco, Russian Federation) supplemented with 10% FCS, 2 mM L-glutamine, 2 mM sodium pyruvate (PanEco, Russian Federation), 2 mM antibiotic-antimycotic (Sigma-Aldrich, St. Louis, MO, USA). K562 and K562-mbIL21 cell lines were maintained in a RPMI-1640 medium (PanEco, Russian Federation) supplemented with 10% fetal calf serum (FCS, HyClone Labs, Logan, UT, USA), 2 mM L-glutamine (PanEco, Russian Federation), and 2 mM antibiotic-antimycotic at a concentration of 2–6 × 10
5 cells/mL. The surface expression of IL-21 in K562-mbIL21 cells was periodically verified by flow cytometry using anti-IL-21-PE antibodies (clone 3A3-N2, BioLegend, San Diego, CA, USA). To prepare feeder cells, K562 or K562-mbIL21 cells were γ-irradiated at 100 Gy, frozen, and stored at –135 °C.
2.2. Isolation, Activation, and Culture of NK Cells
Blood samples were obtained from healthy individuals of different gender and age. All participants gave verbal informed consents prior to the study, which was approved by the local ethics committee (Pirogov Russian National Research Medical University). Peripheral blood mononuclear cells (PBMC) were obtained by gradient centrifugation on a 1.077 g/mL Ficoll gradient (PanEco, RF). NK cells were isolated from PBMC by negative magnetic separation using an NK cell isolation kit (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer’s protocol. The purity of isolated cells was typically at least 97%.
Freshly isolated NK cells were grown in 24-well plates in NK MACS Medium (Miltenyi Biotech, Bergisch Gladbach, Germany) supplemented with 100 U/mL IL-2 (Hoffmann La-Roche, Basel, Switzerland) and irradiated K562-mbIL21 feeder cells at an NK: feeder ratio of 4:5. The cells were cultured at 37 °C with 5% of CO2. After six days in culture, the medium was partially replaced with a fresh one, the concentration of IL-2 was adjusted to 100 U/mL, and the cells were transferred to the new 24-well plate and grown for another 4–5 days before transduction.
2.3. Generation of NK Cell Clones
The FACSVantage DiVa cell sorter (Beckton Dickinson, Franklin Lakes, NJ 07417, USA), equipped with 405, 488, and 643 nm lasers and a corresponding set of detectors and filters, was used for cell sorting. To generate clonal NK cell cultures, freshly isolated cells were pre-stained with fluorochrome-conjugated monoclonal antibodies against CD56 and CD3, and sorted into 96-well round-bottom plates at one cell per well, in the “single cell” cell sorter mode [
18]. At least 120 cells were sorted. Plates prepared for cell deposition contained K562-mbIL21 feeder cells at a concentration of 104 cells per ml in the complete cloning medium (DMEM supplemented with 20% ExVivo medium (Thermo Fisher Scientific, San Jose, CA, USA) and 100 U/mL recombinant human IL-2 (Hoffmann La-Roche, Basel, Switzerland). Half of the spent medium was replaced one time after three weeks of incubation at 37 °C (5% CO
2), and then once weekly. The clones that attained the cell number of 2 × 10
5 cells were expanded to 24-well plates and maintained at a concentration of 0.5 × 10
6 cells/mL. In clonal cultures with low proliferative activity, the medium was changed weekly, while the medium in the cultures with good growth was changed as required to maintain the cultures in a healthy state.
2.4. Antibodies Used for Flow Cytometry and Cell Sorting
The following mouse monoclonal antibodies were used: CD56-APC (clone N901, Beckman Coulter, Miami, FL, USA), CD56-Brilliant Violet 421 (clone HCD56, Sony Biotechnology, San Jose, CA, USA), CD56-PE (clone N901 (HLDA6), Beckman Coulter, Miami, FL, USA), CD57-PE (clone TB01, eBioscience, San Diego, CA, USA), CD57-FITC (clone TB03, Miltenyi Biotec, Bergisch Gladbach, Germany), CD57-APC (clone TB03, Miltenyi Biotec, Bergisch Gladbach, Germany), CD16-PE (Sorbent, RF), CD2-PE-Cy7 (clone TS1/8, Sony Biotechnology, San Jose, CA, USA), anti-NKG2A-PE (clone 131411, R&D Systems, Minneapolis, MN, USA), anti-KIR2DL2/DL3-PE (clone DX27, Miltenyi Biotec, Bergisch Gladbach, Germany), anti-HLA-DR-FITC (clone B8.12.2, Beckman Coulter, Miami, FL, CA, USA), NKG2A-PE (clone 131411), NKG2C-AlexaFluor488 (clone 108724), NKG2C-PE (clone 134591; R&D Systems, Minneapolis, MN, USA), NKp46-FITC (clone 9E2; Sony Biotechnology, San Jose, CA, USA), anti-NKG2D-PE (clone REA1175, Miltenyi Biotec, Bergisch Gladbach, Germany). Staining was performed according to the manufacturer’s instructions.
2.5. Flow Cytometry
A MACSQuant 10 cytometer (Milteniy Biotech, Bergisch Gladbach, Germany) equipped with 405 nm, 488 nm, and 635 nm lasers was used for the acquisition of flow cytometry data. The results were processed using Flowing Software version 2.5.1 (Perttu Terho, Turku Center for Biotechnology, Turku, Finland) or FlowJo software version 7.6 (TreeStar Williamson Way, Ashland, OR, USA). Statistical analysis was performed using SigmaPlot 12 (SYSTAT Software Inc., Washington St, Chicago, IL, USA) and GraphPad Prism 7 (StatSoft Inc., Tulsa, OK, USA) software.
2.6. NK Cell Degranulation Assay
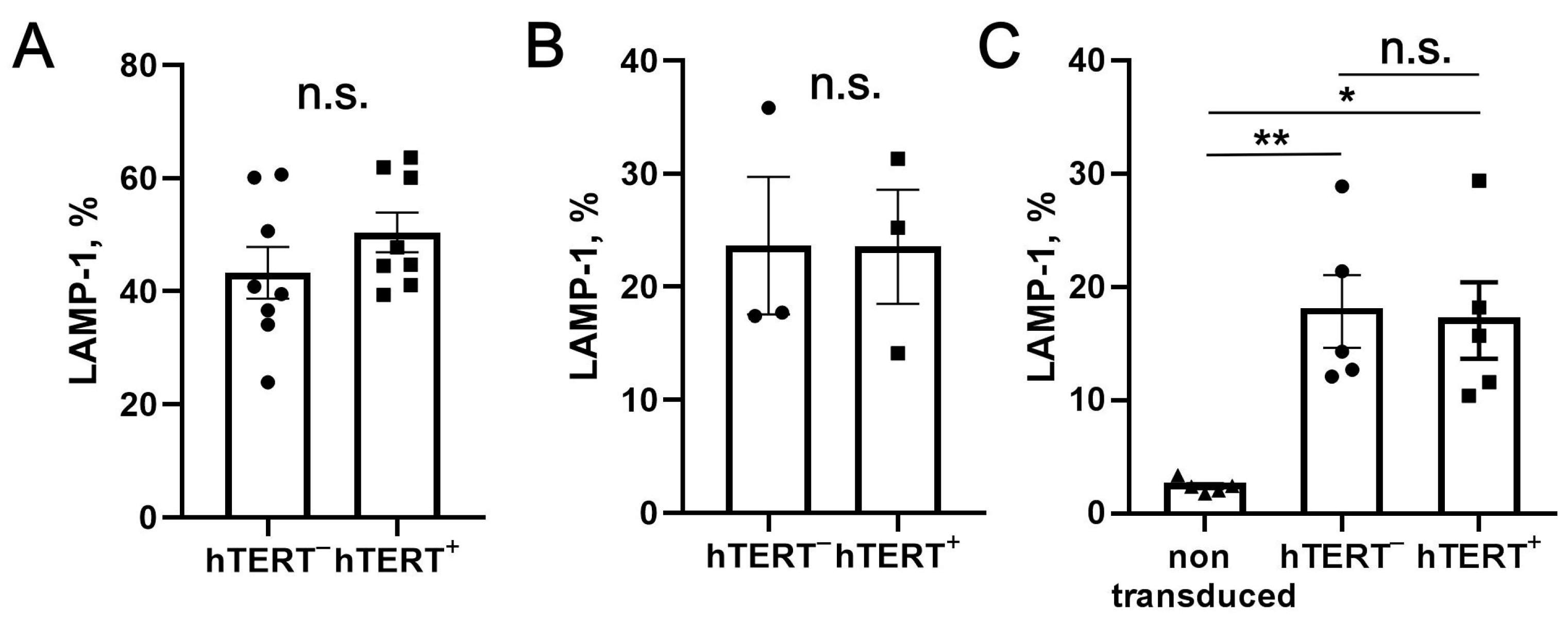
To estimate the level of effector cell degranulation, K562 cells were mixed with NK cells at a 1:1 ratio in the culture medium supplemented with brefeldin A (10 μg/mL, Invitrogen, San Jose, CA, USA) and anti-CD107a antibody (Milteniy Biotech, Germany). The samples were incubated for 2.5 h at 37 °C (5% CO2), followed by washing to remove unbound antibodies and staining for surface markers. The surface levels of CD107a on NK cells were quantified using the MACSQuant 10 flow cytometer.
2.7. Cell Viability Assay
The analysis of cell viability was performed by staining cells with SYTOXRed Dead Cell Stain solution (Invitrogen, San Jose, CA, USA) for 2–5 min in the dark. Alternatively, staining with fluorescent annexin V conjugate (Invitrogen, San Jose, CA, USA) was performed for 15 min at room temperature, followed by adding the propidium iodide (PI, Sigma-Aldrich, St. Louis, MO, USA) solution (2 μg/mL).
2.8. Analysis of Cell Cycle with PI DNA Staining
The NK cell cycle analysis was performed by quantification of DNA amount per cell with PI staining. The cells were harvested, washed in the phosphate-buffered saline (PBS), and fixed in cold 70% ethanol for 1 h, at −4 °C. Subsequently, the cells were centrifuged at 300× g for 10 min, washed with PBS, treated with RNAse (Thermo Fisher, San Jose, CA, USA), and stained with PI according to a standard protocol. Stained cells were analyzed by flow cytometry using MACSQuant 10.
2.9. Analysis of Proliferative Activity
The proliferation of NK cells was evaluated by measuring the percentage of Ki67+ cells in cultures. The cells were harvested, washed in PBS, stained by fluorescent antibodies against CD56 and CD57 for 25 min, washed in PBS, and fixed in cold 70% ethanol for 1 h, at −4 °C. Fixed cells were centrifuged at 300× g, rinsed by PBS, and stained by fluorescent antibodies against Ki67. Stained NK cells were washed in PBS and analyzed in MACSQuant 10 flow cytometer.
2.10. Cell Counting
NK cells were counted by automatic cell counter TC20 (Bio-Rad, Hercules, CA, USA) using the manufacturer-recommended protocol.
2.11. Retroviral Transduction
The hTERT cDNA was fused to the 3’ terminus of the GFP DNA via P2A self-cleaving peptide for bi-cistronic expression [
19]. Retroviral vector xlox-GFP-hTERT expressing GFP-P2A-hTERT fusion protein was used for NK cell transduction (plasmids were kindly provided by Frederick National Laboratory for Cancer Research, Frederick, MD, USA) [
20]. The vector was pseudotyped with RD114 envelope glycoprotein to maximize transduction efficiency [
21]. Pure plasmid DNA was isolated by using GeneJET Plasmid Maxiprep Kit (ThermoFisher Scientific, San Jose, CA, USA) according to the manufacturer’s instructions. High titer retroviral vector stocks were prepared by co-transfecting GP2-293 cells with xlox-GFP-P2A-hTERT DNA and RD114 expression construct in 100 mm Petri dishes treated with poly-L-lysine solution (Sigma-Aldrich, St. Louis, MO, USA), using a calcium phosphate transfection kit (ThermoFisher Scientific, San Jose, CA, USA) according to the manufacturer’s instructions. The cell culture medium containing packaged vector was harvested at 48 and 72 h post-transduction, filtered through Millex-HV-0.45 µm PES filter (Millipore, Burlington, MA, USA), and concentrated on sterile cones with 100 nm pores (Millipore, Burlington, MA, USA). The transduction of bulk and clonal NK cells was performed in 24-well plates treated with a Retronectin solution (Clontech/Takara, Terra Bella Ave. Mountain View, CA USA) in PBS at a final concentration of 20 μg/mL, according to manufacturer’s recommendations. The transduction efficiency was measured as a percentage of GFP+ NK cells in 3–5 days after the transduction.
2.12. Immunophenotyping of GFP-P2A-hTERT-Transduced NK Cells
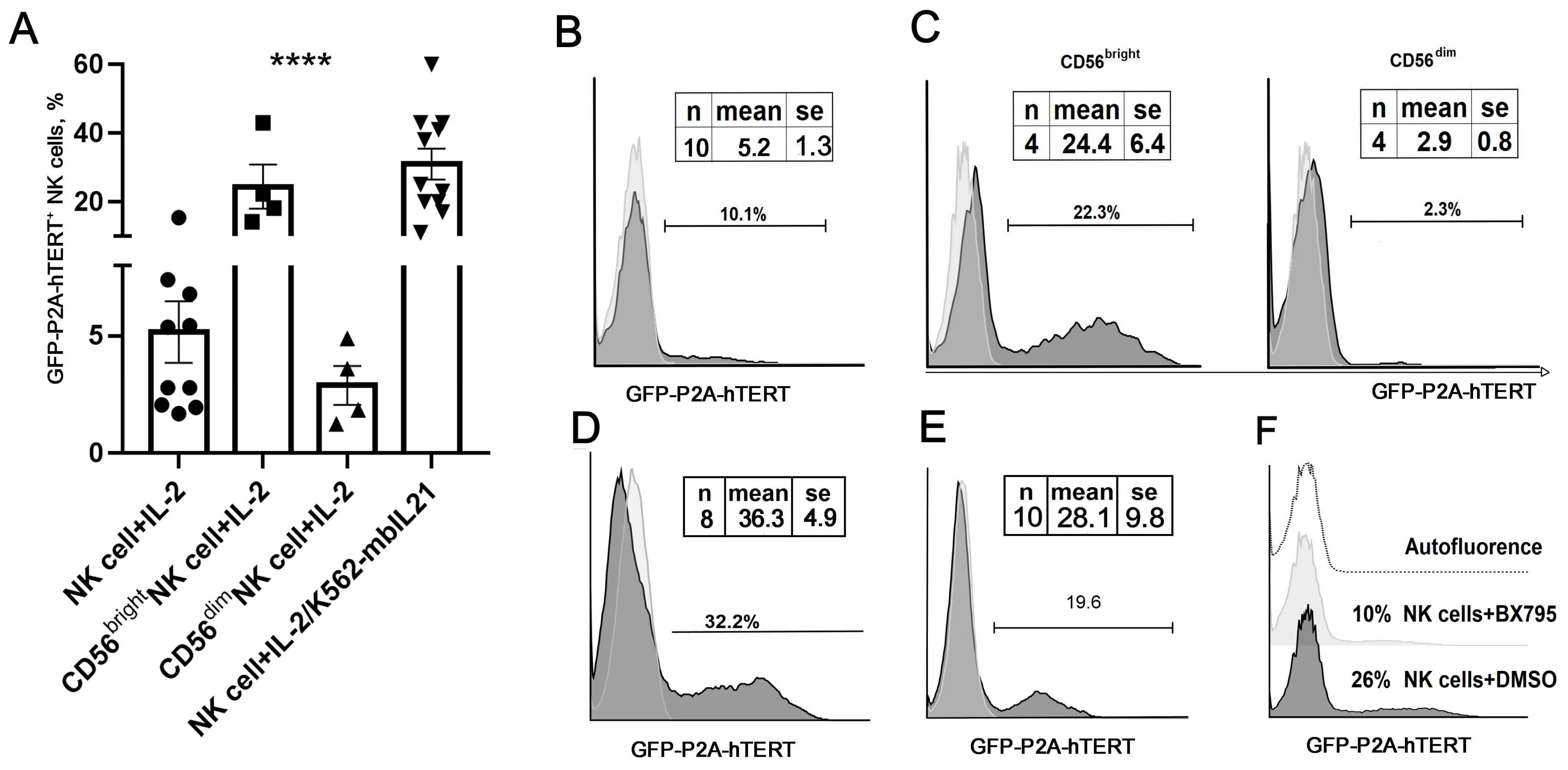
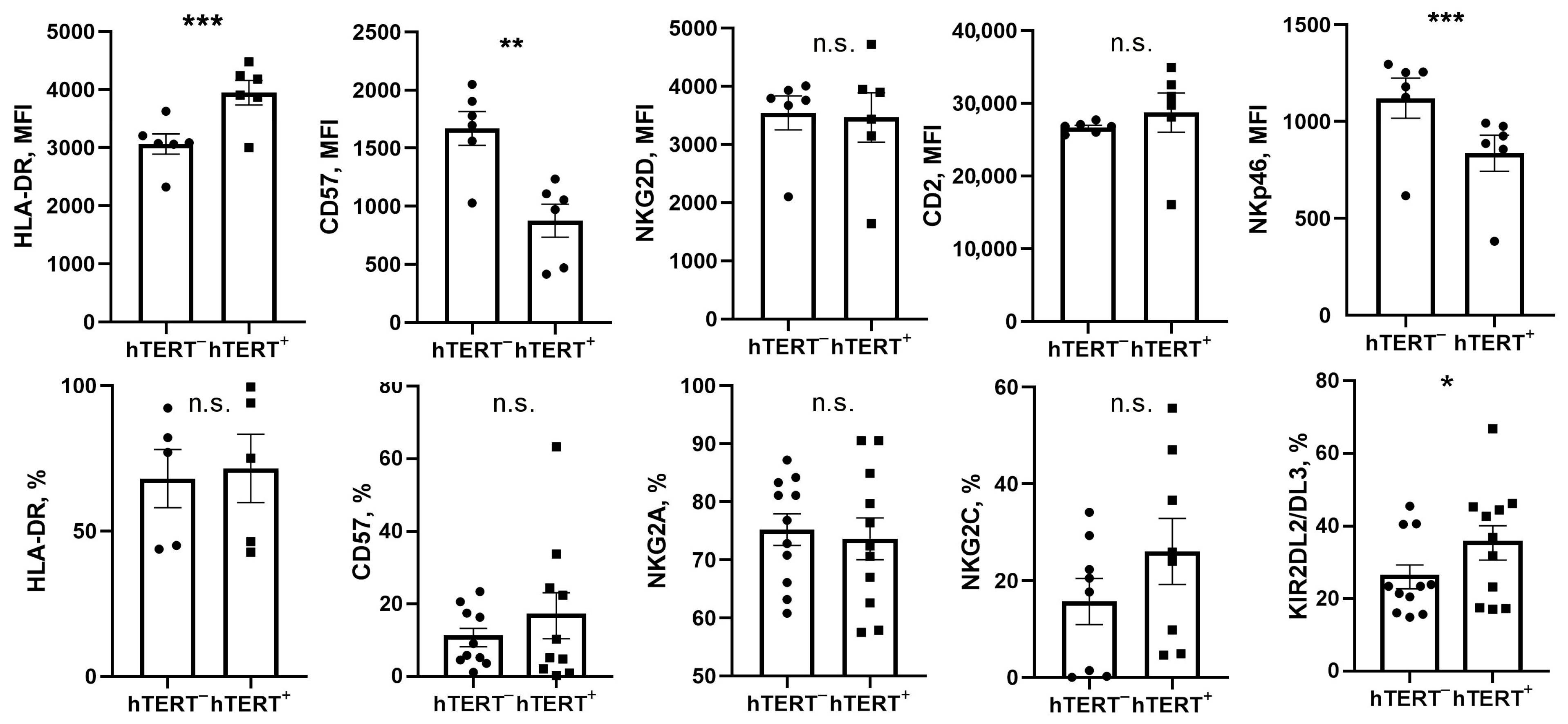
The surface expression of NK-specific markers was determined by flow cytometry analysis of untransduced and transduced NK cells. Since the transduction efficiency in vector-infected cultures never reached 100%, an untransduced fraction of NK cells (GFP-hTERT-) was used as a convenient internal reference. For this, NK cells in vector-infected cultures were stained with appropriate surface marker antibodies and gated on GFP positivity to discriminate GFP-positive transduced (hTERT+) and GFP-negative (hTERT−) cells. The surface marker expression was then measured in each fraction and compared between fractions.
2.13. Evaluation of Telomerase Activity
The NK cells in transduced and untransduced cultures were counted. To evaluate and compare hTERT enzymatic activity between these cultures, all samples were normalized to contain equal cell numbers per volume. Nuclear extracts were prepared from samples, each containing an equal number of NK cells. Enzymatic activity of hTERT was measured in nuclear extracts by telomere repeat amplification protocol (TRAP) [
22] using TRAPeze Telomerase Detection Kit (Millipore, Burlington, MA, USA), according to the manufacturer’s instructions. Telomerase activity was evaluated by visual comparison of width and number of telomere repeat DNA bands with positive control provided in the kit.
4. Discussion
Genetic engineering of NK cells toward creating better products for cancer cell therapy is becoming an increasingly widespread approach. A number of important questions pertinent to engineering of these cells with retroviral and lentiviral vectors remain unresolved. As the retroviral/lentiviral transduction of NK cells is subpar in efficiency in comparison to T cells, it is still unclear what conditions would make NK cells more susceptible to these vectors. In particular, the best methodology for pre-transduction activation of NK cells is not yet established. In this study, we have shown that, although the activation of NK cells by IL-2 is necessary, it alone is not sufficient to achieve high transduction efficiency. This may be caused by the low proliferation activity of IL-2-stimulated NK cells, since active proliferation is a pre-requisite for gamma-retroviral vector integration [
26]. Circulating NK cells are a heterogeneous population of lymphocytes, some of them being less sensitive to IL-2 [
27], which is reflected in the total level of activation and proliferation in bulk NK populations. NK cells with CD56
bright phenotype demonstrate greater proliferative activity under IL-2 stimulation compared to CD56
dim cells, due to expressing high-affinity IL-2 receptors (in some cases, at a higher density) [
28]. In our study, we observed better efficiency of CD56
bright NK cell transduction in comparison with CD56
dim cells, which corresponds to a higher proliferative activity of the CD56
bright subset upon IL-2 stimulation. However, a low percentage of CD56
bright subset (rarely exceeding 10–15% in peripheral blood NK cells), typically seen in healthy individuals [
24,
29], makes producing therapeutically adequate numbers of these cells impractical. In addition to CD56
dim cells, another NK cell subset (CD57
+) is difficult to transduce, as most of the cells in this subset are in the G0 phase of the cell cycle [
23].
We looked for some additional stimulation conditions that could increase the transduction efficiency of bulk NK populations. Previous work demonstrated that stimulation of NK cells with K562-mbIL21 feeder cells leads to a greater expansion of NK cells in vitro than stimulation with K562-mbIL15 feeders [
17]. In our laboratory, using clonal cultures of NK cells, we showed that K562-mbIL21 cells in combination with IL-2 promoted active proliferation of both CD56
bright and CD56
dim subsets. The largest expansion was observed in the clones derived from CD56
dim NK cells negative for CD57, the replicative senescence marker [
15]. This method of combined mbIL-21 and IL-2 stimulation was recently successfully applied to generate NK cells expressing CAR [
30].
Retroviral vectors can integrate into virtually any genomic locus, carrying the risk of insertional mutagenesis and subsequent transformation. If the genes involved in cell cycle regulation are destroyed by vector insertion, or genes responsible for apoptosis and survival are activated, down-regulation of proliferation can potentially occur in transduced cells, which may then die post transduction. Some other insertional mutagenesis events may induce cell division, thus conveying proliferative advantage to the transduced cells. Accordingly, immunophenotyping of transduced primary cells is best performed at least a week post transduction, to give the cells carrying insertional mutagenesis-activated or destroyed genes the time to expand or to die.
As we have shown here, hTERT-transduced cells showed an increased expression of the activation marker HLA-DR, while displaying higher proliferative activity. Decreased CD57 expression on the surface of transduced NK cells is also characteristic of more rapidly dividing cells. NK cells with higher proliferative activity are expected to be more susceptible for the GFP-P2A-hTERT retroviral transduction, as we reported earlier [
23]. Alternatively, this may be attributed to post-transduction selection of better proliferating cells due to insertional mutagenesis. Finally, overexpression of hTERT in transduced NK cells may lead to an increase in HLA-DR expression and a decrease in CD57 levels. The observed increase of the KIR2DL2/DL3
+ population in transduced cells can also be explained by its increased rate of division, as this population possesses a more differentiated phenotype, proliferates faster, and is more efficiently transduced. It is also possible that the increase in the percentage of KIR2DL2/DL3-positive cells occurred due to their better survival or enhanced proliferation. According to earlier work, KIR expression is a relatively stable characteristic of cultured NK cells [
18,
31]. Thus, while it cannot be completely ruled out, it seems less likely that an increase in the percentage of KIR2DL2/DL3
+ NK cells after transduction occurs due to the induction of these receptors during long-term culture. Rather, it may be a direct consequence of hTERT-stimulated proliferation. The higher percentage of more-differentiated KIR2DL2/DL3-positive fraction in the total population of transduced cells can potentially lead to an increased degranulation in hTERT-engineered NK cells compared with control unmodified cells. However, we did not detect a significant difference in the degranulation activity between GFP-P2A-hTERT-positive and negative NK cells.
Ectopic expression of hTERT enables cells to overcome the Hayflick limit by allowing more cell divisions and preventing growth arrest [
32]. However, hTERT-induced unlimited proliferative potential is not necessarily directly linked with the modulation of the proliferation rate. Rather, hTERT prevents cells from reaching senescence via continuous restoration of telomeres, as well as through other non-telomeric-related mechanisms (e.g., interfering with apoptosis). Various effects of hTERT unrelated to telomerase activity (induction of growth factor receptor expression, alterations in epigenetic and apoptotic mechanisms, signal transduction, energy metabolism, and protein synthesis [
33]) have been described for several cell types. It was demonstrated that overexpression of hTERT in cancer cells could decrease intracellular ROS production, thus reducing ROS-mediated apoptosis [
34]. A genome-wide study also showed that hTERT ubiquitously bound to regions that encode tRNAs and RNA Pol III subunit RPC32, enhancing tRNAs expression and promoting increased protein synthesis and cell growth [
35]. To what extent the non-telomeric activities of overexpressed hTERT could have contributed to the hTERT effect on physiology, proliferation, and life span of NK cells observed in our work, is not clear. While being out of scope for this study, these activities in the context of NK biology certainly warrant further exploration, as they may be intricately involved in the NK cell life span extension that we observed. Studies with catalytically inactive hTERT mutants will be of great interest and importance, especially when considering the safety of hTERT-engineered NK cells in cell therapy settings.
Of note, constitutive hTERT expression is linked to immortality in some cell types (e.g., stem or cancer cells), while only extending the life span beyond physiological limit in the others (e.g., hTERT-engineered somatic primary cells). In this study, we observed substantial enzymatic activity of hTERT in engineered NK cells, which was above the physiologic levels. However, while moderately extending their life span, hTERT overexpression failed to induce limitless division capacity and true immortalization. This is consistent with earlier observations showing that the lifespan of hTERT-NK cells in two donors out of four exceeded the control values by only 1.33 and 1.5 times [
12]. In our work, the average lifespan of NK cells engineered with hTERT turned out to be somewhat lower, possibly due to the differences in culture conditions between the studies. Initially, we stimulated transduced cells with IL-2 alone, while Fujisaki et al. used a combination of K562-mb15-41BBL feeder cells and IL-2 [
12]. By adding of K562-mbIL21 feeder cells, we were able to further prolong the expansion of hTERT-NK cell cultures. It should also be noted that, as we have previously shown, the weekly addition of feeder cells leads to a decrease in the cytotoxic activity of NK cells, potentially due to the depletion of their degranulating ability [
15]. Thus, to create an optimal microenvironment that promotes the accumulation of genetically engineered NK cells with a high cytotoxic potential, further fine-tuning of culture conditions and testing of additional feeder-based culture protocols will be required.
One important aspect of using hTERT-engineered NK cells in cell therapy is safety, as the inability of ectopically expressed hTERT to cause oncogenic transformation of NK cells has not been firmly established so far. In our experiments with NK, we observed an extension of the natural life span rather than complete immortalization; the fact that the effect of hTERT on NK survival and longevity ex vivo is limited may open the way for the use of wild type (or mutant) hTERT to engineer NK-based cell therapeutics. However, further work is required to establish the safety and efficacy of hTERT-modified NK cells as a new generation anti-cancer drug.