Novel Treatment Strategy Using Second-Generation Androgen Receptor Inhibitors for Non-Metastatic Castration-Resistant Prostate Cancer
Abstract
1. Introduction
2. Definition of nmCRPC
3. Phase III Clinical Trials Assessing the Effect of Second-Generation ARi for nmCRPC
3.1. SPARTAN (Apalutamide)
3.2. PROSPER (Enzalutamide)
3.3. ARAMIS (Darolutamide)
4. Comparison of Efficacy and Safety of Second-Generation ARi
4.1. MFS and PSA Progression-Free Survival
4.2. OS
4.3. Safety
4.4. Health-Related Quality of Life Outcomes
5. PSMA-PET Imaging in nmCRPC
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Byar, D.P.; Corle, D.K. Hormone therapy for prostate cancer: Results of the Veterans Administration Cooperative Urological Research Group studies. NCI Monogr. 1988, 7, 165–170. [Google Scholar] [CrossRef]
- Sun, M.; Choueiri, T.K.; Hamnvik, O.P.; Preston, M.A.; De Velasco, G.; Jiang, W.; Loeb, S.; Nguyen, P.L.; Trinh, Q.D. Comparison of Gonadotropin-Releasing Hormone Agonists and Orchiectomy: Effects of Androgen-Deprivation Therapy. JAMA Oncol. 2016, 2, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, M.A.; Lepor, H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 2007, 9 (Suppl. S1), S3–S8. [Google Scholar]
- Shafi, A.A.; Yen, A.E.; Weigel, N.L. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharm. 2013, 140, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Kabbinavar, F.; Saad, F.; Hussain, A.; Gittelman, M.C.; Bilhartz, D.L.; Wynne, C.; Murray, R.; Zinner, N.R.; Schulman, C.; et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J. Clin. Oncol. 2005, 23, 2918–2925. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; Phung, B.S.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef]
- Kelly, W.K.; Scher, H.I. Prostate specific antigen decline after antiandrogen withdrawal: The flutamide withdrawal syndrome. J. Urol. 1993, 149, 607–609. [Google Scholar] [CrossRef]
- Scher, H.I.; Kelly, W.K. Flutamide withdrawal syndrome: Its impact on clinical trials in hormone-refractory prostate cancer. J. Clin. Oncol. 1993, 11, 1566–1572. [Google Scholar] [CrossRef]
- Scher, H.I.; Liebertz, C.; Kelly, W.K.; Mazumdar, M.; Brett, C.; Schwartz, L.; Kolvenbag, G.; Shapiro, L.; Schwartz, M. Bicalutamide for advanced prostate cancer: The natural versus treated history of disease. J. Clin. Oncol. 1997, 15, 2928–2938. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, Y.; Kawahara, T.; Miyoshi, Y.; Otani, M.; Yamanaka, S.; Teranishi, J.; Noguchi, K.; Yao, M.; Uemura, H. Efficacy of Immediate Switching from Bicalutamide to Flutamide as Second-Line Combined Androgen Blockade. BioMed Res. Int. 2016, 2016, 4083183. [Google Scholar] [CrossRef]
- Soifer, H.S.; Souleimanian, N.; Wu, S.; Voskresenskiy, A.M.; Collak, F.K.; Cinar, B.; Stein, C.A. Direct regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cells. J. Biol. Chem. 2012, 287, 3777–3787. [Google Scholar] [CrossRef]
- Richards, J.; Lim, A.C.; Hay, C.W.; Taylor, A.E.; Wingate, A.; Nowakowska, K.; Pezaro, C.; Carreira, S.; Goodall, J.; Arlt, W.; et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: A rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012, 72, 2176–2182. [Google Scholar] [CrossRef]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front. Oncol. 2019, 9, 801. [Google Scholar] [CrossRef]
- Messner, E.A.; Steele, T.M.; Tsamouri, M.M.; Hejazi, N.; Gao, A.C.; Mudryj, M.; Ghosh, P.M. The Androgen Receptor in Prostate Cancer: Effect of Structure, Ligands and Spliced Variants on Therapy. Biomedicines 2020, 8. [Google Scholar] [CrossRef]
- Scher, H.I.; Sawyers, C.L. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 2005, 23, 8253–8261. [Google Scholar] [CrossRef]
- Brave, M.; Weinstock, C.; Brewer, J.R.; Chi, D.C.; Suzman, D.L.; Cheng, J.; Zhang, L.; Sridhara, R.; Ibrahim, A.; Kluetz, P.G.; et al. An FDA Review of Drug Development in Nonmetastatic Castration-resistant Prostate Cancer. Clin. Cancer Res. 2020, 26, 4717–4722. [Google Scholar] [CrossRef]
- Janssen Pharmaceuticals, Inc. Highlight of Prescribing Information. Available online: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/ERLEADA-pi.pdf (accessed on 1 May 2021).
- Astellas Pharma, Inc. Highlight of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203415lbl.pdf (accessed on 1 May 2021).
- Bayer HealthCare Pharmaceuticals, Inc. Highlight of Prescribing Information. Available online: http://labeling.bayerhealthcare.com/html/products/pi/Nubeqa_PI.pdf (accessed on 1 May 2021).
- Rathkopf, D.E.; Morris, M.J.; Fox, J.J.; Danila, D.C.; Slovin, S.F.; Hager, J.H.; Rix, P.J.; Chow Maneval, E.; Chen, I.; Gonen, M.; et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 2013, 31, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Jeong, J.W.; Song, J.H.; Lee, K.R.; Ahn, S.; Ahn, S.H.; Kim, S.; Koo, T.S. Pharmacokinetics of enzalutamide, an anti-prostate cancer drug, in rats. Arch. Pharm. Res. 2015, 38, 2076–2082. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Massard, C.; Bono, P.; Jones, R.; Kataja, V.; James, N.; Garcia, J.A.; Protheroe, A.; Tammela, T.L.; Elliott, T.; et al. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): An open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 2014, 15, 975–985. [Google Scholar] [CrossRef]
- Gibbons, J.A.; Ouatas, T.; Krauwinkel, W.; Ohtsu, Y.; van der Walt, J.S.; Beddo, V.; de Vries, M.; Mordenti, J. Clinical Pharmacokinetic Studies of Enzalutamide. Clin. Pharm. 2015, 54, 1043–1055. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline Part I. J. Urol. 2021, 205, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J. Natl. Compr. Canc. Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef]
- Smith, M.R.; Cook, R.; Lee, K.A.; Nelson, J.B. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer 2011, 117, 2077–2085. [Google Scholar] [CrossRef]
- Moreira, D.M.; Howard, L.E.; Sourbeer, K.N.; Amarasekara, H.S.; Chow, L.C.; Cockrell, D.C.; Hanyok, B.T.; Aronson, W.J.; Kane, C.J.; Terris, M.K.; et al. Predictors of Time to Metastasis in Castration-resistant Prostate Cancer. Urology 2016, 96, 171–176. [Google Scholar] [CrossRef]
- Howard, L.E.; Moreira, D.M.; De Hoedt, A.; Aronson, W.J.; Kane, C.J.; Amling, C.L.; Cooperberg, M.R.; Terris, M.K.; Freedland, S.J. Thresholds for PSA doubling time in men with non-metastatic castration-resistant prostate cancer. BJU Int. 2017, 120, E80–E86. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Fizazi, K.; Gillessen, S.; Heidenreich, A.; Perez-Lopez, R.; Oyen, W.J.G.; Shore, N.; Smith, M.; Sweeney, C.; Tombal, B.; et al. Managing Nonmetastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 75, 285–293. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Oudard, S.; Shore, N.; Fizazi, K.; Sieber, P.; Tombal, B.; Damiao, R.; Marx, G.; Miller, K.; et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: Exploratory analyses by baseline prostate-specific antigen doubling time. J. Clin. Oncol. 2013, 31, 3800–3806. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide and Overall Survival in Prostate Cancer. Eur. Urol. 2021, 79, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; Fizazi, K.; Saad, F.; Shore, N.D.; De Giorgi, U.; Penson, D.F.; Ferreira, U.; Efstathiou, E.; Madziarska, K.; Kolinsky, M.P.; et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Shore, N.D.; Tammela, T.; Kuss, I.; Le Berre, M.A.; Mohamed, A.F.; Odom, D.; Bartsch, J.; Snapir, A.; Sarapohja, T.; et al. Impact of darolutamide (DARO) on pain and quality of life (QoL) in patients (Pts) with nonmetastatic castrate-resistant prostate cancer (nmCRPC). J. Clin. Oncol. 2019, 37. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N. Engl. J. Med. 2020, 383, 1040–1049. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juarez Soto, A.; Merseburger, A.S.; Ozguroglu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juarez, A.; Merseburger, A.S.; Ozguroglu, M.; Uemura, H.; et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Higano, C.S.; Beer, T.M.; Taplin, M.E.; Efstathiou, E.; Hirmand, M.; Forer, D.; Scher, H.I. Long-term Safety and Antitumor Activity in the Phase 1-2 Study of Enzalutamide in Pre- and Post-docetaxel Castration-Resistant Prostate Cancer. Eur. Urol. 2015, 68, 795–801. [Google Scholar] [CrossRef]
- Pilon, D.; Behl, A.S.; Ellis, L.A.; Robitaille, M.N.; Lefebvre, P.; Dawson, N.A. Assessment of Real-World Central Nervous System Events in Patients with Advanced Prostate Cancer Using Abiraterone Acetate, Bicalutamide, Enzalutamide, or Chemotherapy. Am. Health Drug Benefits 2017, 10, 143–153. [Google Scholar]
- Slovin, S.; Clark, W.; Carles, J.; Krivoshik, A.; Park, J.W.; Wang, F.; George, D. Seizure Rates in Enzalutamide-Treated Men With Metastatic Castration-Resistant Prostate Cancer and Risk of Seizure: The UPWARD Study. JAMA Oncol. 2018, 4, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Labrize, F.; Cany, L.; Massard, C.; Loriot, Y.; Sargos, P.; Gross-Goupil, M.; Roubaud, G. Enzalutamide and sleep apnea: An emerging central nervous system side-effect? Ann. Oncol. 2016, 27, 206. [Google Scholar] [CrossRef]
- Shore, N.D.; Tammela, T.L.; Massard, C.; Bono, P.; Aspegren, J.; Mustonen, M.; Fizazi, K. Safety and Antitumour Activity of ODM-201 (BAY-1841788) in Chemotherapy-naive and CYP17 Inhibitor-naive Patients: Follow-up from the ARADES and ARAFOR Trials. Eur. Urol. Focus 2018, 4, 547–553. [Google Scholar] [CrossRef]
- Zurth, C.; Koskinen, M.; Fricke, R.; Prien, O.; Korjamo, T.; Graudenz, K.; Denner, K.; Bairlein, M.; von Buhler, C.J.; Wilkinson, G.; et al. Drug-Drug Interaction Potential of Darolutamide: In Vitro and Clinical Studies. Eur. J. Drug Metab. Pharm. 2019, 44, 747–759. [Google Scholar] [CrossRef]
- Moilanen, A.M.; Riikonen, R.; Oksala, R.; Ravanti, L.; Aho, E.; Wohlfahrt, G.; Nykanen, P.S.; Tormakangas, O.P.; Palvimo, J.J.; Kallio, P.J. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci. Rep. 2015, 5, 12007. [Google Scholar] [CrossRef]
- Hird, A.E.; Magee, D.E.; Bhindi, B.; Ye, X.Y.; Chandrasekar, T.; Goldberg, H.; Klotz, L.; Fleshner, N.; Satkunasivam, R.; Klaassen, Z.; et al. A Systematic Review and Network Meta-analysis of Novel Androgen Receptor Inhibitors in Non-metastatic Castration-resistant Prostate Cancer. Clin. Genitourin. Cancer 2020, 18, 343–350. [Google Scholar] [CrossRef]
- Kumar, J.; Jazayeri, S.B.; Gautam, S.; Norez, D.; Alam, M.U.; Tanneru, K.; Bazargani, S.; Costa, J.; Bandyk, M.; Ganapathi, H.P.; et al. Comparative efficacy of apalutamide darolutamide and enzalutamide for treatment of non-metastatic castrate-resistant prostate cancer: A systematic review and network meta-analysis. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, T.; Ma, Z.; Zheng, S.; Chen, J.; Wu, Z.; Zheng, X.; Li, X.; Liu, Z. Systemic Management for Nonmetastatic Castration-resistant Prostate Cancer: A Systematic Review and Network Meta-Analysis. Am. J. Clin. Oncol. 2020, 43, 288–297. [Google Scholar] [CrossRef]
- Mori, K.; Mostafaei, H.; Pradere, B.; Motlagh, R.S.; Quhal, F.; Laukhtina, E.; Schuettfort, V.M.; Abufaraj, M.; Karakiewicz, P.I.; Kimura, T.; et al. Apalutamide, enzalutamide, and darolutamide for non-metastatic castration-resistant prostate cancer: A systematic review and network meta-analysis. Int. J. Clin. Oncol. 2020, 25, 1892–1900. [Google Scholar] [CrossRef]
- Roumiguie, M.; Paoletti, X.; Neuzillet, Y.; Mathieu, R.; Vincendeau, S.; Kleinclauss, F.; Mejean, A.; Guy, L.; Timsit, M.O.; Lebret, T. Apalutamide, darolutamide and enzalutamide in nonmetastatic castration-resistant prostate cancer: A meta-analysis. Future Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Jiang, S.; Terasawa, E.; Garcia-Horton, V.; Ayyagari, R.; Waldeck, A.R.; Shore, N. Indirect Comparison of Darolutamide versus Apalutamide and Enzalutamide for Nonmetastatic Castration-Resistant Prostate Cancer. J. Urol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.A.; Eigl, B.J.; Murray, R.N.; Kollmannsberger, C.; Chi, K.N. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur. Urol. 2015, 67, 23–29. [Google Scholar] [CrossRef]
- Beekman, K.W.; Fleming, M.T.; Scher, H.I.; Slovin, S.F.; Ishill, N.M.; Heller, G.; Kelly, W.K. Second-line chemotherapy for prostate cancer: Patient characteristics and survival. Clin. Prostate Cancer 2005, 4, 86–90. [Google Scholar] [CrossRef]
- Behl, A.S.; Ellis, L.A.; Pilon, D.; Xiao, Y.; Lefebvre, P. Medication Adherence, Treatment Patterns, and Dose Reduction in Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Abiraterone Acetate or Enzalutamide. Am. Health Drug Benefits 2017, 10, 296–303. [Google Scholar]
- Chung, D.Y.; Kang, D.H.; Kim, J.W.; Kim, D.K.; Lee, J.Y.; Hong, C.H.; Cho, K.S. Comparison of Oncologic Outcomes Between Two Alternative Sequences with Abiraterone Acetate and Enzalutamide in Patients with Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, D.J.; Annala, M.; Taavitsainen, S.; Finch, D.L.; Oja, C.; Vergidis, J.; Zulfiqar, M.; Sunderland, K.; Azad, A.A.; Kollmannsberger, C.K.; et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: A multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019, 20, 1730–1739. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Sciarra, A.; Gentilucci, A.; Silvestri, I.; Salciccia, S.; Cattarino, S.; Scarpa, S.; Gatto, A.; Frantellizzi, V.; Von Heland, M.; Ricciuti, G.P.; et al. Androgen receptor variant 7 (AR-V7) in sequencing therapeutic agents for castratrion resistant prostate cancer: A critical review. Medicine 2019, 98, e15608. [Google Scholar] [CrossRef] [PubMed]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wulfing, C.; Kramer, G.; Eymard, J.C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef]
- Italiano, A.; Ortholan, C.; Oudard, S.; Pouessel, D.; Gravis, G.; Beuzeboc, P.; Bompas, E.; Flechon, A.; Joly, F.; Ferrero, J.M.; et al. Docetaxel-based chemotherapy in elderly patients (age 75 and older) with castration-resistant prostate cancer. Eur. Urol. 2009, 55, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Droz, J.P.; Balducci, L.; Bolla, M.; Emberton, M.; Fitzpatrick, J.M.; Joniau, S.; Kattan, M.W.; Monfardini, S.; Moul, J.W.; Naeim, A.; et al. Management of prostate cancer in older men: Recommendations of a working group of the International Society of Geriatric Oncology. BJU Int. 2010, 106, 462–469. [Google Scholar] [CrossRef]
- Duran, I.; Carles, J.; Bulat, I.; Hellemans, P.; Mitselos, A.; Ward, P.; Jiao, J.; Armas, D.; Chien, C. Pharmacokinetic Drug-Drug Interaction of Apalutamide, Part 1: Clinical Studies in Healthy Men and Patients with Castration-Resistant Prostate Cancer. Clin. Pharm. 2020, 59, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Kocher, J.; Mueller, C.; Rosenzweig, S.; Theile, D. Impact of enzalutamide and its main metabolite N-desmethyl enzalutamide on pharmacokinetically important drug metabolizing enzymes and drug transporters. Biopharm. Drug Dispos. 2017, 38, 517–525. [Google Scholar] [CrossRef]
- Bhatnagar, V.; Garcia, E.P.; O’Connor, D.T.; Brophy, V.H.; Alcaraz, J.; Richard, E.; Bakris, G.L.; Middleton, J.P.; Norris, K.C.; Wright, J.; et al. CYP3A4 and CYP3A5 Polymorphisms and Blood Pressure Response to Amlodipine among African-American Men and Women with Early Hypertensive Renal Disease. Am. J. Nephrol. 2010, 31, 95–103. [Google Scholar] [CrossRef]
- Gibbons, J.A.; de Vries, M.; Krauwinkel, W.; Ohtsu, Y.; Noukens, J.; van der Walt, J.S.; Mol, R.; Mordenti, J.; Ouatas, T. Pharmacokinetic Drug Interaction Studies with Enzalutamide. Clin. Pharm. 2015, 54, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.; Zurth, C.; Fricke, R.; Gieschen, H.; Graudenz, K.; Koskinen, M.; Ploeger, B.; Moss, J.; Prien, O.; Borghesi, G.; et al. Evaluation of Clinically Relevant Drug-Drug Interactions and Population Pharmacokinetics of Darolutamide in Patients with Nonmetastatic Castration-Resistant Prostate Cancer: Results of Pre-Specified and Post Hoc Analyses of the Phase III ARAMIS Trial. Target. Oncol. 2019, 14, 527–539. [Google Scholar] [CrossRef]
- Iacovelli, R.; Verri, E.; Cossu Rocca, M.; Aurilio, G.; Cullura, D.; De Cobelli, O.; Nole, F. The incidence and relative risk of cardiovascular toxicity in patients treated with new hormonal agents for castration-resistant prostate cancer. Eur. J. Cancer 2015, 51, 1970–1977. [Google Scholar] [CrossRef]
- Saltalamacchia, G.; Frascaroli, M.; Bernardo, A.; Quaquarini, E. Renal and Cardiovascular Toxicities by New Systemic Treatments for Prostate Cancer. Cancers 2020, 12. [Google Scholar] [CrossRef]
- Iacovelli, R.; Ciccarese, C.; Bria, E.; Romano, M.; Fantinel, E.; Bimbatti, D.; Muraglia, A.; Porcaro, A.B.; Siracusano, S.; Brunelli, M.; et al. The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, e645–e653. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Shore, N.D.; Morgans, A.K.; Winters-Stone, K.M.; Wefel, J.S.; Ortiz, J.A.; Reeves, J.A.; George, D.J. DaroACT: Darolutamide and enzalutamide effects on physical and neurocognitive function and daily activity in patients with castration-resistant prostate cancer (CRPC). J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Esper, P.; Mo, F.; Chodak, G.; Sinner, M.; Cella, D.; Pienta, K.J. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 1997, 50, 920–928. [Google Scholar] [CrossRef]
- Chowdhury, S.; Oudard, S.; Uemura, H.; Joniau, S.; Pilon, D.; Lefebvre, P.; McQuarrie, K.; Liu, J.; Dearden, L.; Sermon, J.; et al. Matching-Adjusted Indirect Comparison of Health-Related Quality of Life and Adverse Events of Apalutamide Versus Enzalutamide in Non-Metastatic Castration-Resistant Prostate Cancer. Adv. Ther. 2020, 37, 512–526. [Google Scholar] [CrossRef]
- Onal, C.; Guler, O.C.; Torun, N.; Reyhan, M.; Yapar, A.F. The effect of androgen deprivation therapy on 68Ga-PSMA tracer uptake in non-metastatic prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Ost, P. PSMA PET-CT redefines nonmetastatic castration-resistant prostate cancer. Nat. Rev. Urol. 2020, 17, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, C.; Wei, Y.; Meng, J.; Zhang, Y.; Gan, H.; Xu, X.; Wan, F.; Pan, J.; Ma, X.; et al. A Prospective Trial of 68Ga-PSMA and (18)F-FDG PET/CT in Nonmetastatic Prostate Cancer Patients with an Early PSA Progression During Castration. Clin. Cancer Res. 2020, 26, 4551–4558. [Google Scholar] [CrossRef]
- Fendler, W.P.; Weber, M.; Iravani, A.; Hofman, M.S.; Calais, J.; Czernin, J.; Ilhan, H.; Saad, F.; Small, E.J.; Smith, M.R.; et al. Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 7448–7454. [Google Scholar] [CrossRef]
- Fourquet, A.; Aveline, C.; Cussenot, O.; Crehange, G.; Montravers, F.; Talbot, J.N.; Gauthe, M. Ga-68-PSMA-11 PET/CT in restaging castration-resistant nonmetastatic prostate cancer: Detection rate, impact on patients’ disease management and adequacy of impact. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Alipour, R.; Azad, A.; Hofman, M.S. Guiding management of therapy in prostate cancer: Time to switch from conventional imaging to PSMA PET. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef]
- de Bono, J.S.; Fleming, M.T.; Wang, J.S.; Cathomas, R.; Miralles, M.S.; Bothos, J.; Hinrichs, M.J.; Zhang, Q.; He, P.; Williams, M.; et al. Phase I Study of MEDI3726: A Prostate-Specific Membrane Antigen-Targeted Antibody-Drug Conjugate, in Patients with mCRPC after Failure of Abiraterone or Enzalutamide. Clin. Cancer Res. 2021. [Google Scholar] [CrossRef]
| Apalutamide [18] | Enzalutamide [19] | Darolutamide [20] | |
|---|---|---|---|
| Brand name | Erleada | Xtandi | Nubeqa |
| Dose/form | 60 mg/tablet | 40 mg/capsule | 300 mg/film-coated tablet |
| Total daily dosage | 240 mg (once per day) | 160 mg (once per day) | 600 mg (twice per day) |
| Route of administration | Oral administration | Oral administration | Oral administration |
| Approved indication | nmCRPC, mCSPC | nmCRPC, mCSPC, mCRPC | nmCRPC |
| Chemical structure | 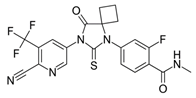 | 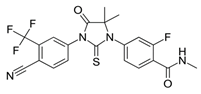 | 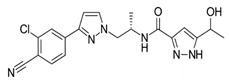 |
| Bioavailability | 100% [21] | Rats: 89.7% [22]; humans: unknown (at least 84.6%; based on recovery from excretion) | ≤30% |
| Metabolites | N-Desmethylapalutamide | N-Desmethylenzalutamide (active) Carboxylic acid derivative metabolite (inactive) | Ketodarolutamide |
| Half-life | 3–4 days (at steady state) | 5.8 days (range 2.8–10.2 days) | 16–20 h [23] |
| Excretion | Urine: 65% Feces: 24% | Urine: 71.0% Bile: 13.6% Feces: 0.39% [24] | Urine: 63.4% Feces: 32.4% |
| Blood–brain barrier penetration | Yes | Yes | Negligible |
| Mechanism of action for AR inhibition | It binds to the ligand-binding domain of the androgen receptor, blocks androgen-receptor nuclear translocation, inhibits DNA binding, and obstructs androgen receptor-mediated transcription. | It prevents the translocation of the AR from the cytoplasm to the nucleus. Within the nucleus, it inhibits AR binding to chromosomal DNA, which prevents further transcription of tumor genes. | It competitively inhibits androgen binding, AR nuclear translocation, and AR-mediated transcription. A major metabolite, keto-darolutamide, exhibited similar in vitro activity to darolutamide. |
| Spartan [7,33] (NCT01946204) | Prosper [6,34] (NCT02003924) | Aramis [36] (NCT02200614) | |
|---|---|---|---|
| Enroll patients | APA (n = 806) vs. PBO (n = 401) | ENZA (n = 933) vs. PBO (n = 468) | DARO (n = 955) vs. PBO (n = 554) |
| Inclusion criteria | M0N0-N1CRPC, PSADT < 10 months | M0N0CRPC, PSADT < 10 months, PSA >2 ng/mL | M0N0-N1CRPC, PSADT < 10 months, PSA >2 ng/mL |
| Median age (range) | APA 74 years (48−94) vs. PBO 74 years (52−97) | ENZA 74 years (50−95) vs. PBO 73 years (53−92) | DARO 74 years (48−95) vs. PBO 74 years (50−92) |
| Median follow-up (final) | 52 months | 48 months | 29 months |
| Median PSA at baseline (ng/mL) | APA 7.78 vs. PBO 7.96 | ENZA 11.1 vs. PBO 10.2 | DARO 9.0 vs. PBO 9.7 |
| Median PSADT (months) | APA 4.4 vs. PBO 4.5 | ENZA 3.8 vs. PBO 3.6 | DARO 4.4 vs. PBO 4.7 |
| Median MFS (months) | APA 40.5 vs. PBO 16.2 24-month MFS benefit | ENZA 36.6 vs. PBO 14.7 22-month MFS benefit | DARO 40.4 vs. PBO 18.4 22-month MFS benefit |
| 72% reduction of distant progression or death; HR 0.28 (95% CI 0.23–0.35); p < 0.001 | 71% reduction of distant progression or death; HR 0.29 (95% CI 0.24–0.35); p < 0.001 | 59% reduction of distant progression or death; HR 0.41 (95% CI 0.34–0.50); p < 0.001 | |
| Final median OS (months) | APA 73.9 vs. PBO 59.9 | ENZA 67.0 vs. PBO 56.3 | Not reached |
| 22% reduction in risk of death HR 0.78 (95% CI 0.64–0.96); p = 0.016 | 27% reduction in risk of death HR 0.73 (95% CI 0.61–0.89); p = 0.001 | 31% reduction in risk of death HR 0.69 (95% CI 0.53–0.88); p = 0.003 | |
| Median time to PSA progression (months) | APA 40.5 vs. PBO 3.7 | ENZA 40.5 vs. PBO 3.7 | DARO 33.2 vs. PBO 7.3 |
| HR 0.07 (95% CI 0.06–0.09); p < 0.0001 | HR 0.07 (95% CI 0.05–0.08); p < 0.001 | HR 0.13 (95% CI 0.11–0.16); p < 0.001 | |
| Median PFS (months) | APA 40.5 vs. PBO 14.7 | NR | DARO 36.8 vs. PBO 14.8 |
| HR 0.29 (95% CI 0.24–0.36); p < 0.0001 | HR 0.38 (95% CI 0.32–0.45); p < 0.001 | ||
| Time to initiation of cytotoxic chemotherapy | HR 0.63 (95% CI 0.49–0.81); p = 0.0002 | NR | HR 0.58 (95% CI 0.44–0.76); p < 0.001 |
| Time to initiation of suqsequent antineoplastic therapy | NR | HR 0.29 (95% CI 0.25–0.34); p < 0.001 | HR 0.36 (95% CI 0.27–0.48); p < 0.001 |
| Safety | Spartan [7,33] (NCT01946204) | Prosper [6,34] (NCT02003924) | Aramis [13] (NCT02200614) | |||
|---|---|---|---|---|---|---|
| APA (n = 803) | PBO (n = 398) | ENZA (n = 930) | PBO (n = 465) | DARO (n = 954) | PBO (n = 554) | |
| Any AE | 781 (97) | 373 (94) | 876 (94) | 380 (82) | 818 (85.7) | 439 (79.2) |
| Grade 3 or 4 AE | 449 (56) | 145 (36) | 292 (31) | 109 (23) | 251 (26.3) | 120 (21.7) |
| Any serious AE | 290 (36) | 99 (25) | 372 (40) | 100 (22) | 249 (26.1) | 121 (21.8) |
| AE leading to discontinuation | 120 (15.0) | 29 (7.3) | 158 (17) | 41 (9) | 85 (8.9) | 48 (8.7) |
| AE leading to death | 24 (3.0) | 2 (0.5) | 51 (5.0) | 3 (1.0) | 38 (4.0) | 19 (3.4) |
| Fatigue * | 265 (33) | 83 (21) | 424 (46) | 103 (22) | 126 (13.2) | 46 (8.3) |
| Hypertension * | 225 (28) | 83 (21) | 161 (17) | 27 (6) | 74 (7.8) | 36 (6.5) |
| Falls * | 177 (22) | 38 (9.5) | 164 (18) | 25 (5) | 50 (5.2) | 27 (4.9 |
| Bone fracture * | 145 (18) | 30 (7.5) | 168 (18) | 29 (6) | 52 (5.5) | 20 (3.6) |
| Arthralgia * | 160 (20) | 33 (8.3) | 119 (13) | 36 (8) | 86 (9.0) | 52 (9.4) |
| Constipation * | not reported | 121 (13) | 39 (8) | 66 (6.9) | 36 (6.5) | |
| Diarrhea * | 184 (23) | 60 (15) | 112 (12) | 47 (10) | 71 (7.4) | 31 (5.6) |
| Hot flush * | 120 (15) | 34 (8.5) | 121 (13) | 36 (8) | 57 (6.0) | 25 (4.5) |
| Mental impairment disorder * | 41 (5.1) | 12 (3) | 73 (8) | 10 (2) | 19 (2.0) | 10 (1.8) |
| Rash * | 212 (26) | 25 (6.3) | 38 (4) | 13 (3) | 30 (3.1) | 6 (1.1) |
| Seizure * | 5 (0.6) | 0 | 3 (<1) | 0 | 2 (0.2) | 1 (0.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, D.Y.; Ha, J.S.; Cho, K.S. Novel Treatment Strategy Using Second-Generation Androgen Receptor Inhibitors for Non-Metastatic Castration-Resistant Prostate Cancer. Biomedicines 2021, 9, 661. https://doi.org/10.3390/biomedicines9060661
Chung DY, Ha JS, Cho KS. Novel Treatment Strategy Using Second-Generation Androgen Receptor Inhibitors for Non-Metastatic Castration-Resistant Prostate Cancer. Biomedicines. 2021; 9(6):661. https://doi.org/10.3390/biomedicines9060661
Chicago/Turabian StyleChung, Doo Yong, Jee Soo Ha, and Kang Su Cho. 2021. "Novel Treatment Strategy Using Second-Generation Androgen Receptor Inhibitors for Non-Metastatic Castration-Resistant Prostate Cancer" Biomedicines 9, no. 6: 661. https://doi.org/10.3390/biomedicines9060661
APA StyleChung, D. Y., Ha, J. S., & Cho, K. S. (2021). Novel Treatment Strategy Using Second-Generation Androgen Receptor Inhibitors for Non-Metastatic Castration-Resistant Prostate Cancer. Biomedicines, 9(6), 661. https://doi.org/10.3390/biomedicines9060661






