HOXB5 Overexpression Is Associated with Neuroendocrine Differentiation and Poor Prognosis in Prostate Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Immunohistochemistry
2.3. In Silico Analysis
2.4. Cell Lines
2.5. Western Blotting
2.6. Generation of HOXB5-Expressing Cells
2.7. RNA Interference
2.8. Cell Growth and Invasion Assays
2.9. Generation of RB1 Knockout Cells
2.10. Enzalutamide and Apalutamide Treatment
2.11. Statistical Analysis
3. Results
3.1. Expression of HOXB5 in Localized PCa
3.2. Expression of HOXB5 in Metastatic PCa
3.3. HOXB5 Promotes Cell Growth and Invasion in PCa
3.4. Association between HOXB5 and RET in PCa
3.5. Involvement of HOXB5 in Neuroendocrine Differentiation in PCa
3.6. HOXB5 Expression Is Upregulated by RB1 and p53 Modulation
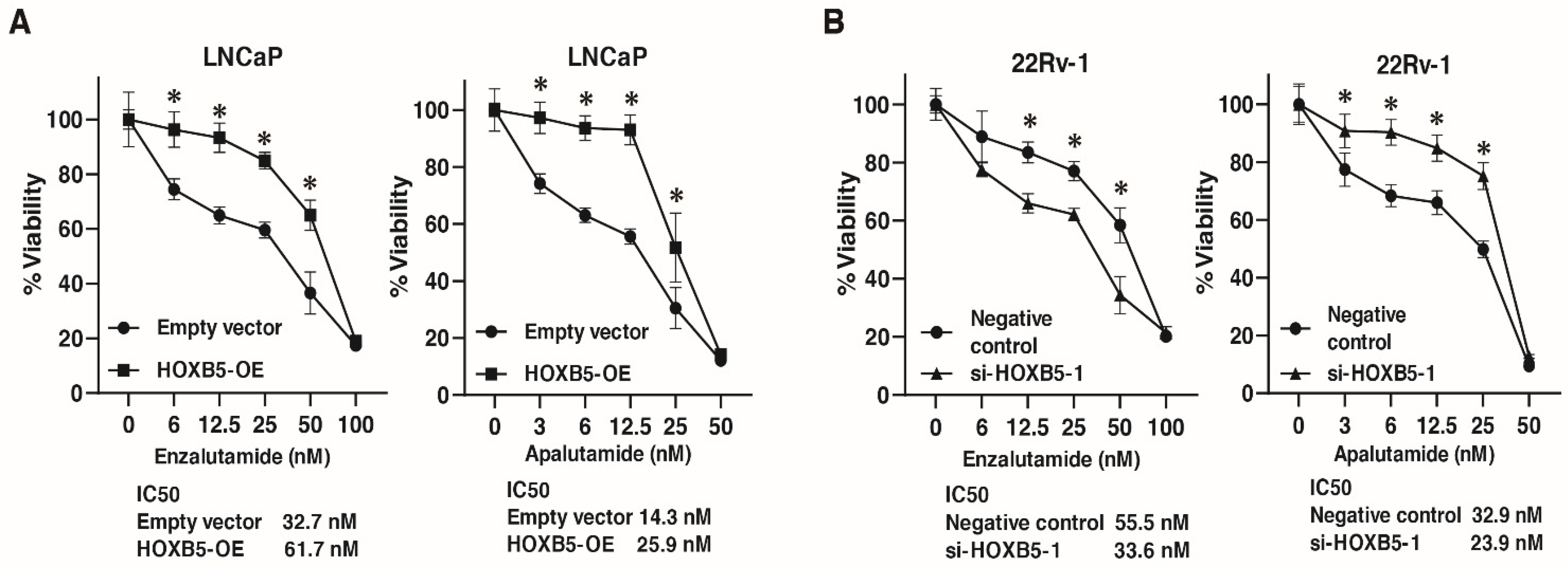
3.7. Involvement of HOXB5 in Enzalutamide and Apalutamide Resistance in PCa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Karantanos, T.; Evans, C.P.; Tombal, B.; Thompson, T.C.; Montironi, R.; Isaacs, W.B. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur. Urol. 2015, 67, 470–479. [Google Scholar] [CrossRef] [Green Version]
- Mohler, M.L.; Sikdar, A.; Ponnusamy, S.; Hwang, D.J.; He, Y.; Miller, D.D.; Narayanan, R. An Overview of Next-Generation Androgen Receptor-Targeted Therapeutics in Development for the Treatment of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 2124. [Google Scholar] [CrossRef] [PubMed]
- Carceles-Cordon, M.; Kelly, W.K.; Gomella, L.; Knudsen, K.E.; Rodriguez-Bravo, V.; Domingo-Domenech, J. Cellular rewiring in lethal prostate cancer: The architect of drug resistance. Nat. Rev. Urol. 2020, 17, 292–307. [Google Scholar] [CrossRef]
- Kaarijarvi, R.; Kaljunen, H.; Ketola, K. Molecular and Functional Links between Neurodevelopmental Processes and Treatment-Induced Neuroendocrine Plasticity in Prostate Cancer Progression. Cancers 2021, 13, 692. [Google Scholar] [CrossRef]
- Mallo, M.; Alonso, C.R. The regulation of Hox gene expression during animal development. Development 2013, 140, 3951–3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatlekar, S.; Fields, J.Z.; Boman, B.M. HOX genes and their role in the development of human cancers. J. Mol. Med. (Berl) 2014, 92, 811–823. [Google Scholar] [CrossRef]
- Brotto, D.B.; Siena, A.D.D.; de Barros, I.I.; Carvalho, S.; Muys, B.R.; Goedert, L.; Cardoso, C.; Placa, J.R.; Ramao, A.; Squire, J.A.; et al. Contributions of HOX genes to cancer hallmarks: Enrichment pathway analysis and review. Tumour Biol. 2020, 42. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Zhu, S.; Zhang, H.; Li, L.; Niu, Y. Germline homeobox B13 (HOXB13) G84E mutation and prostate cancer risk in European descendants: A meta-analysis of 24,213 cases and 73, 631 controls. Eur. Urol. 2013, 64, 173–176. [Google Scholar] [CrossRef]
- Dong, Y.; Cai, Y.; Liu, B.; Jiao, X.; Li, Z.T.; Guo, D.Y.; Li, X.W.; Wang, Y.J.; Yang, D.K. HOXA13 is associated with unfavorable survival and acts as a novel oncogene in prostate carcinoma. Future Oncol. 2017, 13, 1505–1516. [Google Scholar] [CrossRef]
- Hamid, A.R.; Hoogland, A.M.; Smit, F.; Jannink, S.; van Rijt-van de Westerlo, C.; Jansen, C.F.; van Leenders, G.J.; Verhaegh, G.W.; Schalken, J.A. The role of HOXC6 in prostate cancer development. Prostate 2015, 75, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Kam, M.K.; Lui, V.C. Roles of Hoxb5 in the development of vagal and trunk neural crest cells. Dev. Growth Differ. 2015, 57, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Lui, V.C.; Cheng, W.W.; Leon, T.Y.; Lau, D.K.; Garcia-Barcelo, M.M.; Miao, X.P.; Kam, M.K.; So, M.T.; Chen, Y.; Wall, N.A.; et al. Perturbation of hoxb5 signaling in vagal neural crests down-regulates ret leading to intestinal hypoganglionosis in mice. Gastroenterology 2008, 134, 1104–1115. [Google Scholar] [CrossRef]
- He, M.X.; Cuoco, M.S.; Crowdis, J.; Bosma-Moody, A.; Zhang, Z.; Bi, K.; Kanodia, A.; Su, M.J.; Ku, S.Y.; Garcia, M.M.; et al. Transcriptional mediators of treatment resistance in lethal prostate cancer. Nat. Med. 2021, 27, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Buyyounouski, M.K.; Choyke, P.L.; McKenney, J.K.; Sartor, O.; Sandler, H.M.; Amin, M.B.; Kattan, M.W.; Lin, D.W. Prostate cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Freedland, S.J.; Sutter, M.E.; Dorey, F.; Aronson, W.J. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology 2003, 61, 365–369. [Google Scholar] [CrossRef]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Sekino, Y.; Oue, N.; Mukai, S.; Shigematsu, Y.; Goto, K.; Sakamoto, N.; Sentani, K.; Hayashi, T.; Teishima, J.; Matsubara, A.; et al. Protocadherin B9 promotes resistance to bicalutamide and is associated with the survival of prostate cancer patients. Prostate 2019, 79, 234–242. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [Green Version]
- Varambally, S.; Yu, J.; Laxman, B.; Rhodes, D.R.; Mehra, R.; Tomlins, S.A.; Shah, R.B.; Chandran, U.; Monzon, F.A.; Becich, M.J.; et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 2005, 8, 393–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlins, S.A.; Mehra, R.; Rhodes, D.R.; Cao, X.; Wang, L.; Dhanasekaran, S.M.; Kalyana-Sundaram, S.; Wei, J.T.; Rubin, M.A.; Pienta, K.J.; et al. Integrative molecular concept modeling of prostate cancer progression. Nat. Genet. 2007, 39, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Wang, H.; He, H.H.; Chen, S.; He, L.; Ma, F.; Mucci, L.; Wang, Q.; Fiore, C.; Sowalsky, A.G.; et al. ERG induces androgen receptor-mediated regulation of SOX9 in prostate cancer. J. Clin. Investig. 2013, 123, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Derosa, C.A.; Furusato, B.; Shaheduzzaman, S.; Srikantan, V.; Wang, Z.; Chen, Y.; Seifert, M.; Ravindranath, L.; Young, D.; Nau, M.; et al. Elevated osteonectin/SPARC expression in primary prostate cancer predicts metastatic progression. Prostate Cancer Prostatic Dis. 2012, 15, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Tzelepi, V.; Zhang, J.; Lu, J.F.; Kleb, B.; Wu, G.; Wan, X.; Hoang, A.; Efstathiou, E.; Sircar, K.; Navone, N.M.; et al. Modeling a lethal prostate cancer variant with small-cell carcinoma features. Clin. Cancer Res. 2012, 18, 666–677. [Google Scholar] [CrossRef] [Green Version]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Long, Q.; Xu, J.; Osunkoya, A.O.; Sannigrahi, S.; Johnson, B.A.; Zhou, W.; Gillespie, T.; Park, J.Y.; Nam, R.K.; Sugar, L.; et al. Global transcriptome analysis of formalin-fixed prostate cancer specimens identifies biomarkers of disease recurrence. Cancer Res. 2014, 74, 3228–3237. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Coleman, I.M.; Brown, L.G.; True, L.D.; Kollath, L.; Lucas, J.M.; Lam, H.M.; Dumpit, R.; Corey, E.; Chery, L.; et al. SRRM4 Expression and the Loss of REST Activity May Promote the Emergence of the Neuroendocrine Phenotype in Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2015, 21, 4698–4708. [Google Scholar] [CrossRef] [Green Version]
- Ross-Adams, H.; Lamb, A.D.; Dunning, M.J.; Halim, S.; Lindberg, J.; Massie, C.M.; Egevad, L.A.; Russell, R.; Ramos-Montoya, A.; Vowler, S.L.; et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: A discovery and validation cohort study. EBioMedicine 2015, 2, 1133–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Coleman, I.; Morrissey, C.; Zhang, X.; True, L.D.; Gulati, R.; Etzioni, R.; Bolouri, H.; Montgomery, B.; White, T.; et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 2016, 22, 369–378. [Google Scholar] [CrossRef]
- Tsai, H.K.; Lehrer, J.; Alshalalfa, M.; Erho, N.; Davicioni, E.; Lotan, T.L. Gene expression signatures of neuroendocrine prostate cancer and primary small cell prostatic carcinoma. BMC Cancer 2017, 17, 759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labrecque, M.P.; Coleman, I.M.; Brown, L.G.; True, L.D.; Kollath, L.; Lakely, B.; Nguyen, H.M.; Yang, Y.C.; da Costa, R.M.G.; Kaipainen, A.; et al. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J. Clin. Investig. 2019, 129, 4492–4505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyquist, M.D.; Corella, A.; Coleman, I.; De Sarkar, N.; Kaipainen, A.; Ha, G.; Gulati, R.; Ang, L.; Chatterjee, P.; Lucas, J.; et al. Combined TP53 and RB1 Loss Promotes Prostate Cancer Resistance to a Spectrum of Therapeutics and Confers Vulnerability to Replication Stress. Cell Rep. 2020, 31, 107669. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Cyrta, J.; Heller, G.; Prandi, D.; Armenia, J.; Coleman, I.; Cieslik, M.; Benelli, M.; Robinson, D.; Van Allen, E.M.; et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl Acad Sci. USA 2019, 116, 11428–11436. [Google Scholar] [CrossRef] [Green Version]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Tsherniak, A.; Vazquez, F.; Montgomery, P.G.; Weir, B.A.; Kryukov, G.; Cowley, G.S.; Gill, S.; Harrington, W.F.; Pantel, S.; Krill-Burger, J.M.; et al. Defining a Cancer Dependency Map. Cell 2017, 170, 564–576.e16. [Google Scholar] [CrossRef] [Green Version]
- Sekino, Y.; Sakamoto, N.; Sentani, K.; Oue, N.; Teishima, J.; Matsubara, A.; Yasui, W. miR-130b Promotes Sunitinib Resistance through Regulation of PTEN in Renal Cell Carcinoma. Oncology 2019, 97, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Garcia-Barcelo, M.M.; Tam, P.K.; Lui, V.C. HOXB5 cooperates with NKX2-1 in the transcription of human RET. PLoS ONE 2011, 6, e20815. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.J.; Kam, M.K.; Garcia-Barcelo, M.M.; Tam, P.K.; Lui, V.C. HOXB5 binds to multi-species conserved sequence (MCS+9.7) of RET gene and regulates RET expression. Int. J. Biochem. Cell Biol. 2014, 51, 142–149. [Google Scholar] [CrossRef]
- VanDeusen, H.R.; Ramroop, J.R.; Morel, K.L.; Bae, S.Y.; Sheahan, A.V.; Sychev, Z.; Lau, N.A.; Cheng, L.C.; Tan, V.M.; Li, Z.; et al. Targeting RET Kinase in Neuroendocrine Prostate Cancer. Mol. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.S.; Clegg, N.; Arnold, H.; Ferguson, C.; Bonham, M.; White, J.; Hood, L.; Lin, B. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc. Natl Acad Sci. USA 2002, 99, 11890–11895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, P.; Zhang, Z.; Benelli, M.; Karthaus, W.R.; Hoover, E.; Chen, C.C.; Wongvipat, J.; Ku, S.Y.; Gao, D.; Cao, Z.; et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 2017, 355, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Fei, X.; Kong, L.; Tan, X. HOXB5 promotes proliferation, migration, and invasion of pancreatic cancer cell through the activation of the GSK3beta/beta-catenin pathway. Anticancer Drugs 2020, 31, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, N.; Zhang, H. Knockdown of Homeobox B5 (HOXB5) Inhibits Cell Proliferation, Migration, and Invasion in Non-Small Cell Lung Cancer Cells Through Inactivation of the Wnt/beta-Catenin Pathway. Oncol. Res. 2018, 26, 37–44. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hur, H.; Yun, H.J.; Kim, Y.; Yang, S.; Kim, S.I.; Kim, M.H. HOXB5 Promotes the Proliferation and Invasion of Breast Cancer Cells. Int. J. Biol. Sci. 2015, 11, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Chang, J.W.; Oh, C.; Liu, L.; Jung, S.N.; Won, H.R.; Kim, Y.I.; Rha, K.S.; Koo, B.S. HOXB5 acts as an oncogenic driver in head and neck squamous cell carcinoma via EGFR/Akt/Wnt/beta-catenin signaling axis. Eur. J. Surg. Oncol. 2019. [Google Scholar] [CrossRef]
- Ban, K.; Feng, S.; Shao, L.; Ittmann, M. RET Signaling in Prostate Cancer. Clin. Cancer Res. 2017, 23, 4885–4896. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, R.; Huang, J.; Alumkal, J.J.; Zhang, L.; Feng, F.Y.; Thomas, G.V.; Weinstein, A.S.; Friedl, V.; Zhang, C.; Witte, O.N.; et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J. Clin. Oncol. 2018, 36, 2492–2503. [Google Scholar] [CrossRef]
- Namekawa, T.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Application of Prostate Cancer Models for Preclinical Study: Advantages and Limitations of Cell Lines, Patient-Derived Xenografts, and Three-Dimensional Culture of Patient-Derived Cells. Cells 2019, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, Y.H.; Lee, D.S.; Chung, J.K.; Kim, S. In vivo imaging of functional targeting of miR-221 in papillary thyroid carcinoma. J. Nucl. Med. 2008, 49, 1686–1693. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Lothe, R.A.; Ahlquist, T.; Silins, I.; Trope, C.G.; Micci, F.; Nesland, J.M.; Suo, Z.; Lind, G.E. DNA methylation profiling of ovarian carcinomas and their in vitro models identifies HOXA9, HOXB5, SCGB3A1, and CRABP1 as novel targets. Mol. Cancer 2007, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abida, W.; Armenia, J.; Gopalan, A.; Brennan, R.; Walsh, M.; Barron, D.; Danila, D.; Rathkopf, D.; Morris, M.; Slovin, S.; et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis Oncol. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
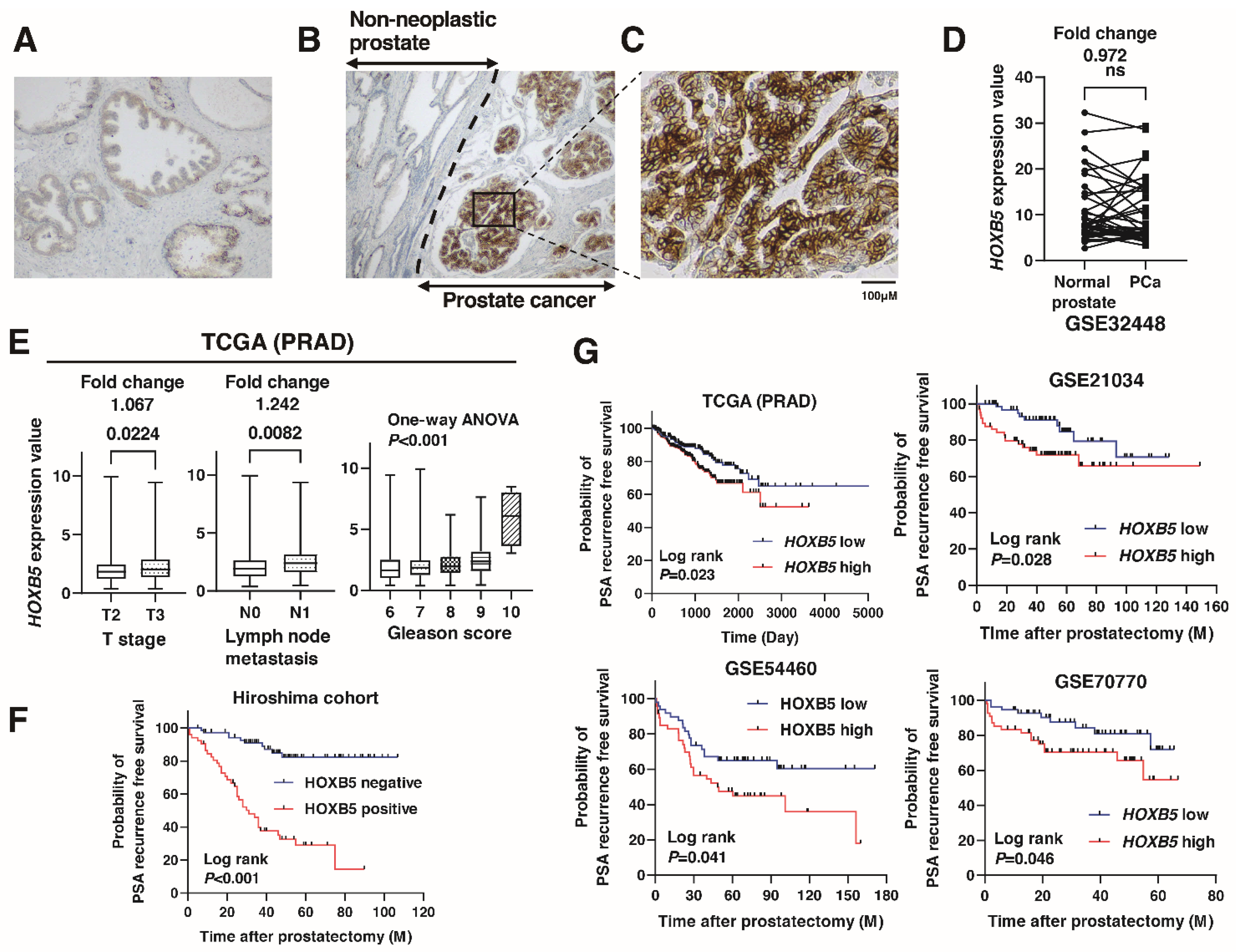
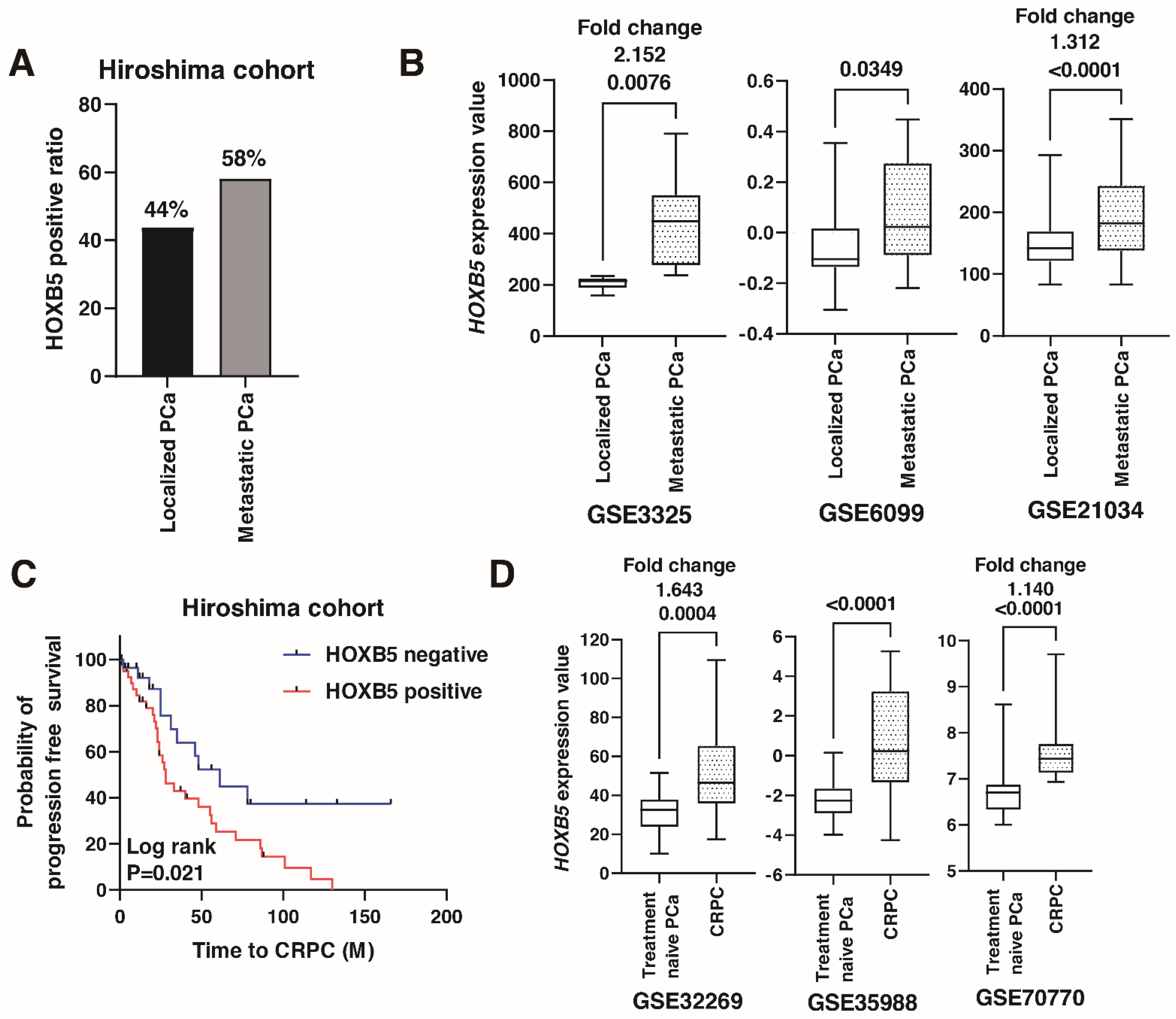


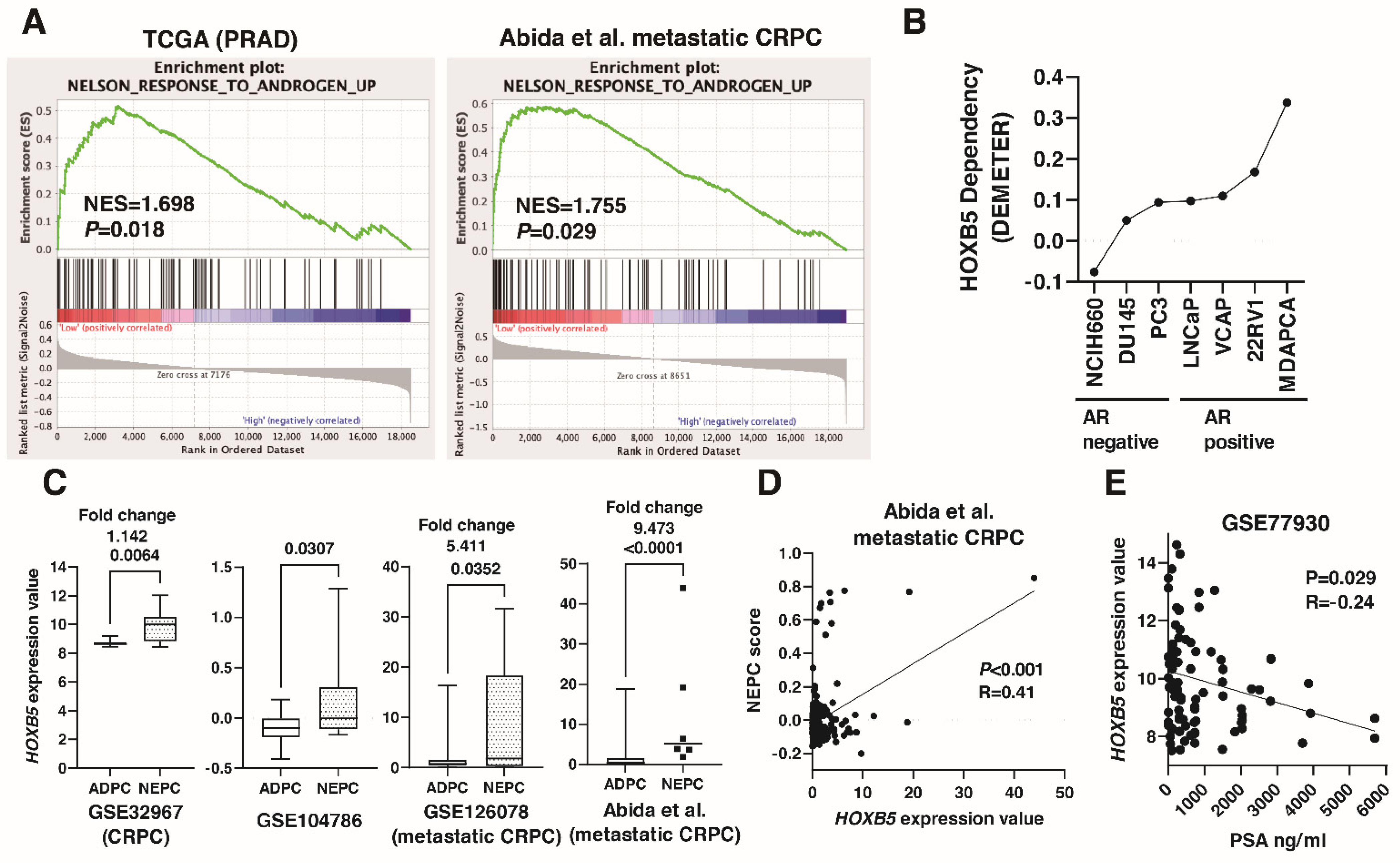
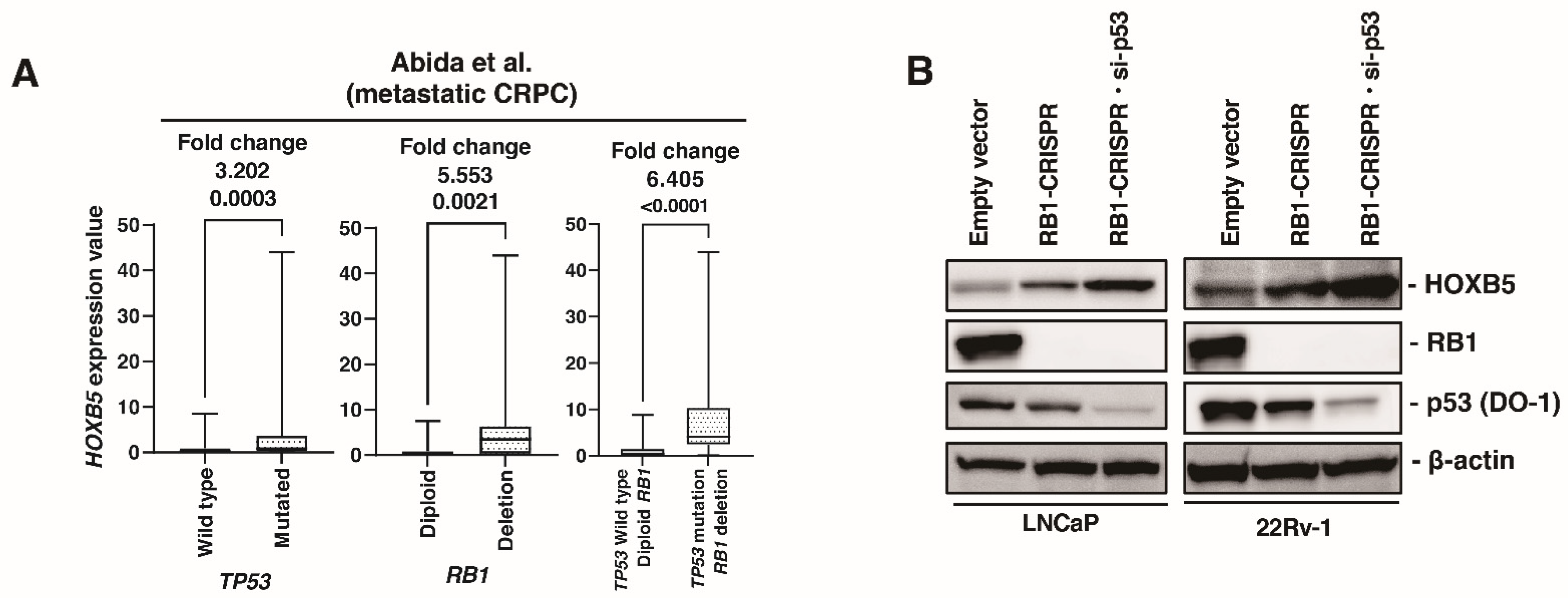
| HOXB5 Expression | p-Value a | ||
|---|---|---|---|
| Positive (n = 56) (%) | Negative (n = 72) (%) | ||
| Age | |||
| ≤65 (n = 53) | 23 (41%) | 30 (42%) | N.S. |
| ≥66 (n = 75) | 33 (59%) | 42 (58%) | |
| Preoperative PSA (ng/mL) | |||
| ≥10 (n = 78) | 29 (52%) | 49 (68%) | N.S. |
| <10 (n = 50) | 27 (48%) | 23 (31%) | |
| Gleason score | |||
| 6/7 (n = 84) | 28 (50%) | 56 (78%) | 0.001 |
| 8/9/10 (n = 44) | 28 (50%) | 16 (22%) | |
| Gleason grade (ISUP) | |||
| 1/2 (n = 45) | 12 (21%) | 33 (46%) | 0.001 |
| 3/4/5 (n = 83) | 44 (79%) | 39 (54%) | |
| pT stage | |||
| pT2 (n = 105) | 39 (70%) | 66 (92%) | 0.001 |
| pT3 (n = 23) | 17 (30%) | 6 (8%) | |
| Seminal vesicle invasion | |||
| Negative (n = 123) | 51 (91%) | 72 (100%) | 0.003 |
| Positive (n = 5) | 5 (9%) | 0 (0%) | |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Preoperative PSA (ng/mL) | ||||
| ≤20 | 1 (Ref.) | 1 (Ref.) | ||
| >20 | 3.181 (1.348–7.506) | 0.008 | 1.501 (0.6112–3.687) | 0.3755 |
| pT stage | ||||
| pT2 | 1 (Ref.) | 1 (Ref.) | ||
| pT3 | 3.137 (1.717–5.730) | <0.001 | 1.608 (0.855–3.028) | 0.140 |
| Gleason score | ||||
| 6/7 | 1 (Ref.) | 1 (Ref.) | ||
| 8/9/10 | 2.691 (1.533–4.723) | <0.001 | 1.806 (1.006–3.242) | 0.048 |
| HOXB5 | ||||
| Negative | 1 (Ref.) | 1 (Ref.) | ||
| Positive | 7.802 (3.848–15.819) | <0.001 | 6.032 (2.876–12.648) | <0.001 |
| RET Alteration | p-Value a | ||
|---|---|---|---|
| No (n = 66) | Yes (n = 41) | ||
| HOXB5 alteration | |||
| No (n = 56) | 51 (91%) | 5 (9%) | <0.001 |
| Yes (n = 51) | 15 (29%) | 36 (71%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekino, Y.; Pham, Q.T.; Kobatake, K.; Kitano, H.; Ikeda, K.; Goto, K.; Inoue, S.; Hayashi, T.; Shiota, M.; Yasui, W.; et al. HOXB5 Overexpression Is Associated with Neuroendocrine Differentiation and Poor Prognosis in Prostate Cancer. Biomedicines 2021, 9, 893. https://doi.org/10.3390/biomedicines9080893
Sekino Y, Pham QT, Kobatake K, Kitano H, Ikeda K, Goto K, Inoue S, Hayashi T, Shiota M, Yasui W, et al. HOXB5 Overexpression Is Associated with Neuroendocrine Differentiation and Poor Prognosis in Prostate Cancer. Biomedicines. 2021; 9(8):893. https://doi.org/10.3390/biomedicines9080893
Chicago/Turabian StyleSekino, Yohei, Quoc Thang Pham, Kohei Kobatake, Hiroyuki Kitano, Kenichiro Ikeda, Keisuke Goto, Shogo Inoue, Tetsutaro Hayashi, Masaki Shiota, Wataru Yasui, and et al. 2021. "HOXB5 Overexpression Is Associated with Neuroendocrine Differentiation and Poor Prognosis in Prostate Cancer" Biomedicines 9, no. 8: 893. https://doi.org/10.3390/biomedicines9080893
APA StyleSekino, Y., Pham, Q. T., Kobatake, K., Kitano, H., Ikeda, K., Goto, K., Inoue, S., Hayashi, T., Shiota, M., Yasui, W., & Teishima, J. (2021). HOXB5 Overexpression Is Associated with Neuroendocrine Differentiation and Poor Prognosis in Prostate Cancer. Biomedicines, 9(8), 893. https://doi.org/10.3390/biomedicines9080893








