Inhibition of Hsp90 Counteracts the Established Experimental Dermal Fibrosis Induced by Bleomycin
Abstract
1. Introduction
2. Methods
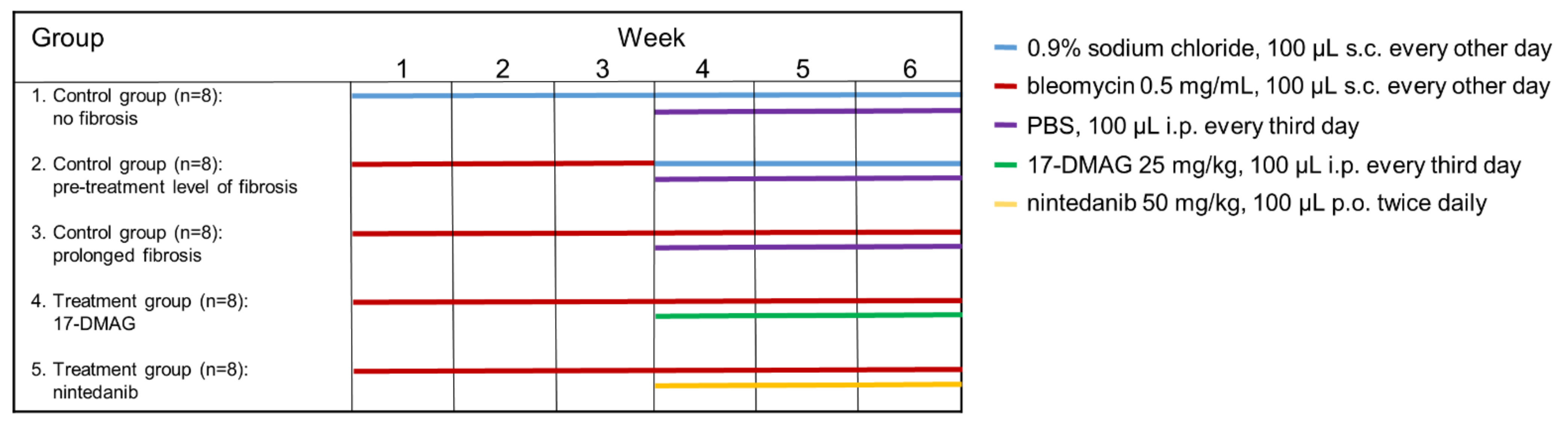
2.1. Treating Established Bleomycin-Induced Dermal Fibrosis
- (1)
- The first control group was administered subcutaneous injections of 100 μL 0.9% NaCl every other day for six weeks, and served as a control for treatment with bleomycin (n = 8).
- (2)
- The second control group of mice (n = 8) was subcutaneously injected with bleomycin for the first three weeks and with 0.9% NaCl for the last three weeks. The level of achieved dermal fibrosis in this group after the first three weeks of subcutaneous bleomycin injections represents the pretreatment level of established dermal fibrosis.
- (3)
- Dermal fibrosis was induced by subcutaneous injections of bleomycin (Bleomedac, Medac GmbH, Wedel, Germany) dissolved in 0.9% sodium chloride (NaCl, B. Braun Medical s.r.o., Prague, Czech Republic) at a concentration of 0.5 mg/mL [38,39]. One hundred microliters of bleomycin was administered into the defined area of 1 cm2 at the upper back every other day for six weeks (n = 8 mice) [38]. Vehicle treatment in groups 1–3 was performed with Dulbecco’s Phosphate Buffered Saline (PBS, Lonza, Walkersville, MD, USA), 100 μL intraperitoneally, every third day in the last three weeks of the six-week experiment.
- (4)
- The main treatment group was challenged with bleomycin for six weeks as described above. In the last three weeks of this six-week period, mice (n = 8) were treated intraperitoneally every third day with 100 μL of 17-DMAG (InvivoGen, San Diego, CA, USA) at a concentration of 25 mg/kg (5 mg/mL in PBS, Lonza) [26]. The dose of 17-DMAG used in this study had previously been shown to effectively inhibit Hsp90 in vivo [40], and to be well-tolerated in a chronic dose regimen of up to 180 days in vivo [41].
- (5)
- For the control treatment, we chose a small-molecule competitive inhibitor of nonreceptor tyrosine kinases (nRTKs), nintedanib, as an established antifibrotic agent (kindly provided by Boehringer Ingelheim Pharma GmbH & Co.KG, Ingelheim am Rhein, Germany). These mice (n = 8) were challenged with bleomycin for six weeks as described above, and in the last three weeks of this six-week period, nintedanib 50 mg/kg (100 μL diluted in deionized water) was administered twice daily perorally [28,42].
2.2. Histological Analysis of Dermal Thickness
2.3. Assessment of the Number of Infiltrating Leukocytes
2.4. Hydroxyproline Assay
2.5. Immunohistochemistry Staining for Aplha-Smooth Muscle Actin (aSMA)
2.6. Immunofluorescence Staining
2.7. Measurement of Inflammatory Cytokines/Chemokines in the Serum
2.8. Safety of 17-DMAG in Mice
2.9. Statistical Analysis
3. Results
3.1. Treatment with 17-DMAG Prevents Progression and May Induce Regression of Preestablished Bleomycin-Induced Skin Fibrosis
3.2. 17-DMAG Reduces the Activation of TGF-β Smad Signaling in Bleomycin-Induced Dermal Fibrosis
3.3. 17-DMAG Treatment Reduces Local and Systemic Inflammation in Bleomycin-Induced Dermal Fibrosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Hsp90 | heat shock protein 90 |
| SSc | systemic sclerosis |
| 17-DMAG | 17-dimethylaminoethylamino-17-demethoxy-geldanamycin |
| pSmad3 | phosphorylated small mothers against decapentaplegic homolog 3 |
| ECM | extracellular matrix |
| TGF-β | transforming growth factor-beta |
| aSMA | alpha-smooth muscle actin |
| TβRI | transforming growth factor-beta type I |
| TβRII | transforming growth factor-beta type II |
| PDGF | platelet-derived growth factor |
| IL | interleukin |
| MCP-1 | monocyte chemoattractant protein 1 |
| CCL | CC chemokine |
| Hsp | heat shock proteins |
| TNF | tumor necrosis factor |
| Src | proto-oncogene tyrosine-protein kinase Src |
| C57BL/6 | laboratory strain of mice referred to as black 6 |
| NaCl | sodium chloride |
| PBS | phosphate buffered saline |
| nRTKs | nonreceptor tyrosine kinases |
| Lck | lymphocyte-specific protein tyrosine kinase |
| Lyn | tyrosine-protein kinase Lyn |
| RTKs | receptor tyrosine kinases |
| FGF | fibroblast growth factor |
| VEGF | vascular endothelial growth factor |
| FLT-3 | fms-like tyrosine kinase 3 |
| FDA | Food and Drug Administration |
| ILD | interstitial lung disease |
| AZV | Czech Health Research Council |
| MSMT | Ministry of Education, Youth and Sports of the Czech Republic |
| 3Rs | Replacement of animals by alternatives wherever possible, Reduction in number of animals used, and Refinement of experimental conditions and procedures to minimize the harm to animals |
| HCl | hydrogen chloride |
| pH | potential of hydrogen |
| NaOH | sodium hydroxide |
| H2O2 | hydrogen peroxide |
| HRP | horseradish peroxidase |
| DAPI | 4’,6-diamidino-2-phenylindole |
| G-CSF | granulocyte colony-stimulating factor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| IFN-γ | interferon-γ |
| KC | keratinocytes-derived chemokine |
| CXCL1 | chemokine C-X-C motif ligand 1 |
| MIP-1α | macrophage inflammatory protein-1α, also referred to as CCL3 |
| MIP-1β | macrophage inflammatory protein-1β, also referred to as CCL4 |
| RANTES | regulated on activation/normal T cell expressed and secreted, also referred to as CCL5 |
| SEM | standard error of the mean |
| P | p-value |
| 17-AAG | 17-N-allylamino-17-demethoxy-geldanamycin |
| PBMCs | peripheral blood mononuclear cells |
| BAL | bronchoalveolar lavage, w: week |
| BLM | bleomycin, NINT: nintedanib |
| N | number of mice |
| s.c. | subcutaneous |
| i.p. | intraperitoneal |
| p.o. | peroral |
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Stern, E.P.; Denton, C.P. The pathogenesis of systemic sclerosis. Rheum. Dis. Clin. N. Am. 2015, 41, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.W.; Gyorfi, A.H.; Ramanujam, M.; Whitfield, M.L.; Konigshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Bond, J.E.; Ho, T.Q.; Selim, M.A.; Hunter, C.L.; Bowers, E.V.; Levinson, H. Temporal spatial expression and function of non-muscle myosin ii isoforms iia and iib in scar remodeling. Lab. Investig. 2011, 91, 499–508. [Google Scholar] [CrossRef]
- Van Caam, A.; Vonk, M.; van den Hoogen, F.; van Lent, P.; van der Kraan, P. Unraveling ssc pathophysiology; the myofibroblast. Front. Immunol. 2018, 9, 2452. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Raja, J.; Denton, C.P. Cytokines in the immunopathology of systemic sclerosis. Semin. Immunopathol. 2015, 37, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.; Feghali-Bostwick, C.; Soare, A.; Asano, Y.; Distler, O.; Abraham, D.J. Review: Frontiers of antifibrotic therapy in systemic sclerosis. Arthritis Rheumatol. 2017, 69, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Poudel, D.R.; Derk, C.T. Mortality and survival in systemic sclerosis: A review of recent literature. Curr. Opin. Rheumatol. 2018, 30, 588–593. [Google Scholar] [CrossRef]
- Schlesinger, M.J. Heat shock proteins. J. Biol. Chem. 1990, 265, 12111–12114. [Google Scholar] [CrossRef]
- Santoro, M.G. Heat shock factors and the control of the stress response. Biochem. Pharmacol. 2000, 59, 55–63. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Biebl, M.M.; Buchner, J. Structure, function, and regulation of the hsp90 machinery. Cold Spring Harb. Perspect. Biol. 2019, 11, a034017. [Google Scholar] [CrossRef]
- Burrows, F.; Zhang, H.; Kamal, A. Hsp90 activation and cell cycle regulation. Cell Cycle 2004, 3, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, P.C.; Picard, D. Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim. Biophys. Acta 2010, 1803, 641–649. [Google Scholar] [CrossRef]
- Jackson, S.E. Hsp90: Structure and function. Top. Curr. Chem. 2013, 328, 155–240. [Google Scholar]
- Li, J.; Buchner, J. Structure, function and regulation of the hsp90 machinery. Biomed. J. 2013, 36, 106–117. [Google Scholar] [PubMed]
- Mahalingam, D.; Swords, R.; Carew, J.S.; Nawrocki, S.T.; Bhalla, K.; Giles, F.J. Targeting hsp90 for cancer therapy. Br. J. Cancer 2009, 100, 1523–1529. [Google Scholar] [CrossRef]
- Wong, D.S.; Jay, D.G. Emerging roles of extracellular hsp90 in cancer. Adv. Cancer Res. 2016, 129, 141–163. [Google Scholar] [PubMed]
- Geller, R.; Taguwa, S.; Frydman, J. Broad action of hsp90 as a host chaperone required for viral replication. Biochim. Biophys. Acta 2012, 1823, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.K.; Kalia, L.V.; McLean, P.J. Molecular chaperones as rational drug targets for parkinson’s disease therapeutics. CNS Neurol. Disord. Drug Targets 2010, 9, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, A.D.; Moses, M.A.; Neckers, L. Heat shock protein 90: Its inhibition and function. Philos. Trans. R Soc. Lond. B Biol. Sci. 2018, 373, 20160527. [Google Scholar] [CrossRef] [PubMed]
- Koga, F.; Xu, W.; Karpova, T.S.; McNally, J.G.; Baron, R.; Neckers, L. Hsp90 inhibition transiently activates src kinase and promotes src-dependent akt and erk activation. Proc. Natl. Acad. Sci. USA 2006, 103, 11318–11322. [Google Scholar] [CrossRef] [PubMed]
- Skhirtladze, C.; Distler, O.; Dees, C.; Akhmetshina, A.; Busch, N.; Venalis, P.; Zwerina, J.; Spriewald, B.; Pileckyte, M.; Schett, G.; et al. Src kinases in systemic sclerosis: Central roles in fibroblast activation and in skin fibrosis. Arthritis Rheum. 2008, 58, 1475–1484. [Google Scholar] [CrossRef]
- Wrighton, K.H.; Lin, X.; Feng, X.H. Critical regulation of tgfbeta signaling by hsp90. Proc. Natl. Acad. Sci. USA 2008, 105, 9244–9249. [Google Scholar] [CrossRef]
- Tomcik, M.; Zerr, P.; Pitkowski, J.; Palumbo-Zerr, K.; Avouac, J.; Distler, O.; Becvar, R.; Senolt, L.; Schett, G.; Distler, J.H. Heat shock protein 90 (hsp90) inhibition targets canonical tgf-beta signalling to prevent fibrosis. Ann. Rheum. Dis. 2014, 73, 1215–1222. [Google Scholar] [CrossRef]
- Akhmetshina, A.; Venalis, P.; Dees, C.; Busch, N.; Zwerina, J.; Schett, G.; Distler, O.; Distler, J.H. Treatment with imatinib prevents fibrosis in different preclinical models of systemic sclerosis and induces regression of established fibrosis. Arthritis Rheum. 2009, 60, 219–224. [Google Scholar] [CrossRef]
- Huang, J.; Beyer, C.; Palumbo-Zerr, K.; Zhang, Y.; Ramming, A.; Distler, A.; Gelse, K.; Distler, O.; Schett, G.; Wollin, L.; et al. Nintedanib inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. Ann. Rheum. Dis. 2016, 75, 883–890. [Google Scholar] [CrossRef]
- King, J.; Abraham, D.; Stratton, R. Chemokines in systemic sclerosis. Immunol. Lett. 2018, 195, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, Y.N.; Wang, H.Z.; Fang, S.L.; Zhang, M.; Zhao, Q.; Liu, J. Inhibition of heat shock protein 90 as a novel platform for the treatment of cancer. Curr. Pharm. Des. 2019, 25, 849–855. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; You, Q.D.; Xu, X.L. Heat shock protein 90 inhibitors: An update on achievements, challenges, and future directions. J. Med. Chem. 2020, 63, 1798–1822. [Google Scholar] [CrossRef]
- Mellatyar, H.; Talaei, S.; Pilehvar-Soltanahmadi, Y.; Barzegar, A.; Akbarzadeh, A.; Shahabi, A.; Barekati-Mowahed, M.; Zarghami, N. Targeted cancer therapy through 17-dmag as an hsp90 inhibitor: Overview and current state of the art. Biomed. Pharmacother. 2018, 102, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Carter, T.R.; Cohen, M.S.; Blagg, B.S.J. Old and new approaches to target the hsp90 chaperone. Curr. Cancer Drug Targets 2020, 20, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Schett, G.; Distler, O.; Distler, J.H. Animal models of systemic sclerosis: Prospects and limitations. Arthritis Rheum. 2010, 62, 2831–2844. [Google Scholar] [CrossRef]
- Beyer, C.; Reich, N.; Schindler, S.C.; Akhmetshina, A.; Dees, C.; Tomcik, M.; Hirth-Dietrich, C.; von Degenfeld, G.; Sandner, P.; Distler, O.; et al. Stimulation of soluble guanylate cyclase reduces experimental dermal fibrosis. Ann. Rheum. Dis. 2012, 71, 1019–1026. [Google Scholar] [CrossRef]
- Dees, C.; Zerr, P.; Tomcik, M.; Beyer, C.; Horn, A.; Akhmetshina, A.; Palumbo, K.; Reich, N.; Zwerina, J.; Sticherling, M.; et al. Inhibition of notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum. 2011, 63, 1396–1404. [Google Scholar] [CrossRef]
- Dees, C.; Akhmetshina, A.; Zerr, P.; Reich, N.; Palumbo, K.; Horn, A.; Jungel, A.; Beyer, C.; Kronke, G.; Zwerina, J.; et al. Platelet-derived serotonin links vascular disease and tissue fibrosis. J. Exp. Med. 2011, 208, 961–972. [Google Scholar] [CrossRef]
- Distler, J.H.; Jungel, A.; Huber, L.C.; Schulze-Horsel, U.; Zwerina, J.; Gay, R.E.; Michel, B.A.; Hauser, T.; Schett, G.; Gay, S.; et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007, 56, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Takagawa, S.; Katayama, I.; Yamazaki, K.; Hamazaki, Y.; Shinkai, H.; Nishioka, K. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J. Investig. Dermatol. 1999, 112, 456–462. [Google Scholar] [CrossRef]
- Lang, S.A.; Klein, D.; Moser, C.; Gaumann, A.; Glockzin, G.; Dahlke, M.H.; Dietmaier, W.; Bolder, U.; Schlitt, H.J.; Geissler, E.K.; et al. Inhibition of heat shock protein 90 impairs epidermal growth factor-mediated signaling in gastric cancer cells and reduces tumor growth and vascularization in vivo. Mol. Cancer Ther. 2007, 6, 1123–1132. [Google Scholar] [CrossRef]
- Hertlein, E.; Wagner, A.J.; Jones, J.; Lin, T.S.; Maddocks, K.J.; Towns, W.H., 3rd; Goettl, V.M.; Zhang, X.; Jarjoura, D.; Raymond, C.A.; et al. 17-dmag targets the nuclear factor-kappab family of proteins to induce apoptosis in chronic lymphocytic leukemia: Clinical implications of hsp90 inhibition. Blood 2010, 116, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Maier, C.; Zhang, Y.; Soare, A.; Dees, C.; Beyer, C.; Harre, U.; Chen, C.W.; Distler, O.; Schett, G.; et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the fra2 mouse model of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1941–1948. [Google Scholar] [CrossRef]
- Avouac, J.; Furnrohr, B.G.; Tomcik, M.; Palumbo, K.; Zerr, P.; Horn, A.; Dees, C.; Akhmetshina, A.; Beyer, C.; Distler, O.; et al. Inactivation of the transcription factor stat-4 prevents inflammation-driven fibrosis in animal models of systemic sclerosis. Arthritis Rheum. 2011, 63, 800–809. [Google Scholar] [CrossRef]
- Woessner, J.F., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef]
- Tomcik, M.; Palumbo-Zerr, K.; Zerr, P.; Sumova, B.; Avouac, J.; Dees, C.; Distler, A.; Becvar, R.; Distler, O.; Schett, G.; et al. Tribbles homologue 3 stimulates canonical tgf-beta signalling to regulate fibroblast activation and tissue fibrosis. Ann. Rheum. Dis 2016, 75, 609–616. [Google Scholar] [CrossRef]
- Zheng, Z.; Nguyen, C.; Zhang, X.; Khorasani, H.; Wang, J.Z.; Zara, J.N.; Chu, F.; Yin, W.; Pang, S.; Le, A.; et al. Delayed wound closure in fibromodulin-deficient mice is associated with increased tgf-beta3 signaling. J. Investig. Dermatol. 2011, 131, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Kropackova, T.; Vernerova, L.; Storkanova, H.; Horvathova, V.; Vokurkova, M.; Klein, M.; Oreska, S.; Spiritovic, M.; Hermankova, B.; Kubinova, K.; et al. Clusterin is upregulated in serum and muscle tissue in idiopathic inflammatory myopathies and associates with clinical disease activity and cytokine profile. Clin. Exp. Rheumatol. 2020. Epub ahead of print. [Google Scholar]
- Noh, H.; Kim, H.J.; Yu, M.R.; Kim, W.Y.; Kim, J.; Ryu, J.H.; Kwon, S.H.; Jeon, J.S.; Han, D.C.; Ziyadeh, F. Heat shock protein 90 inhibitor attenuates renal fibrosis through degradation of transforming growth factor-beta type ii receptor. Lab. Investig. 2012, 92, 1583–1596. [Google Scholar] [CrossRef]
- Prescott, R.J.; Freemont, A.J.; Jones, C.J.; Hoyland, J.; Fielding, P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J. Pathol. 1992, 166, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, A.; Iwata, Y.; Komura, K.; Ogawa, F.; Hara, T.; Muroi, E.; Takenaka, M.; Shimizu, K.; Hasegawa, M.; Fujimoto, M.; et al. Cd19 regulates skin and lung fibrosis via toll-like receptor signaling in a model of bleomycin-induced scleroderma. Am. J. Pathol. 2008, 172, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Sontake, V.; Wang, Y.; Kasam, R.K.; Sinner, D.; Reddy, G.B.; Naren, A.P.; McCormack, F.X.; White, E.S.; Jegga, A.G.; Madala, S.K. Hsp90 regulation of fibroblast activation in pulmonary fibrosis. JCI Insight 2017, 2, e91454. [Google Scholar] [CrossRef]
- Dong, H.; Luo, L.; Zou, M.; Huang, C.; Wan, X.; Hu, Y.; Le, Y.; Zhao, H.; Li, W.; Zou, F.; et al. Blockade of extracellular heat shock protein 90alpha by 1g6-d7 attenuates pulmonary fibrosis through inhibiting erk signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L1006–L1015. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, H.; Liang, L.; Bi, Z.; Wang, Y.; Gao, S.; Wang, M.; Li, H.; Miao, Y.; Deng, R.; et al. Myricetin ameliorates bleomycin-induced pulmonary fibrosis in mice by inhibiting tgf-beta signaling via targeting hsp90beta. Biochem. Pharmacol. 2020, 178, 114097. [Google Scholar] [CrossRef]
- Marinova, M.; Solopov, P.; Dimitropoulou, C.; Colunga Biancatelli, R.M.L.; Catravas, J.D. Post-treatment with a heat shock protein 90 inhibitor prevents chronic lung injury and pulmonary fibrosis, following acute exposure of mice to hcl. Exp. Lung Res. 2020, 46, 203–216. [Google Scholar] [CrossRef]
- Sibinska, Z.; Tian, X.; Korfei, M.; Kojonazarov, B.; Kolb, J.S.; Klepetko, W.; Kosanovic, D.; Wygrecka, M.; Ghofrani, H.A.; Weissmann, N.; et al. Amplified canonical transforming growth factor-beta signalling via heat shock protein 90 in pulmonary fibrosis. Eur. Respir J. 2017, 49, 1501941. [Google Scholar] [CrossRef]
- Solopov, P.; Biancatelli, R.; Marinova, M.; Dimitropoulou, C.; Catravas, J.D. The hsp90 inhibitor, auy-922, ameliorates the development of nitrogen mustard-induced pulmonary fibrosis and lung dysfunction in mice. Int. J. Mol. Sci. 2020, 21, 4740. [Google Scholar] [CrossRef]
- Caceres, R.A.; Chavez, T.; Maestro, D.; Palanca, A.R.; Bolado, P.; Madrazo, F.; Aires, A.; Cortajarena, A.L.; Villar, A.V. Reduction of cardiac tgfbeta-mediated profibrotic events by inhibition of hsp90 with engineered protein. J. Mol. Cell Cardiol. 2018, 123, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Wollin, L.; Wex, E.; Pautsch, A.; Schnapp, G.; Hostettler, K.E.; Stowasser, S.; Kolb, M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir J. 2015, 45, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef]
- Yamamoto, T. The bleomycin-induced scleroderma model: What have we learned for scleroderma pathogenesis? Arch. Dermatol. Res. 2006, 297, 333–344. [Google Scholar] [CrossRef]
- Dello Russo, C.; Polak, P.E.; Mercado, P.R.; Spagnolo, A.; Sharp, A.; Murphy, P.; Kamal, A.; Burrows, F.J.; Fritz, L.C.; Feinstein, D.L. The heat-shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin suppresses glial inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J. Neurochem. 2006, 99, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.W.; Veal, J.M.; Fadden, R.P.; Barabasz, A.F.; Partridge, J.M.; Barta, T.E.; Dubois, L.G.; Huang, K.H.; Mabbett, S.R.; Silinski, M.A.; et al. Small molecule inhibitors of hsp90 potently affect inflammatory disease pathways and exhibit activity in models of rheumatoid arthritis. Arthritis Rheum. 2008, 58, 3765–3775. [Google Scholar] [CrossRef]
- Han, J.M.; Kwon, N.H.; Lee, J.Y.; Jeong, S.J.; Jung, H.J.; Kim, H.R.; Li, Z.; Kim, S. Identification of gp96 as a novel target for treatment of autoimmune disease in mice. PLoS ONE 2010, 5, e9792. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Muller, R.; Manz, R.; Magens, M.; Hammers, C.M.; Somlai, C.; Westermann, J.; Schmidt, E.; Zillikens, D.; Ludwig, R.J.; et al. Heat-shock protein 90 inhibition in autoimmunity to type vii collagen: Evidence that nonmalignant plasma cells are not therapeutic targets. Blood 2011, 117, 6135–6142. [Google Scholar] [CrossRef] [PubMed]
- Storkanova, H.; Oreska, S.; Spiritovic, M.; Hermankova, B.; Bubova, K.; Komarc, M.; Pavelka, K.; Vencovsky, J.; Dislter, J.; Senolt, L.; et al. Plasma hsp90 levels in patients with systemic sclerosis and relation to lung and skin involvement: A cross-sectional and longitudinal study. Sci. Rep. 2020, 11, 1. [Google Scholar] [CrossRef]
- Degryse, A.L.; Lawson, W.E. Progress toward improving animal models for idiopathic pulmonary fibrosis. Am. J. Med. Sci. 2011, 341, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Hara, M.; Wright, T.M. Endogenous il-1alpha from systemic sclerosis fibroblasts induces il-6 and pdgf-a. J. Clin. Investig. 1999, 103, 1253–1260. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; McCarthy, S.A.; Watkins, S.C.; Wright, T.M. Autocrine activation by interleukin 1alpha induces the fibrogenic phenotype of systemic sclerosis fibroblasts. J. Rheumatol. 2004, 31, 1946–1954. [Google Scholar]
- Khanna, D.; Denton, C.P.; Jahreis, A.; van Laar, J.M.; Frech, T.M.; Anderson, M.E.; Baron, M.; Chung, L.; Fierlbeck, G.; Lakshminarayanan, S.; et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (fasscinate): A phase 2, randomised, controlled trial. Lancet 2016, 387, 2630–2640. [Google Scholar] [CrossRef]
- Khanna, D.; Denton, C.P.; Lin, C.J.F.; van Laar, J.M.; Frech, T.M.; Anderson, M.E.; Baron, M.; Chung, L.; Fierlbeck, G.; Lakshminarayanan, S.; et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: Results from the open-label period of a phase ii randomised controlled trial (fasscinate). Ann. Rheum. Dis. 2018, 77, 212–220. [Google Scholar] [CrossRef]
- Furuse, S.; Fujii, H.; Kaburagi, Y.; Fujimoto, M.; Hasegawa, M.; Takehara, K.; Sato, S. Serum concentrations of the cxc chemokines interleukin 8 and growth-regulated oncogene-alpha are elevated in patients with systemic sclerosis. J. Rheumatol. 2003, 30, 1524–1528. [Google Scholar] [PubMed]
- Tukaj, S.; Gruner, D.; Zillikens, D.; Kasperkiewicz, M. Hsp90 blockade modulates bullous pemphigoid igg-induced il-8 production by keratinocytes. Cell Stress Chaperones 2014, 19, 887–894. [Google Scholar] [CrossRef]
- Chung, S.W.; Lee, J.H.; Choi, K.H.; Park, Y.C.; Eo, S.K.; Rhim, B.Y.; Kim, K. Extracellular heat shock protein 90 induces interleukin-8 in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2009, 378, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Park, H.K.; Lee, K.M.; Lee, K.J.; Kim, J.H.; Cho, S.W.; Hahm, K.B. Blockage of hsp 90 modulates helicobacter pylori-induced il-8 productions through the inactivation of transcriptional factors of ap-1 and nf-kappab. Biochem. Biophys. Res. Commun. 2004, 320, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, S.; Amoreo, C.A.; Nuvoli, B.; Galati, R.; Strano, S.; Facciolo, F.; Alessandrini, G.; Pass, H.I.; Ciliberto, G.; Blandino, G.; et al. Hsp90 inhibition alters the chemotherapy-driven rearrangement of the oncogenic secretome. Oncogene 2018, 37, 1369–1385. [Google Scholar] [CrossRef]
- Hartman, M.L.; Rogut, M.; Mielczarek-Lewandowska, A.; Wozniak, M.; Czyz, M. 17-aminogeldanamycin inhibits constitutive nuclear factor-kappa b (nf-kappab) activity in patient-derived melanoma cell lines. Int. J. Mol. Sci. 2020, 21, 3749. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štorkánová, H.; Štorkánová, L.; Navrátilová, A.; Bečvář, V.; Hulejová, H.; Oreská, S.; Heřmánková, B.; Špiritović, M.; Bečvář, R.; Pavelka, K.; et al. Inhibition of Hsp90 Counteracts the Established Experimental Dermal Fibrosis Induced by Bleomycin. Biomedicines 2021, 9, 650. https://doi.org/10.3390/biomedicines9060650
Štorkánová H, Štorkánová L, Navrátilová A, Bečvář V, Hulejová H, Oreská S, Heřmánková B, Špiritović M, Bečvář R, Pavelka K, et al. Inhibition of Hsp90 Counteracts the Established Experimental Dermal Fibrosis Induced by Bleomycin. Biomedicines. 2021; 9(6):650. https://doi.org/10.3390/biomedicines9060650
Chicago/Turabian StyleŠtorkánová, Hana, Lenka Štorkánová, Adéla Navrátilová, Viktor Bečvář, Hana Hulejová, Sabína Oreská, Barbora Heřmánková, Maja Špiritović, Radim Bečvář, Karel Pavelka, and et al. 2021. "Inhibition of Hsp90 Counteracts the Established Experimental Dermal Fibrosis Induced by Bleomycin" Biomedicines 9, no. 6: 650. https://doi.org/10.3390/biomedicines9060650
APA StyleŠtorkánová, H., Štorkánová, L., Navrátilová, A., Bečvář, V., Hulejová, H., Oreská, S., Heřmánková, B., Špiritović, M., Bečvář, R., Pavelka, K., Vencovský, J., Distler, J. H. W., Šenolt, L., & Tomčík, M. (2021). Inhibition of Hsp90 Counteracts the Established Experimental Dermal Fibrosis Induced by Bleomycin. Biomedicines, 9(6), 650. https://doi.org/10.3390/biomedicines9060650






