Role of VEGF Polymorphisms in the Susceptibility and Severity of Interstitial Lung Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. VEGF Polymorphisms Selection and Genotyping
2.3. Serum VEGF Determination
2.4. Statistical Analysis
3. Results
3.1. Analysis of VEGF Genotype, Allele and Carrier Frequencies
3.2. Haplotype Analysis of VEGF
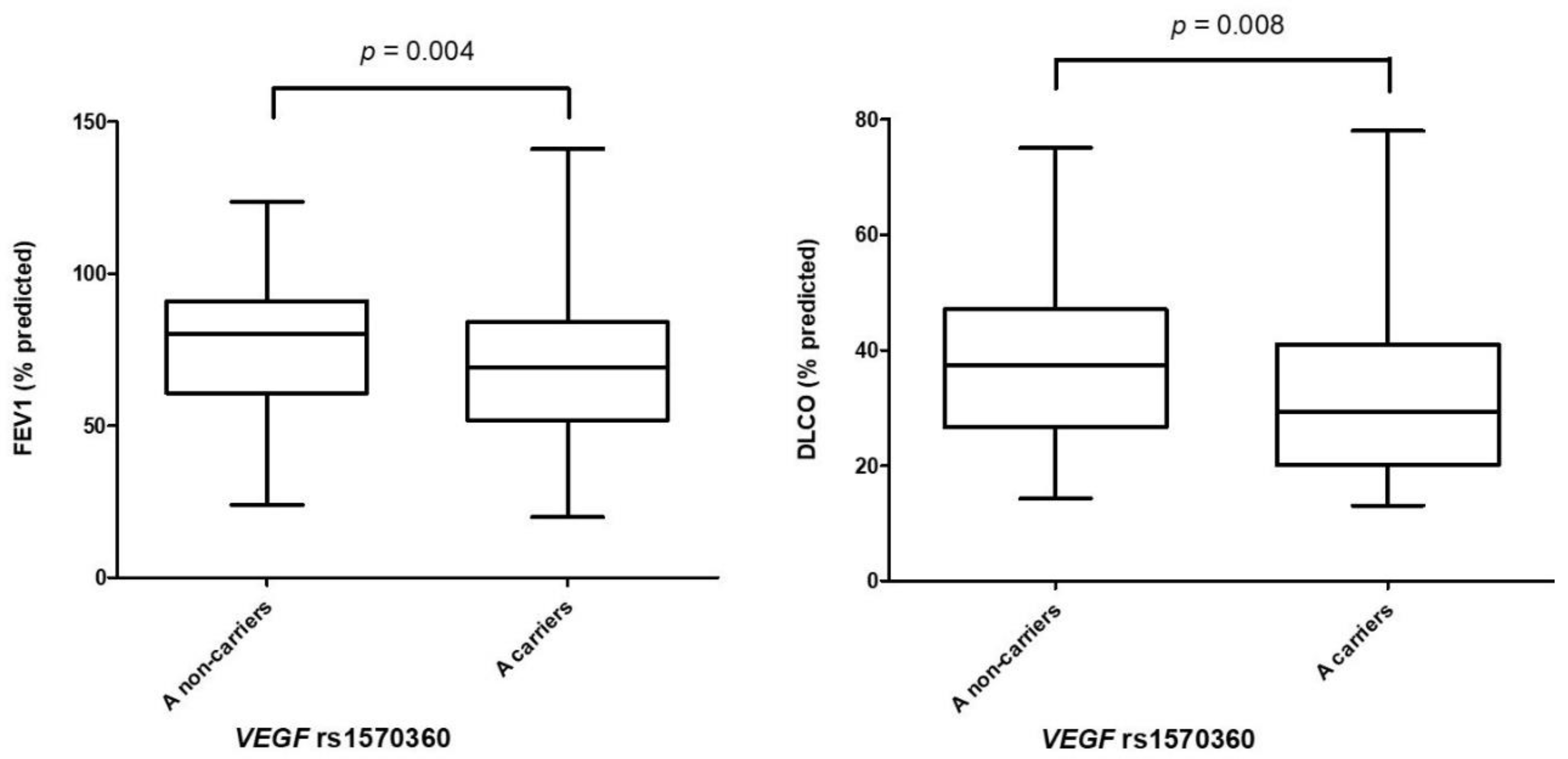
3.3. Association of VEGF Polymorphisms with Pulmonary Function Tests
3.4. Influence of VEGF Polymorphisms on VEGF Serum Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behr, J. Approach to the Diagnosis of Interstitial Lung Disease. Clin. Chest Med. 2012, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Hirani, N.A.; Hotchkin, D.L.; Nambiar, A.M.; Ogura, T.; Otaola, M.; Skowasch, D.; Park, J.S.; Poonyagariyagorn, H.K.; Wuyts, W.; et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur. Respir. Rev. 2018, 27, 180076. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Borie, R.; Le Guen, P.; Ghanem, M.; Taillé, C.; Dupin, C.; Dieudé, P.; Kannengiesser, C.; Crestani, B. The genetics of interstitial lung diseases. Eur. Respir. Rev. 2019, 28, 190053. [Google Scholar] [CrossRef] [PubMed]
- Atienza-Mateo, B.; Remuzgo-Martínez, S.; Cuesta, V.M.M.; Iturbe-Fernández, D.; Fernández-Rozas, S.; Prieto-Peña, D.; Calderón-Goercke, M.; Corrales, A.; Rodríguez, G.B.B.; Gómez-Román, J.J.; et al. The Spectrum of Interstitial Lung Disease Associated with Autoimmune Diseases: Data of a 3.6-Year Prospective Study from a Referral Center of Interstitial Lung Disease and Lung Transplantation †. J. Clin. Med. 2020, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Moermans, C.; Henket, M.; Corhay, J.-L.; Louis, R. Blood Biomarkers in Idiopathic Pulmonary Fibrosis. Lung 2017, 195, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Drakopanagiotakis, F.; Wujak, L.; Wygrecka, M.; Markart, P. Biomarkers in idiopathic pulmonary fibrosis. Matrix Biol. 2018, 68–69, 404–421. [Google Scholar] [CrossRef]
- Prasse, A.; Müller-Quernheim, J. Non-invasive biomarkers in pulmonary fibrosis. Respirol. 2009, 14, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Byrne, A.M.; Bouchier-Hayes, D.; Harmey, J. Angiogenic and cell survival functions of Vascular Endothelial Growth Factor (VEGF). J. Cell. Mol. Med. 2005, 9, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, S.A.J.; Kim, J.; Ganapathi, M.K.; Ganapathi, R. Vascular Endothelial Growth Factor Polymorphisms: Role in Response and Toxicity of Tyrosine Kinase Inhibitors. Curr. Oncol. Rep. 2010, 12, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Pierik, M.; Henckaerts, L.; Joossens, M.; Claes, K.; Van Schuerbeek, N.; Vlietinck, R.; Rutgeerts, P.; Van Assche, G.; Vermeire, S. The role of vascular endothelial growth factor (VEGF) in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 870–878. [Google Scholar] [CrossRef]
- Carvalho, J.F.; Blank, M.; Shoenfeld, Y. Vascular Endothelial Growth Factor (VEGF) in Autoimmune Diseases. J. Clin. Immunol. 2007, 27, 246–256. [Google Scholar] [CrossRef]
- Rueda, B.; Perez-Armengol, C.; Lopez-Lopez, S.; Garcia-Porrua, C.; Martín, J.; A Gonzalez-Gay, M. Association between functional haplotypes of vascular endothelial growth factor and renal complications in Henoch-Schönlein purpura. J. Rheumatol. 2006, 33, 69–73. [Google Scholar]
- Salvarani, C.; Boiardi, L.; Casali, B.; Olivieri, I.; Cantini, F.; Salvi, F.; Malatesta, R.; La Corte, R.; Triolo, G.; Ferrante, A.; et al. Vascular endothelial growth factor gene polymorphisms in Behçet’s disease. J. Rheumatol. 2004, 31, 1785–1789. [Google Scholar]
- Laddha, A.P.; Kulkarni, Y.A. VEGF and FGF-2: Promising targets for the treatment of respiratory disorders. Respir. Med. 2019, 156, 33–46. [Google Scholar] [CrossRef]
- Barratt, S.L.; Flower, V.A.; Pauling, J.D.; Millar, A.B. VEGF (Vascular Endothelial Growth Factor) and Fibrotic Lung Disease. Int. J. Mol. Sci. 2018, 19, 1269. [Google Scholar] [CrossRef]
- Kaner, R.J.; Crystal, R.G. Compartmentalization of Vascular Endothelial Growth Factor to the Epithelial Surface of the Human Lung. Mol. Med. 2001, 7, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, N.F.; Vandivier, R.W.; Tuder, R.M. Vascular endothelial growth factor in the lung. Am. J. Physiol. Cell. Mol. Physiol. 2006, 290, L209–L221. [Google Scholar] [CrossRef]
- Hanumegowda, C.; Farkas, L.; Kolb, M. Angiogenesis in Pulmonary Fibrosis. Chest 2012, 142, 200–207. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zeng, Q.; Zhang, P.; Li, G.; Xie, Q.; Cheng, Y. VEGFPromoter Polymorphism Confers an Increased Risk of Pulmonary Arterial Hypertension in a Chinese Population. Yonsei Med. J. 2017, 58, 305–311. [Google Scholar] [CrossRef]
- Baz-Dávila, R.; Espinoza-Jimenez, A.; Rodríguez-Pérez, M.D.C.; Zulueta, J.; Varo, N.; Montejo, Á.; Almeida-González, D.; Aguirre-Jaime, A.; Cordoba-Lanus, E.; Casanova, C. Role of HIF1A, VEGFA and VEGFR2 SNPs in the Susceptibility and Progression of COPD in a Spanish Population. PLoS ONE 2016, 11, e0154998. [Google Scholar] [CrossRef]
- Zhai, R.; Gong, M.N.; Zhou, W.; Thompson, T.B.; Kraft, P.; Su, L.; Christiani, D.C. Genotypes and haplotypes of the VEGF gene are associated with higher mortality and lower VEGF plasma levels in patients with ARDS. Thorax 2007, 62, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qin, Z.; Shao, C.; Liu, W.; Ma, L.; Shu, Y.; Shen, H. Association between VEGF Gene Polymorphisms and the Susceptibility to Lung Cancer: An Updated Meta-Analysis. BioMed Res. Int. 2018, 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.; Custovic, A.; Tepper, R.; Graves, P.; Stern, D.A.; Jones, M.; Hankinson, J.; Curtin, J.A.; Wu, J.; Blekic, M.; et al. Genetic Variation in Vascular Endothelial Growth Factor-A and Lung Function. Am. J. Respir. Crit. Care Med. 2012, 185, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Vij, R.; Noth, I. Peripheral blood biomarkers in idiopathic pulmonary fibrosis. Transl. Res. 2012, 159, 218–227. [Google Scholar] [CrossRef]
- Ando, M.; Miyazaki, E.; Ito, T.; Hiroshige, S.; Nureki, S.-I.; Ueno, T.; Takenaka, R.; Fukami, T.; Kumamoto, T. Significance of Serum Vascular Endothelial Growth Factor Level in Patients with Idiopathic Pulmonary Fibrosis. Lung 2010, 188, 247–252. [Google Scholar] [CrossRef]
- Grimminger, F.; Günther, A.; Vancheri, C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1426–1433. [Google Scholar] [CrossRef]
- A Mikolasch, T.; Garthwaite, H.S.; Porter, J.C. Update in diagnosis and management of interstitial lung disease. Clin. Med. 2017, 17, 146–153. [Google Scholar] [CrossRef]
- Yu, Z.-G.; Wang, B.-Z.; Cheng, Z.-Z. The association of genetic polymorphisms of hypoxia inducible factor-1 alpha and vascular endothelial growth factor with increased risk of chronic obstructive pulmonary disease: A case—Control study. Kaohsiung J. Med. Sci. 2017, 33, 433–441. [Google Scholar] [CrossRef]
- Almawi, W.Y.; Saldanha, F.L.; Mahmood, N.A.; Al-Zaman, I.; Sater, M.S.; Mustafa, F.E. Relationship between VEGFA polymorphisms and serum VEGF protein levels and recurrent spontaneous miscarriage. Hum. Reprod. 2013, 28, 2628–2635. [Google Scholar] [CrossRef]
- Liu, L.; Dai, H.-P.; Xiao, B.; Zhang, S.; Ban, C.-J.; Xin, P. Association of ENA-78, IP-10 and VEGF gene polymorphism with idiopathic pulmonary fibrosis. Zhonghua Yi Xue Za Zhi 2009, 89, 2690–2694. [Google Scholar]
- Rueda, B.; González-Gay, M.A.; López-Nevot, M.A.; García, A.; Fernández-Arquero, M.; Balsa, A.; Pablos, J.L.; Pascual-Salcedo, D.; de la Concha, E.G.; González-Escribano, M.F. Analysis of vascular endothelial growth factor (VEGF) functional variants in rheumatoid arthritis. Hum Immunol. 2005, 66, 864–868. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, L.; Bermudez, M.G.; González-Juanatey, C.; Vazquez-Rodriguez, T.R.; Miranda-Filloy, J.A.; Fernandez-Gutierrez, B.; Llorca, J.; Martin, J.; González-Gay, M.A. Vascular endothelial growth factor A and cardiovascular disease in rheumatoid arthritis patients. Tissue Antigens 2011, 77, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santiago, R.; Sharma, M.; Mueller, J.C.; Gohlke, H.; Illig, T.; Anneser, J.; Munch, C.; Ludolph, A.; Kamm, C.; Gasser, T. Possible gender-dependent association of vascular endothelial growth factor (VEGF) gene and ALS. Neurology 2006, 66, 1929–1931. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Hong, S.H.; Kim, J.W.; Oh, D.; Hwang, S.G.; An, H.J.; Kim, U.-K.; Kim, N.K. Gender-specific association of the VEGF -2578C > A polymorphism in Korean patients with colon cancer. Anticancer. Res. 2007, 27, 2535–2539. [Google Scholar]
- Bae, S.J.; Kim, J.W.; Kang, H.; Hwang, S.G.; Oh, D.; Kim, N.K. Gender-specific association between polymorphism of vascular endothelial growth factor (VEGF 936 C>T) gene and colon cancer in Korea. Anticancer. Res. 2008, 28, 1271–1276. [Google Scholar]
- Bae, S.J.; Ahn, D.H.; Hong, S.P.; Kang, H.; Hwang, S.G.; Oh, D.; Kim, N.K. Gender-specific Association between Polymorphism of Vascular Endothelial Growth Factor (VEGF 936C > T) Gene and Patients with Stomach Cancer. Yonsei Med. J. 2008, 49, 783. [Google Scholar] [CrossRef]
| Characteristic | Whole Cohort of ILD Patients (n = 436) | IIP Patients (n = 244) | Non-IIP Patients (n = 192) |
|---|---|---|---|
| Sex (men/women), n (%) | 294/142 (67.4/32.6) | 190/54 (77.9/22.1) | 104/88 (54.2/45.8) |
| Age at the time of the study (years), mean ± SD | 61.1 ± 10.2 | 62.8 ± 9.4 | 59.0 ± 10.8 |
| Smoking history, n (%) | 294 (69.2) | 178 (74.2) | 116 (62.7) |
| Packs of cigarettes per year, mean ± SD | 30.7 ± 20.2 | 33.8 ± 20.7 | 25.8 ± 18.5 |
| Pulmonary function tests | |||
| FVC (% predicted), mean ± SD | 76.8 ± 24.1 | 73.0 ± 22.7 | 81.7 ± 25.0 |
| FEV1 (% predicted), mean ± SD | 73.7 ± 23.6 | 72.8 ± 22.4 | 74.8 ± 25.0 |
| DLCO (% predicted), mean ± SD | 36.4 ± 15.7 | 35.2 ± 15.0 | 37.9 ± 16.6 |
| VEGF Polymorphism | Genotype/Allele/ Carriers | Whole Cohort of ILD Patients % (n/N) | Healthy Controls % (n/N) | OR [95% CI] | p-Value |
|---|---|---|---|---|---|
| rs833061 | TT | 32.6 (142/435) | 28.8 (151/524) | Reference | - |
| TC | 47.6 (207/435) | 48.7 (255/524) | 0.86 [0.64–1.16] | 0.33 | |
| CC | 19.8 (86/435) | 22.5 (118/524) | 0.78 [0.54–1.11] | 0.17 | |
| T | 56.4 (491/870) | 53.1 (557/1048) | Reference | - | |
| C | 43.6 (379/870) | 46.9 (491/1048) | 0.88 [0.73–1.05] | 0.15 | |
| C non-carriers | 32.6 (142/435) | 28.8 (151/524) | Reference | - | |
| C carriers | 67.4 (293/435) | 71.2 (373/524) | 0.84 [0.63–1.10] | 0.20 | |
| rs1570360 | GG | 53.5 (232/434) | 48.3 (250/518) | Reference | - |
| GA | 38.0 (165/434) | 41.9 (217/518) | 0.82 [0.63–1.07] | 0.15 | |
| AA | 8.5 (37/434) | 9.8 (51/518) | 0.78 [0.49–1.24] | 0.29 | |
| G | 72.5 (629/868) | 69.2 (717/1036) | Reference | - | |
| A | 27.5 (239/868) | 30.8 (319/1036) | 0.85 [0.70–1.04] | 0.12 | |
| A non-carriers | 53.5 (232/434) | 48.3 (250/518) | Reference | - | |
| A carriers | 46.5 (202/434) | 51.7 (268/518) | 0.81 [0.63–1.05] | 0.11 | |
| rs2010963 | GG | 43.0 (187/435) | 43.7 (231/528) | Reference | - |
| GC | 43.2 (188/435) | 46.6 (246/528) | 0.94 [0.72–1.24] | 0.68 | |
| CC | 13.8 (60/435) | 9.7 (51/528) | 1.45 [0.95–2.21] | 0.08 | |
| G | 64.6 (562/870) | 67.0 (708/1056) | Reference | - | |
| C | 35.4 (308/870) | 33.0 (348/1056) | 1.11 [0.92–1.35] | 0.26 | |
| C non-carriers | 43.0 (187/435) | 43.7 (231/528) | Reference | - | |
| C carriers | 57.0 (248/435) | 56.3 (297/528) | 1.03 [0.80–1.33] | 0.81 | |
| rs3025020 | CC | 51.9 (225/434) | 54.4 (285/524) | Reference | - |
| CT | 39.6 (172/434) | 37.0 (194/524) | 1.12 [0.86–1.47] | 0.40 | |
| TT | 8.5 (37/434) | 8.6 (45/524) | 1.04 [0.65–1.66] | 0.87 | |
| C | 71.7 (622/868) | 72.9 (764/1048) | Reference | - | |
| T | 28.3 (246/868) | 27.1 (284/1048) | 1.06 [0.87–1.30] | 0.55 | |
| T non-carriers | 51.8 (225/434) | 54.4 (285/524) | Reference | - | |
| T carriers | 48.2 (209/434) | 45.6 (239/524) | 1.11 [0.86–1.43] | 0.43 | |
| rs3025039 | CC | 74.5 (325/436) | 79.0 (418/529) | Reference | - |
| CT | 22.7 (99/436) | 18.9 (100/529) | 1.27 [0.93–1.73] | 0.13 | |
| TT | 2.8 (12/436) | 2.1 (11/529) | 1.40 [0.61–3.22] | 0.42 | |
| C | 85.9 (749/872) | 88.5 (936/1058) | Reference | - | |
| T | 14.1 (123/872) | 11.5 (122/1058) | 1.26 [0.96–1.65] | 0.09 | |
| T non-carriers | 74.5 (325/436) | 79.0 (418/529) | Reference | - | |
| T carriers | 25.5 (111/436) | 21.0 (111/529) | 1.29 [0.95–1.74] | 0.10 |
| VEGF Polymorphism | Genotype/Allele/ Carriers | IIP Patients % (n/N) | Non-IIP Patients % (n/N) | OR [95% CI] * | p-Value * |
|---|---|---|---|---|---|
| rs833061 | TT | 28.7 (70/244) | 37.7 (72/191) | Reference | - |
| TC | 49.2 (120/244) | 45.6 (87/191) | 1.32 [0.82–2.11] | 0.25 | |
| CC | 22.1 (54/244) | 16.7 (32/191) | 1.73 [0.95–3.16] | 0.07 | |
| T | 53.3 (260/488) | 60.5 (231/382) | Reference | - | |
| C | 46.7 (228/488) | 39.5 (151/382) | 1.33 [0.99–1.79] | 0.06 | |
| C non-carriers | 28.7 (70/244) | 37.7 (72/191) | Reference | - | |
| C carriers | 71.3 (174/244) | 62.3 (119/191) | 1.43 [0.92–2.22] | 0.12 | |
| rs1570360 | GG | 47.5 (116/244) | 61.1 (116/190) | Reference | - |
| GA | 43.4 (106/244) | 31.1 (59/190) | 1.79 [1.14–2.81] | 0.01 | |
| AA | 9.1 (22/244) | 7.8 (15/190) | 1.50 [0.68–3.31] | 0.31 | |
| G | 69.3 (338/488) | 76.6 (291/380) | Reference | - | |
| A | 30.7 (150/488) | 23.4 (89/380) | 1.46 [1.04–2.05] | 0.03 | |
| A non-carriers | 47.5 (116/244) | 61.1 (116/190) | Reference | - | |
| A carriers | 52.5 (128/244) | 38.9 (74/190) | 1.74 [1.14–2.65] | 0.01 | |
| rs2010963 | GG | 44.7 (109/244) | 40.8 (78/191) | Reference | - |
| GC | 42.2 (103/244) | 44.5 (85/191) | 0.80 [0.51–1.25] | 0.32 | |
| CC | 13.1 (32/244) | 14.7 (28/191) | 0.66 [0.34–1.25] | 0.20 | |
| G | 65.8 (321/488) | 63.1 (241/382) | Reference | - | |
| C | 34.2 (167/488) | 36.9 (141/382) | 0.80 [0.59–1.08] | 0.15 | |
| C non-carriers | 44.7 (109/244) | 40.8 (78/191) | Reference | - | |
| C carriers | 55.3 (135/244) | 59.2 (113/191) | 0.76 [0.50–1.16] | 0.20 | |
| rs3025020 | CC | 50.4 (123/244) | 53.7 (102/190) | Reference | - |
| CT | 39.8 (97/244) | 39.5 (75/190) | 1.13 [0.73–1.76] | 0.57 | |
| TT | 9.8 (24/244) | 6.8 (13/190) | 1.57 [0.69–3.54] | 0.28 | |
| C | 70.3 (343/488) | 73.4 (279/380) | Reference | - | |
| T | 29.7 (145/488) | 26.6 (101/380) | 1.20 [0.86–1.67] | 0.28 | |
| T non-carriers | 50.4 (123/244) | 53.7 (102/190) | Reference | - | |
| T carriers | 49.6 (121/244) | 46.3 (88/190) | 1.20 [0.79–1.82] | 0.40 | |
| rs3025039 | CC | 77.5 (189/244) | 70.8 (136/192) | Reference | - |
| CT | 20.5 (50/244) | 25.5 (49/192) | 0.63 [0.38–1.04] | 0.07 | |
| TT | 2.0 (5/244) | 3.7 (7/192) | 1.09 [0.24–5.02] | 0.91 | |
| C | 87.7 (428/488) | 83.6 (321/384) | Reference | - | |
| T | 12.3 (60/488) | 16.4 (63/384) | 0.72 [0.47–1.12] | 0.14 | |
| T non-carriers | 77.5 (189/244) | 70.8 (136/192) | Reference | - | |
| T carriers | 22.5 (55/244) | 29.2 (56/192) | 0.66 [0.41–1.06] | 0.09 |
| Whole Cohort of ILD Patients versus Controls | IIP versus Non-IIP Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Haplotype * | Frequency in ILD % (n/N) | Frequency in Controls % (n/N) | OR [95% CI] | p-Value | Frequency in IIP % (n/N) | Frequency in Non-IIP % (n/N) | OR [95% CI] ‡ | p-Value ‡ |
| TGGCC | 31.4 (271/862) | 32.6 (330/1012) | Reference | - | 30.7 (150/488) | 32.4 (121/374) | Reference | - |
| TGCCC | 10.7 (92/862) | 9.8 (99/1012) | 1.13 [0.82–1.57] | 0.46 | 10.0 (49/488) | 11.5 (43/374) | 0.81 [0.48–1.36] | 0.42 |
| CAGCC | 7.1 (61/862) | 9.3 (94/1012) | 0.79 [0.55–1.13] | 0.20 | 7.8 (38/488) | 6.2 (23/374) | 1.11 [0.60–2.05] | 0.74 |
| CGGCC | 7.5 (65/862) | 7.7 (78/1012) | 1.01 [0.70–1.46] | 0.94 | 8.6 (42/488) | 6.2 (23/374) | 1.56 [0.86–2.86] | 0.15 |
| CAGTC | 8.1 (70/862) | 7.6 (77/1012) | 1.11 [0.77–1.59] | 0.58 | 9.4 (46/488) | 6.4 (24/374) | 1.67 [0.92–3.05] | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remuzgo-Martínez, S.; Genre, F.; Pulito-Cueto, V.; Atienza-Mateo, B.; Mora Cuesta, V.M.; Iturbe Fernández, D.; Fernández Rozas, S.M.; Lera-Gómez, L.; Alonso Lecue, P.; Ussetti, M.P.; et al. Role of VEGF Polymorphisms in the Susceptibility and Severity of Interstitial Lung Disease. Biomedicines 2021, 9, 458. https://doi.org/10.3390/biomedicines9050458
Remuzgo-Martínez S, Genre F, Pulito-Cueto V, Atienza-Mateo B, Mora Cuesta VM, Iturbe Fernández D, Fernández Rozas SM, Lera-Gómez L, Alonso Lecue P, Ussetti MP, et al. Role of VEGF Polymorphisms in the Susceptibility and Severity of Interstitial Lung Disease. Biomedicines. 2021; 9(5):458. https://doi.org/10.3390/biomedicines9050458
Chicago/Turabian StyleRemuzgo-Martínez, Sara, Fernanda Genre, Verónica Pulito-Cueto, Belén Atienza-Mateo, Víctor Manuel Mora Cuesta, David Iturbe Fernández, Sonia María Fernández Rozas, Leticia Lera-Gómez, Pilar Alonso Lecue, María Piedad Ussetti, and et al. 2021. "Role of VEGF Polymorphisms in the Susceptibility and Severity of Interstitial Lung Disease" Biomedicines 9, no. 5: 458. https://doi.org/10.3390/biomedicines9050458
APA StyleRemuzgo-Martínez, S., Genre, F., Pulito-Cueto, V., Atienza-Mateo, B., Mora Cuesta, V. M., Iturbe Fernández, D., Fernández Rozas, S. M., Lera-Gómez, L., Alonso Lecue, P., Ussetti, M. P., Laporta, R., Berastegui, C., Solé, A., Pérez, V., De Pablo Gafas, A., Gualillo, O., Cifrián, J. M., López-Mejías, R., & González-Gay, M. Á. (2021). Role of VEGF Polymorphisms in the Susceptibility and Severity of Interstitial Lung Disease. Biomedicines, 9(5), 458. https://doi.org/10.3390/biomedicines9050458









