Antiviral Effects of Polyphenols from Marine Algae
Abstract
1. Introduction
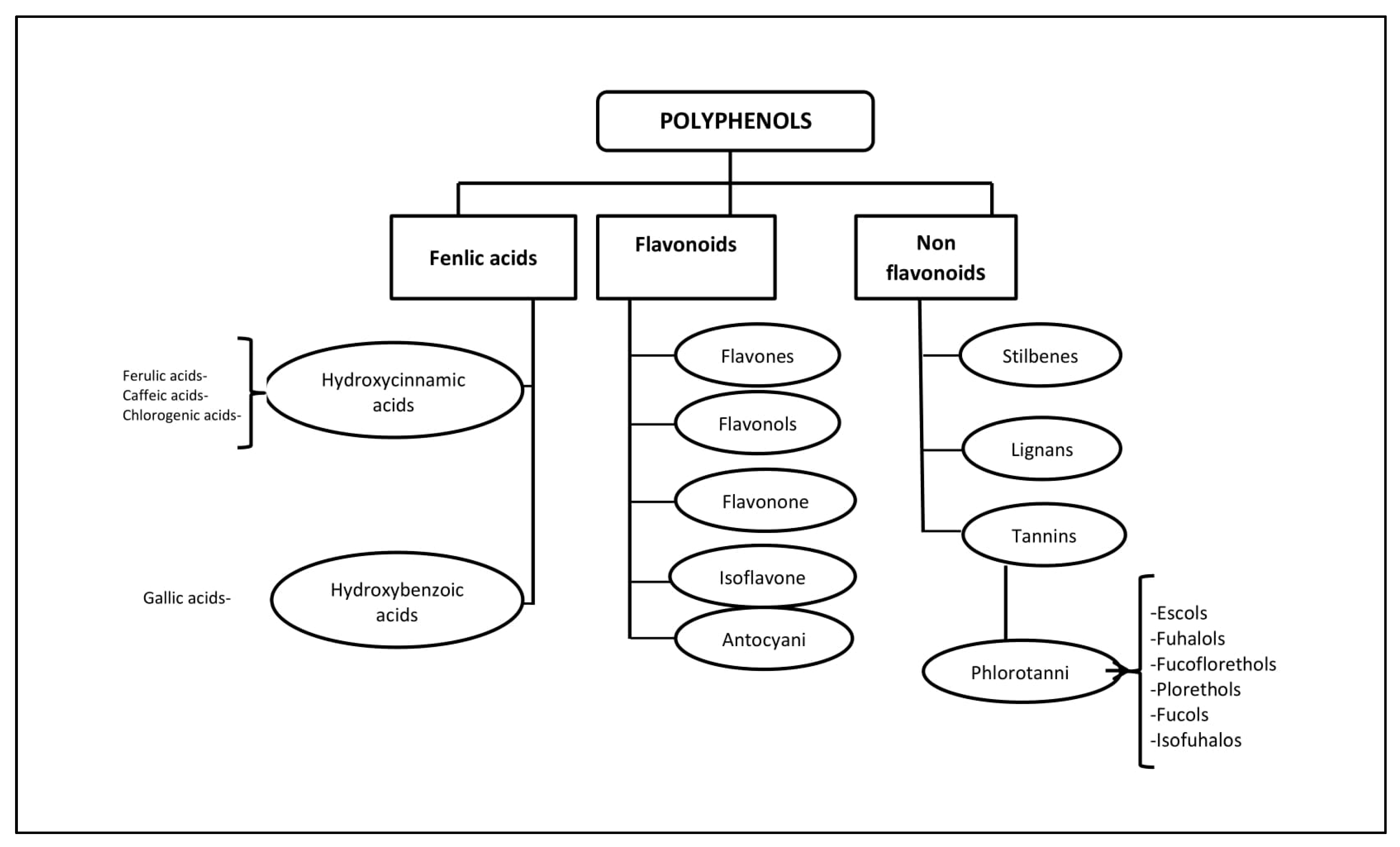
2. General Characteristics of the Polyphenolic Compounds of Seaweed
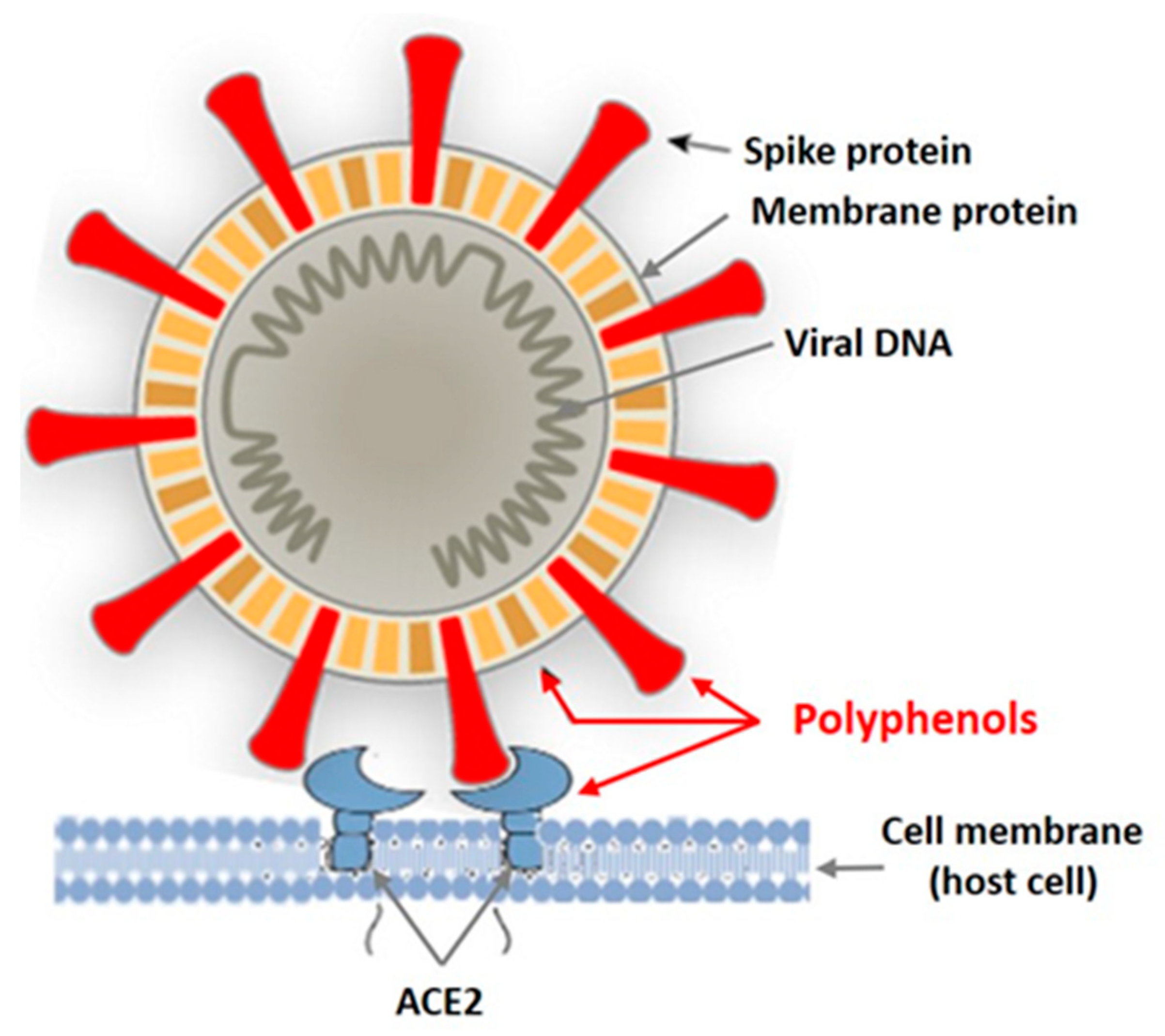
3. Interaction of Seaweed Polyphenols with Enveloped and Nonenveloped Viruses
- A.
- Interaction of Polyphenols of Seaweed with Enveloped Viruses
- B.
- Interaction of PTs of Algae with Nonenveloped Viruses
4. Seaweed Polyphenols and Their Inhibition of Vital Viral Proteins
5. Synergism of Algae-Derived Phlorotannins and Antiviral Drugs
- -
- reduction in individual doses of drugs;
- -
- reduction in the number and severity of side effects of antiviral drugs; and
- -
- prevention, in some cases, of the emergence of drug-resistant virus variants [69].
6. The Effect of PT on Pathogenetic Targets of Viral Infections in a Macroorganism
The Role of Anti-Inflammatory Action of Polyphenols in Protection against Viral Infections
7. In Vivo Efficacy of Polyphenolic Compounds
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ryu, W.-S. Virus Life Cycle. Mol. Virol. Hum. Pathog. Viruses 2017, 5, 31–45. [Google Scholar]
- Abdullah, A.A.; Abdullah, R.; Nazariah, Z.A.; Balakrishnan, K.N.; Abdullah, F.F.J.; Bala, J.A.; Mohd-Lila, M.-A. Cyclophilin A as a target in the treatment of cytomegalovirus infections. Antivir. Chem. Chemother. 2018, 26. [Google Scholar] [CrossRef]
- Strasfeld, L.; Chou, S. Antiviral Drug Resistance: Mechanisms and Clinical Implications. Infect. Dis. Clin. N. Am. 2010, 24, 413–437. [Google Scholar] [CrossRef]
- Irwin, K.K.; Renzette, N.; Kowalik, T.F.; Jensen, J.D. Antiviral drug resistance as an adaptive process. Virus Evol. 2016, 2, vew014. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Pedrosa, R.; Gaudêncio, S.P.; Vasconcelos, V. XVI International Symposium on Marine Natural Products|XI European Conference on Marine Natural Products. Mar. Drugs 2020, 18, 40. [Google Scholar] [CrossRef]
- Moghaddam, J.A.; Dávila-Céspedes, A.; Kehraus, S.; Crüsemann, M.; Köse, M.; Müller, C.E.; König, G.M. Cyclopropane-Containing Fatty Acids from the Marine Bacterium Labrenzia sp. 011 with Antimicrobial and GPR84 Activity. Mar. Drugs 2018, 16, 369. [Google Scholar] [CrossRef]
- Santhi, L.S.; Vssl, P.T.; Sy, N.; Radha Krishna, E. Bioactive Compounds from Marine Sponge Associates: Antibiotics from Bacillus sp. Nat. Prod. Chem. Res. 2017, 5, 4. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2019, 18, 2. [Google Scholar] [CrossRef]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.; Diop, A.; Rainville, L.-C.; Barnabé, S. Bioextracting Polyphenols from the Brown Seaweed Ascophyllum nodosum from Québec’s North Shore Coastline. Ind. Biotechnol. 2019, 15, 212–218. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Имбс, Т.; Звягинцева, Т. ФЛОРОТАННИНЫ - ПОЛИФЕНОЛЬНЫЕ МЕТАБОЛИТЫ БУРЫХ ВОДОРОСЛЕЙ. Биoлoгия мoря 2018, 44, 217–227. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; Soler-Villa, A.; Fitzgerald, R.J.; Brunton, N.P. Phenolic content and antioxidant activity of fractions obtained from selected Irish macroalgae species (Laminaria digitate, Fucus serratus, Gracillaria gracilis and Codium fragile). J. Appl. Phycol. 2014, 27, 519–530. [Google Scholar] [CrossRef]
- Shibata, T.; Kawaguchi, S.; Hama, Y.; Inagaki, M.; Yamaguchi, K.; Nakamura, T. Local and chemical distribution of phlorotannins in brown algae. Environ. Biol. Fishes 2004, 16, 291–296. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Sathya, M.; Kokilavani, R. Phitochemical screening and in vivo antioxidant activity of Saccharum spontaneous linn. Int. J. Pharm. Sci. Rev. Res. 2013, 18, 75–79. [Google Scholar]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Singh, R.; Akhtar, N.; Haqqi, T.M. Green tea polyphenol epigallocatechi3-gallate: Inflammation and arthritis. Life Sci. 2010, 86, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Melanson, J.E.; MacKinnon, S.L. Characterization of Phlorotannins from Brown Algae by LC-HRMS. Methods Mol. Biol. 2015, 1308, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef]
- Swallah, M.S.; Fu, H.; Sun, H.; Affoh, R.; Yu, H. The Impact of Polyphenol on General Nutrient Metabolism in the Monogastric Gastrointestinal Tract. J. Food Qual. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Clifford, M.N. Diet-Derived Phenols in Plasma and Tissues and their Implications for Health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef]
- Lewandowska, U.; Szewczyk, K.; Hrabec, E.; Janecka, A.; Gorlach, S. Overview of Metabolism and Bioavailability Enhancement of Polyphenols. J. Agric. Food Chem. 2013, 61, 12183–12199. [Google Scholar] [CrossRef] [PubMed]
- Ragan, M.A.; Glombitza, K.W. Phlorotannins, brown algal polyphenols. Prog. Phycol. Res. 1986, 4, 129–241. [Google Scholar]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A. Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Mannino, A.M.; Micheli, C. Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, K.R.; Aderogba, M.A.; Amoo, S.O.; Stirk, W.A.; Van Staden, J. Potential antiradical and alpha-glucosidase inhibitors from Ecklonia maxima (Osbeck) Papenfuss. Food Chem. 2013, 141, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.-R.; Shin, T.-S.; Lee, M.-S.; Park, J.-Y.; Park, K.-E.; Yoon, N.-Y.; Kim, J.-S.; Choi, J.-S.; Jang, B.-C.; Byun, D.-S.; et al. Isolation and Identification of Phlorotannins fromEcklonia stoloniferawith Antioxidant and Anti-inflammatory Properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Li, Y.; Lee, S.-H.; Le, Q.-T.; Kim, M.-M.; Kim, S.-K. Anti-allergic Effects of Phlorotannins on Histamine Release via Binding Inhibition between IgE and FcεRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Yoon, K.-D.; Min, S.-Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.-G.; Huh, H.; Kim, J. Inhibition of HIV-1 Reverse Transcriptase and Protease by Phlorotannins from the Brown Alga Ecklonia cava. Biol. Pharm. Bull. 2004, 27, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jeon, Y.-J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef]
- Koivikko, R.; Eranen, J.K.; Loponen, J.; Jormalainen, Y. Variation of phlorotannins among three populations of Fucus vesic-ulosus as revealed by HPLC and colorimetric quantification. J. Chem. Ecol. 2008, 34, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Firquet, S.; Beaujard, S.; Lobert, P.-E.; Sané, F.; Caloone, D.; Izard, D.; Hober, D. Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces. Microbes Environ. 2015, 30, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Potential of DNA intercalating alcaloids and other plant secondary metabolites against SARS-CoV-2 causing COVID-19. Diversity 2020, 12, 175. [Google Scholar] [CrossRef]
- Venkatesan, J.; Keekan, K.K.; Anil, S.; Bhatnagar, I.; Kim, S.-K. Phlorotannins. Encycl. Food Chem. 2019, 27, 515–527. [Google Scholar] [CrossRef]
- Yang, H.-K.; Jung, M.-H.; Avunje, S.; Nikapitiya, C.; Kang, S.Y.; Ryu, Y.B.; Lee, W.S.; Jung, S.-J. Efficacy of algal Ecklonia cava extract against viral hemorrhagic septicemia virus (VHSV). Fish Shellfish. Immunol. 2018, 72, 273–281. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Ryu, Y.B.; Kim, Y.-M.; Song, N.; Kim, C.Y.; Rho, M.-C.; Jeong, J.-H.; Cho, K.-O.; Lee, W.S.; Park, S.-J. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorgan. Med. Chem. 2013, 21, 4706–4713. [Google Scholar] [CrossRef]
- Ueda, K.; Kawabata, R.; Irie, T.; Nakai, Y.; Tohya, Y.; Sakaguchi, T. Inactivation of Pathogenic Viruses by Plant-Derived Tannins: Strong Effects of Extracts from Persimmon (Diospyros kaki) on a Broad Range of Viruses. PLOS ONE 2013, 8, e55343. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.M.; Doan, T.P.; Ha, T.K.Q.; Kim, H.W.; Lee, B.W.; Pham, H.T.T.; Cho, T.O.; Oh, W.K. Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (qTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia cava. Mar. Drugs 2019, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, N.I.; Kapustin, D.V.; Krasnova, E.I.; Izvekova, I.Y.I. NOROVIRUS INFECTION (SYSTEMATIC REVIEW). J. Infectol. 2018, 10, 5–14. [Google Scholar] [CrossRef]
- La Rosa, G.; Muscillo, M. Molecular detection of viruses in water and sewage. In Viruses in Food and Water; Elsevier: Amsterdam, The Netherlands, 2013; pp. 97–125. [Google Scholar]
- Atmar, R.L. Noroviruses: State of the Art. Food Environ. Virol. 2010, 2, 117–126. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, E.; Moon, S.; Choi, J.-D.; Lee, M.-S.; Kim, Y.-M. Phaeophyta Extracts Exhibit Antiviral Activity against Feline Calicivirus. Fish. Aquat. Sci. 2014, 17, 155–158. [Google Scholar] [CrossRef][Green Version]
- Eom, S.H.; Moon, S.Y.; Lee, D.S.; Kim, H.J.; Park, K.; Lee, E.W.; Kim, T.H.; Chung, Y.H.; Lee, M.S.; Kim, Y.M. In vitro antiviral activity of dieckol and phlorofucofuroecko-A isolated from edible brown alga Eisenia bicyclis against murine norovirus. Algae 2015, 30, 241–246. [Google Scholar] [CrossRef]
- Bailey-Elkin, B.A.; Knaap, R.C.; Kikkert, M.; Mark, B.L. Structure and Function of Viral Deubiquitinating Enzymes. J. Mol. Biol. 2017, 429, 3441–3470. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-W.; Cherney, M.M.; Liu, J.; James, K.E.; Powers, J.C.; Eltis, L.D.; James, M.N. Crystal Structures Reveal an Induced-fit Binding of a Substrate-like Aza-peptide Epoxide to SARS Coronavirus Main Peptidase. J. Mol. Biol. 2007, 366, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Peng, C.; Shi, Y.; Zhu, Z.; Mu, K.; Wang, X.; Zhu, W. Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an inte-grative approach combining homology modelling, molecular docking and binding free energy calculation. BioRxiv 2020. [Google Scholar] [CrossRef]
- Rathnayake, A.D.; Zheng, J.; Kim, Y.; Perera, K.D.; Mackin, S.; Meyerholz, D.K.; Kashipathy, M.M.; Battaile, K.P.; Lovell, S.; Perlman, S.; et al. 3C-like protease inhibitors block coronavirus replication in vitro and improve survival in MERS-CoV–infected mice. Sci. Transl. Med. 2020, 12, eabc5332. [Google Scholar] [CrossRef]
- Skvortsov, V.; Druzhilovskiy, D.; Veselovsky, A. Potential inhibitors of protease 3CLpro virus COVID-19: Drug reposition. Biomed. Chem. Res. Methods 2020, 3, e00124. [Google Scholar] [CrossRef]
- Ho, T.-Y.; Wu, S.-L.; Chen, J.-C.; Li, C.-C.; Hsiang, C.-Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007, 74, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Hulswit, R.J.; De Haan, C.A.; Bosch, B.J. Coronavirus Spike Protein and Tropism Changes. Adv. Virus Res. 2016, 96, 29–57. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Yuk, H.J.; Ryu, H.W.; Lim, S.H.; Kim, K.S.; Park, K.H.; Ryu, Y.B.; Lee, W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Crescente, G.; Faramarzi, S.; Formato, M.; Pecoraro, M.T.; Pacifico, S. Polyphenols vs. Coronaviruses: How Far Has Research Moved Forward? Molecules 2020, 25, 4103. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, J.M.; Chan, R.W.; Russell, R.J.; Air, G.M.; Peiris, J.M. Evolving complexities of influenza virus and its receptors. Trends Microbiol. 2008, 16, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Kim, J.H.; Kwon, J.M.; Kwon, H.-J.; Jeong, H.J.; Kim, Y.M.; Kim, D.; Lee, W.S.; Ryu, Y.B. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorgan. Med. Chem. 2013, 21, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Liu, W.; Li, X.; Chen, T.; Shafiee, A.; Card, D.; Abruzzo, G.; Flattery, A.; Gill, C.; Thompson, J.R.; et al. Antifungal Spectrum, In Vivo Efficacy, and Structure–Activity Relationship of Ilicicolin H. ACS Med. Chem. Lett. 2012, 3, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Liu, W.; Li, X.; Chen, T.; Shafiee, A.; Dreikorn, S.; Hornak, V.; Meinz, M.; Onishi, J.C. Structure–activity relationship of cytochrome bc1 reductase inhibitor broad spectrum antifungal ilicicolin H. Bioorgan. Med. Chem. Lett. 2013, 23, 3018–3022. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Yoon, S.Y.; Park, J.-Y.; Kim, Y.M.; Park, S.-J.; Rho, M.-C.; Kim, S.-J.; Lee, W.S. Influenza Virus Neuraminidase Inhibitory Activity of Phlorotannins from the Edible Brown AlgaEcklonia cava. J. Agric. Food Chem. 2011, 59, 6467–6473. [Google Scholar] [CrossRef]
- Vo, T.-S.; Kim, S.-K. Potential Anti-HIV Agents from Marine Resources: An Overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Yoon, K.-D.; Kim, C.Y.; Shin, C.-G.; Kim, J.H.; Kim, J. Inhibitory activity on HIV-1 reverse transcriptase and integrase of a carmalol derivative from a brown Alga, Ishige okamurae. Phytotherapy Res. 2006, 20, 711–713. [Google Scholar] [CrossRef]
- Artan, M.; Li, Y.; Karadeniz, F.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Anti-HIV-1 activity of phloroglucinol derivative, 6,6′-bieckol, from Ecklonia cava. Bioorganic Med. Chem. 2008, 16, 7921–7926. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, F.; Kang, K.-H.; Park, J.W.; Park, S.-J.; Kim, S.-K. Anti-HIV-1 activity of phlorotannin derivative 8,4‴-dieckol from Korean brown algaEcklonia cava. Biosci. Biotechnol. Biochem. 2014, 78, 1151–1158. [Google Scholar] [CrossRef]
- Benarba, B.; Pandiella, A. Medicinal Plants as Sources of Active Molecules Against COVID-19. Front. Pharmacol. 2020, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- De Mello, C.P.P.; Drusano, G.L.; Rodriquez, J.L.; Kaushik, A.; Brown, A.N. Antiviral Effects of Clinically-Relevant Interferon-α and Ribavirin Regimens against Dengue Virus in the Hollow Fiber Infection Model (HFIM). Viruses 2018, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Morán-Santibañez, K.; Peña-Hernández, M.A.; Cruz-Suárez, L.E.; Ricque-Marie, D.; Skouta, R.; Vasquez, A.H.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Virucidal and Synergistic Activity of Polyphenol-Rich Extracts of Seaweeds against Measles Virus. Viruses 2018, 10, 465. [Google Scholar] [CrossRef]
- Xi, C.; Zhang, Y.; Marrs, C.F.; Ye, W.; Simon, C.; Foxman, B.; Nriagu, J. Prevalence of Antibiotic Resistance in Drinking Water Treatment and Distribution Systems. Appl. Environ. Microbiol. 2009, 75, 5714–5718. [Google Scholar] [CrossRef] [PubMed]
- Valyi-Nagy, T.; Dermody, T.S. Role of oxidative damage in the pathogenesis of viral infections of the nervous system. Histol. Histopathol. 2005, 20, 957–967. [Google Scholar]
- Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N. Antiviral and Antioxidant Properties of Echinochrome A. Mar. Drugs 2018, 16, 509. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Reactive Oxygen Species and Cellular Defense System. In Free Radicals in Human Health and Disease; Rani, V., Yadav, U.C.S., Eds.; Springer: New Delhi, India, 2015; pp. 17–29. [Google Scholar]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuropro-gression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef]
- Sansone, C.; Brunet, C.; Noonan, D.M.; Albini, A. Marine Algal Antioxidants as Potential Vectors for Controlling Viral Diseases. Antioxidants 2020, 9, 392. [Google Scholar] [CrossRef]
- Gullberg, R.C.; Steel, J.J.; Moon, S.L.; Soltani, E.; Geiss, B.J. Oxidative stress influences positive strand RNA virus genome synthesis and capping. Virology 2015, 475, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Sebastiano, M.; Chastel, O.; De Thoisy, B.; Eens, M.; Costantini, D. Oxidative stress favours herpes virus infection in vertebrates: A meta-analysis. Curr. Zoöl. 2016, 62, 325–332. [Google Scholar] [CrossRef]
- Kavouras, J.H.; Prandovszky, E.; Valyi-Nagy, K.; Kovacs, S.K.; Tiwari, V.; Kovács, M.; Shukla, D.; Valyi-Nagy, T. Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. J. NeuroVirol. 2007, 13, 416–425. [Google Scholar] [CrossRef]
- Chen, X.; Kamranvar, S.A.; Masucci, M.G. Oxidative stress enables Epstein–Barr virus-induced B-cell transformation by posttranscriptional regulation of viral and cellular growth-promoting factors. Oncogene 2016, 35, 3807–3816. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant Therapy: Current Status and Future Prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef]
- Fassina, G.; Buffa, A.; Benelli, R.; Varnier, O.E.; Noonan, D.M.; Albini, A. Polyphenolic antioxidant (–)-epigallocatechin-3-gallate from green tea as a candidate anti-HIV agent. AIDS 2002, 16, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Hsu, W.-L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Di Sotto, A.; Checconi, P.; Celestino, I.; Locatelli, M.; Carissimi, S.; De Angelis, M.; Rossi, V.; Limongi, D.; Toniolo, C.; Martinoli, L.; et al. Antiviral and Antioxidant Activity of a Hydroalcoholic Extract from Humulus lupulus L. Oxidative Med. Cell. Longev. 2018, 2018, 5919237. [Google Scholar] [CrossRef] [PubMed]
- Reshi, M.L.; Su, Y.-C.; Hong, J.-R. RNA Viruses: ROS-Mediated Cell Death. Int. J. Cell Biol. 2014, 2014, 467452. [Google Scholar] [CrossRef]
- Dasuri, K.; Zhang, L.; Keller, J.N. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free. Radic. Biol. Med. 2013, 62, 170–185. [Google Scholar] [CrossRef]
- Palus, M.; Bílý, T.; Elsterová, J.; Langhansová, H.; Salát, J.; Vancová, M.; Růžek, D. Infection and injury of human astrocytes by tick-borne encephalitis virus. J. Gen. Virol. 2014, 95, 2411–2426. [Google Scholar] [CrossRef] [PubMed]
- Krylova, N.V.; Popov, A.M.; Leonova, G.N. Antioxidants as Potential Antiviral Drugs for Flavivirus Infections. Antibiot. Chemother. 2016, 61, 25–30. (In Russia) [Google Scholar]
- Popov, A.M.; Artyukov, A.A.; Krivoshapko, O.N.; Krylova, N.V.; Leonova, G.N.; Kozlovskaja, E.P. An Agent with Antioxidant, Cardioprotective, Antidiabetic, Anti-Inflammatory, Hepatoprotective, Anti-Tumor and Antiviral Effects. RF Patent C1 2432959, 2011. (In Russia). [Google Scholar]
- Krylova, N.V.; Popov, A.M.; Leonova, G.N.; Artiukov, A.A.; Maĭstrovskaia, O.S. Study of the activity of the drug Luromarin in vitro against tick-borne encephalitis virus. Antibiot. Chemother. 2010, 55, 17–19. [Google Scholar]
- Krylova, N.V.; Popov, A.M.; Leonova, G.N.; Artiukov, A.A.; Maĭstrovskaia, O.S. Comparative study of the antiviral activity of luteolin and luteolin 7,3’-disulfate. Antibiot. Chemother. 2011, 56, 7–10. [Google Scholar]
- Lee, S.H.; Eom, S.H.; Yoon, N.Y.; Kim, M.K.; Li, Y.X.; Ha, S.K.; Kim, S.K. Fucofuroeckol-A from Eisenia bicyclis inhibits inflammation in lipopolysaccha-ride-induced mouse macrophages via downregulation of the MAPK/NF-kB signaling pathway. J. Chem. 2016, 6509212. (In Russia) [Google Scholar]
- Montero-Lobato, Z.; Vázquez, M.; Navarro, F.; Fuentes, J.L.; Bermejo, E.; Garbayo, I.; Vílchez, C.; Cuaresma, M. Chemically-Induced Production of Anti-Inflammatory Molecules in Microalgae. Mar. Drugs 2018, 16, 478. [Google Scholar] [CrossRef]
- Sheikh, F.; Dickensheets, H.; Gamero, A.M.; Vogel, S.N.; Donnelly, R.P. An essential role for IFN-β in the induction of IFN-stimulated gene expression by LPS in macrophages. J. Leukoc. Biol. 2014, 96, 591–600. [Google Scholar] [CrossRef]
- Yang, Y.-I.; Jung, S.-H.; Lee, K.-T.; Choi, J.-H. 8,8′-Bieckol, isolated from edible brown algae, exerts its anti-inflammatory effects through inhibition of NF-κB signaling and ROS production in LPS-stimulated macrophages. Int. Immunopharmacol. 2014, 23, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Zhang, M.; Chen, Y.; Zhu, T.; Wang, J. Protective Effect of Eckol against Acute Hepatic Injury Induced by Carbon Tetrachloride in Mice. Mar. Drugs 2018, 16, 300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Y.; Guo, J.; Hu, X.-M.; Zhao, S.-Q.; Li, S.-L.; Wang, J. An in vivo anti-tumor effect of eckol from marine brown algae by improving the immune response. Food Funct. 2019, 10, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Zhen, A.X.; Hyun, Y.J.; Piao, M.J.; Fernando, P.D.S.M.; Kang, K.A.; Ahn, M.J.; Yi, J.M.; Kang, H.K.; Koh, Y.S.; Lee, N.H.; et al. Eckol Inhibits Particulate Matter 2.5-Induced Skin Keratinocyte Damage via MAPK Signaling Pathway. Mar. Drugs 2019, 17, 444. [Google Scholar] [CrossRef]
- Kroon, P.A.; Clifford, M.N.; Crozier, A.; Day, A.J.; Donovan, J.L.; Manach, C.; Williamson, G. How should we assess the effects of exposure to dietary polyphenols in vitro? Am. J. Clin. Nutr. 2004, 80, 15–21. [Google Scholar] [CrossRef]
- Landete, J.M. Updated Knowledge about Polyphenols: Functions, Bioavailability, Metabolism, and Health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.S. Quercetin Monograph. Altern. Med. Rev. 2011, 16, 172–194. [Google Scholar]
- Mehrbod, P.; Hudy, D.; Shyntum, D.; Markowski, J.; Łos, M.J.; Ghavami, S. Quercetin as a Natural Therapeutic Candidate for the Treatment of Influenza Virus. Biomolecules 2020, 11, 10. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, J.H.; Kwon, D.H. Quercetin 3-rhamnoside Exerts Antiinfluenza A Virus Activity in Mice. Phytother. Res. 2011, 26, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Makarova, M.N. Bioavailability and metabolism of flavonoids. Vopr. Pitan. 2011, 80, 33–40. [Google Scholar]
- Msc, T.A.N.R.; Lakshmi, A.N.V.; Anand, T.; Rao, L.V.; Sharma, G. Protective effects of quercetin during influenza virus-induced oxidative stress. Asia Pac. J. Clin. Nutr. 2000, 9, 314–317. [Google Scholar] [CrossRef]
- Savov, V.M.; Galabov, A.S.; Tantcheva, L.P.; Mileva, M.M.; Pavlova, E.L.; Stoeva, E.S.; Braykova, A.A. Effects of rutin and quercetin on monooxygenase activities in experimental influenza virus infection. Exp. Toxicol. Pathol. 2006, 58, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Gangemi, J.D. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R505–R509. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef]
- Newcomb, D.C.; Sajjan, U.S.; Nagarkar, D.R.; Wang, Q.; Nanua, S.; Zhou, Y.; McHenry, C.L.; Hennrick, K.T.; Tsai, W.C.; Bentley, J.K.; et al. Human Rhinovirus 1B Exposure Induces Phosphatidylinositol 3-Kinase–dependent Airway Inflammation in Mice. Am. J. Respir. Crit. Care Med. 2008, 177, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, U.; Ganesan, S.; Comstock, A.T.; Shim, J.; Wang, Q.; Nagarkar, D.R.; Zhao, Y.; Goldsmith, A.M.; Sonstein, J.; Linn, M.J.; et al. Elastase- and LPS-exposed mice display altered responses to rhinovirus infection. Am. J. Physiol. Cell. Mol. Physiol. 2009, 297, L931–L944. [Google Scholar] [CrossRef] [PubMed]
- Galochkina, A.V.; Anikin, V.B.; Babkin, V.A.; Ostrouhova, L.A.; Zarubaev, V.V. Virus-inhibiting activity of dihydroquercetin, a flavonoid from Larix sibirica, against coxsackievirus B4 in a model of viral pancreatitis. Arch. Virol. 2016, 161, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Kroeker, A.; He, S.; Kozak, R.; Audet, J.; Mbikay, M.; Chrétien, M. Prophylactic Efficacy of Quercetin 3-β-O-d-Glucoside against Ebola Virus Infection. Antimicrob. Agents Chemother. 2016, 60, 5182–5188. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and Quercetin: Promising Flavonoids with Chemopreventive Potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, Y.; Tomimori, T.; Yamada, H. Antiviral Activity of Plant Flavonoid, 5,7,4’-Trihydroxy-8-methoxyflavone, from the Roots of Scutellaria baicalensis against Influenza A (H3N2) and B Viruses. Biol. Pharm. Bull. 1995, 18, 295–299. [Google Scholar] [CrossRef]
- Ding, Y.; Dou, J.; Teng, Z.; Yu, J.; Wang, T.; Lu, N.; Wang, H.; Zhou, C. Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Arch. Virol. 2014, 159, 3269–3278. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Xu, L.; Zhang, M.-B.; Chu, Z.-Y.; Wang, Y.-D. Role of Baicalin in Anti-Influenza Virus A as a Potent Inducer of IFN-Gamma. BioMed Res. Int. 2015, 2015, 263630. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Chen, L.; Xu, G.; Zhang, L.; Zhou, H.; Wang, H.; Su, Z.; Ke, M.; Guo, Q.; Zhou, C. Effects of baicalein on Sendai virus in vivo are linked to serum baicalin and its inhibition of hemagglutinin-neuraminidase. Arch. Virol. 2011, 156, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Zang, N.; Xie, X.; Deng, Y.; Wu, S.; Wang, L.; Peng, C.; Li, S.; Ni, K.; Luo, Y.; Liu, E. Resveratrol-Mediated Gamma Interferon Reduction Prevents Airway Inflammation and Airway Hyperresponsiveness in Respiratory Syncytial Virus-Infected Immunocompromised Mice. J. Virol. 2011, 85, 13061–13068. [Google Scholar] [CrossRef]
- Huang, H.; Liao, D.; Zhou, G.; Zhu, Z.; Cui, Y.; Pu, R. Antiviral activities of resveratrol against rotavirus in vitro and in vivo. Phytomedicine 2020, 77, 153230. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yamada, H.; Takuma, N.; Niino, H.; Sagesaka, Y.M. Effects of Green Tea Catechins and Theanine on Preventing Influenza Infection among Healthcare Workers: A Randomized Controlled Trial. BMC Complement. Altern. Med. 2011, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.; Law, J.; Egli, A.; Douglas, D.; Lund, G.; Forester, S.; Lambert, J.; Law, M.; Burton, D.R.; Tyrrell, D.L.J.; et al. Prevention of hepatitis C virus infection using a broad cross-neutralizing monoclonal antibody (AR4A) and epigallocatechin gallate. Liver Transplant. 2015, 22, 324–332. [Google Scholar] [CrossRef] [PubMed]
| Antiviral Drug Class | Antiviral Mechanism of Action | Examples of Available Drugs |
|---|---|---|
| Nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) | Affect the ability of a virus to multiply or reproduce. NRTIs prevent the virus’s reverse transcriptase from accurately copying its RNA into DNA. | Zidovudine (Retrovir), Lamivudine (Epivir), Abacavir sulfate (Ziagen), Didanosine (Videx), Stavudine (Zerit), Emtricitabine (Emtriva) |
| Non-nucleoside reverse transcriptase inhibitors (NNRTIs) | NNRTIs block DNA elongation by directly binding to the reverse transcriptase enzyme | Delavirdine, Efavirenz, Etravirine, Nevirapine, Rilpivirine |
| Protease inhibitors | Protease inhibitor drugs block the action of protease enzymes. This can stop the virus from multiplying. | Atazanavir (Reyataz), Darunavir (Prezista), Fosamprenavir (Lexiva), Indinavir (Crixivan), Nelfinavir (Viracept), Ritonavir (Norvir), Saquinavir (Invirase) |
| Integrase inhibitors | These drugs stop HIV from being able to make integrase, which is necessary for its replication. | Raltegravir (Isentress), Dolutegravir (Tivicay), Elvitegravir, Bictegravir |
| Inhibitors of fusion | Inhibitors of the fusion of HIV to host cells, preventing viral entry. | Enfuvirtide, Maraviroc, Leronlimab, Aplaviroc, Ibalizumab, Temsavir |
| Inhibitors of chemokine receptors | These drugs inhibit chemokine receptors (CXCR4 and CCR5) and block the entry virus into the host cell. | Selzentry (Pro) Maraviroc, Bicyclam derivatives, AMD070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Shchelkanov, M.Y. Antiviral Effects of Polyphenols from Marine Algae. Biomedicines 2021, 9, 200. https://doi.org/10.3390/biomedicines9020200
Besednova NN, Andryukov BG, Zaporozhets TS, Kryzhanovsky SP, Fedyanina LN, Kuznetsova TA, Zvyagintseva TN, Shchelkanov MY. Antiviral Effects of Polyphenols from Marine Algae. Biomedicines. 2021; 9(2):200. https://doi.org/10.3390/biomedicines9020200
Chicago/Turabian StyleBesednova, Natalya N., Boris G. Andryukov, Tatyana S. Zaporozhets, Sergey P. Kryzhanovsky, Ludmila N. Fedyanina, Tatyana A. Kuznetsova, Tatyana N. Zvyagintseva, and Mikhail Yu. Shchelkanov. 2021. "Antiviral Effects of Polyphenols from Marine Algae" Biomedicines 9, no. 2: 200. https://doi.org/10.3390/biomedicines9020200
APA StyleBesednova, N. N., Andryukov, B. G., Zaporozhets, T. S., Kryzhanovsky, S. P., Fedyanina, L. N., Kuznetsova, T. A., Zvyagintseva, T. N., & Shchelkanov, M. Y. (2021). Antiviral Effects of Polyphenols from Marine Algae. Biomedicines, 9(2), 200. https://doi.org/10.3390/biomedicines9020200









