Caffeic Acid, One of the Major Phenolic Acids of the Medicinal Plant Antirhea borbonica, Reduces Renal Tubulointerstitial Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Nephrotoxic Compounds Identification and Quantification of Polyphenols by UPLC-UV-ESI-MS/MS
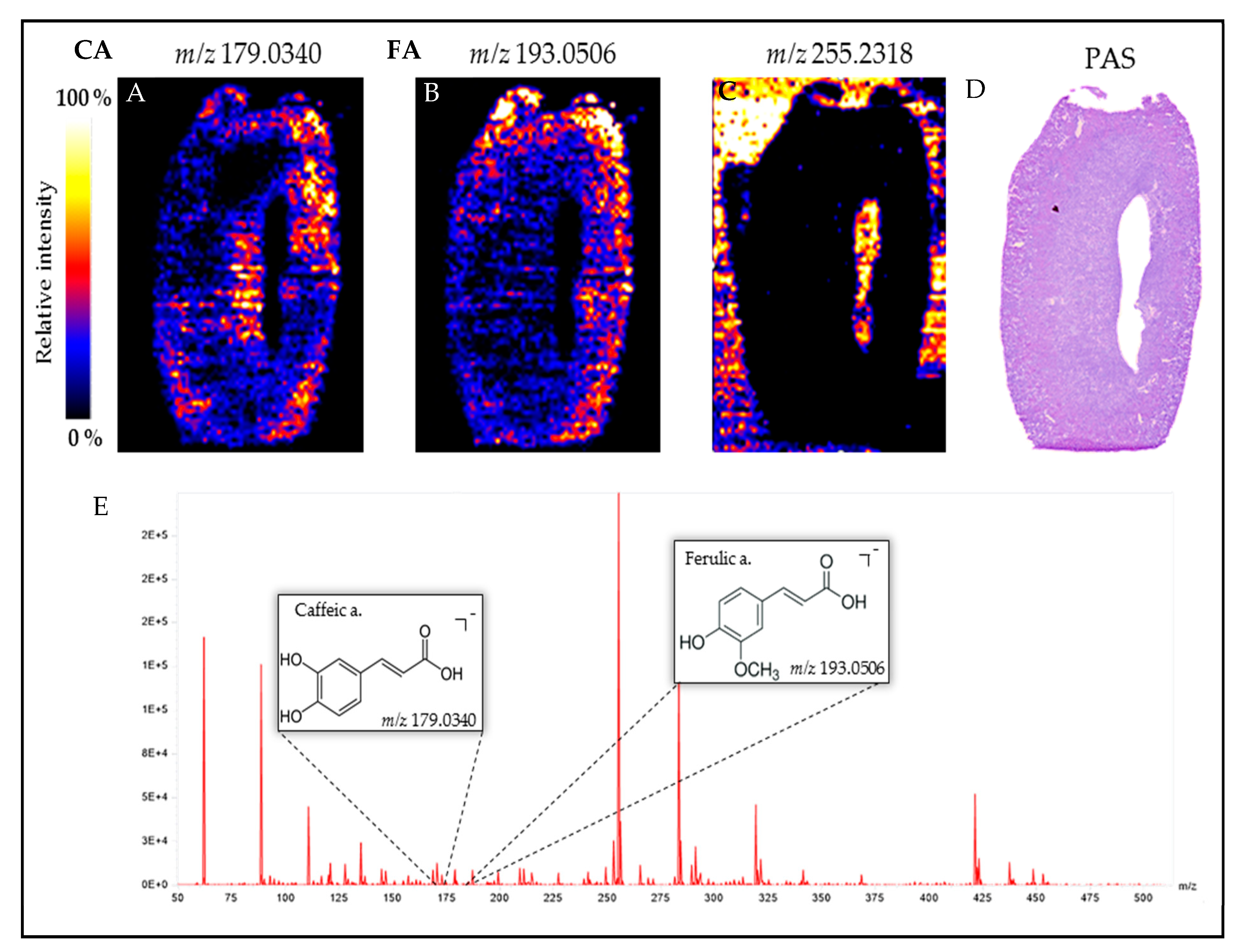
2.4. Desorption Electrospray Ionization-High Resolution/Mass Spectrometry (DESI-HR/MS) Imaging
2.5. Animal Model: Unilateral Ureteral Obstruction (UUO)—Biodistribution and Pharmacokinetic Studies
2.6. Determination of Phenolic and Flavonoid Contents—Measurement of the Total Antioxidant Capacity of Polyphenol-Rich Plant Extract Administered Orally
2.7. Immunohistochemistry and Histological Analysis
2.8. RT-qPCR
2.9. Protein Isolation from Kidney Tissue, Antioxidant Activities (Mn-SOD, Cu/Zn-SOD, GPX, CAT) and Protein Carbonylation
2.10. Statistics
3. Results
3.1. Detection and Identification of Nephrotoxic Components
3.2. Phenolic/Flavonoid Contents and Total Antioxidant Activity of Orally Administered Polyphenol-Rich Extract from A. borbonica.
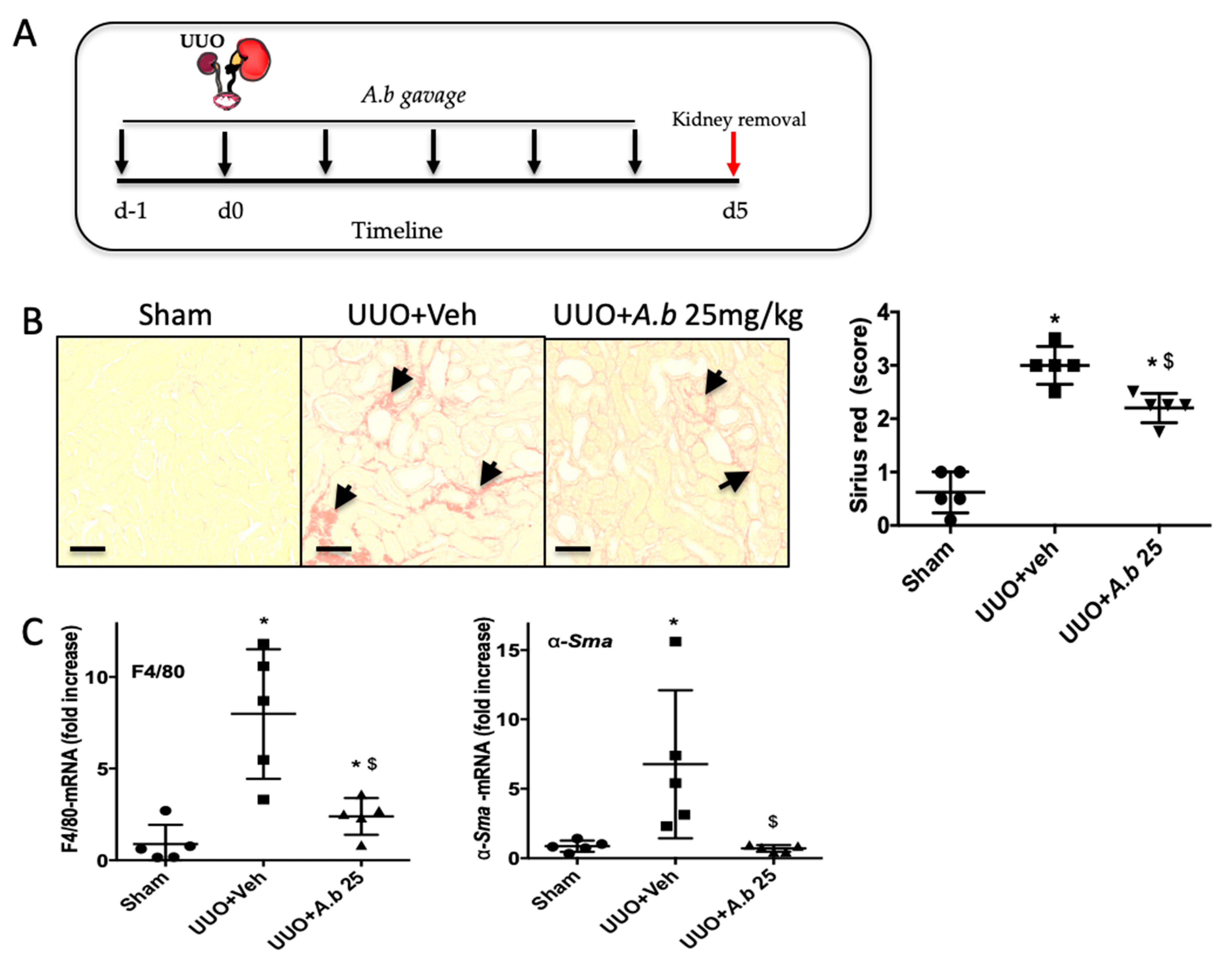
3.3. Preventive Experiment: Effects of Polyphenol-Rich Extract from A. borbonica on Body and Kidney Weight and Diuresis in a UUO Model
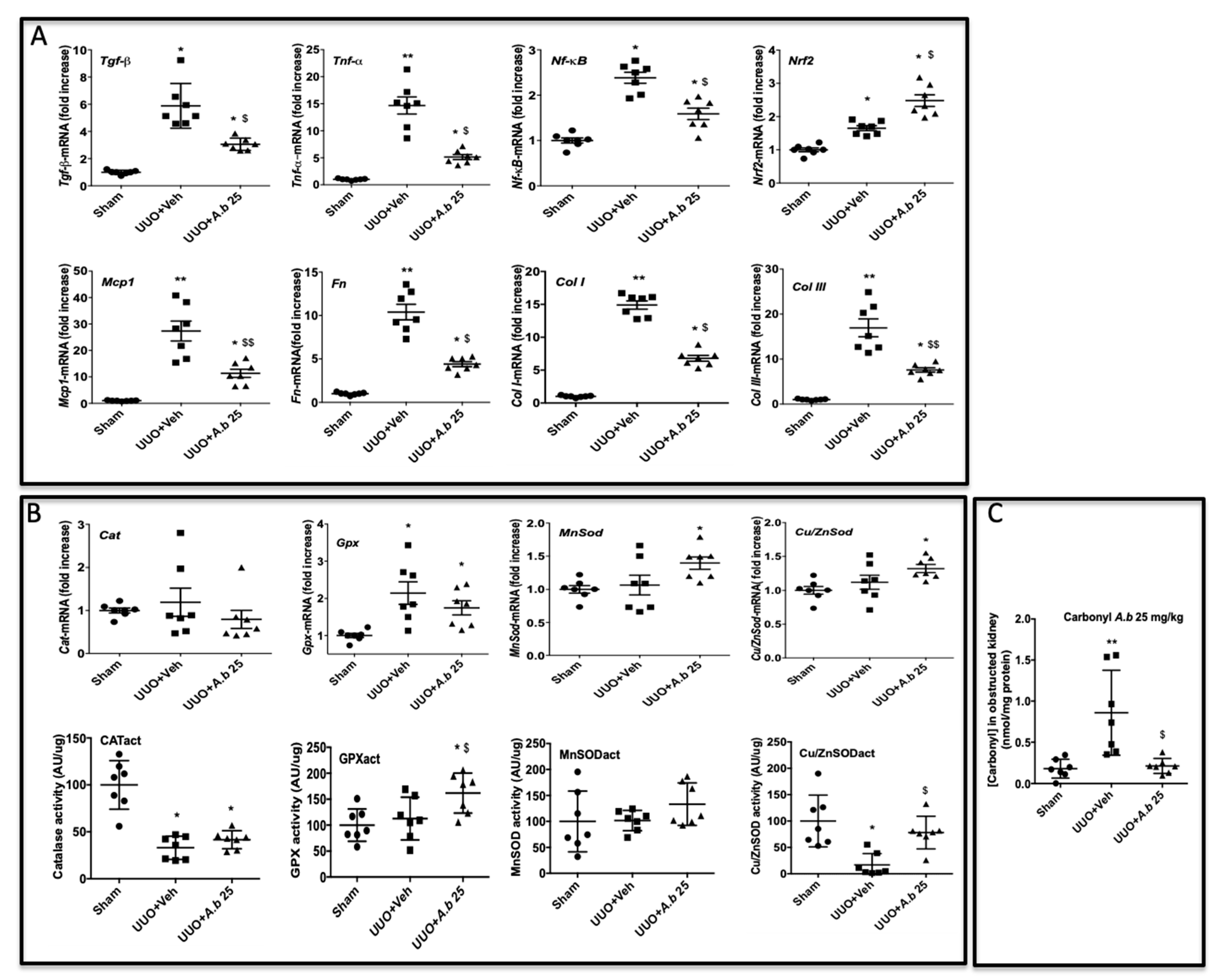
3.4. Preventive Effect of Polyphenol-Rich Extract from A. borbonica in UUO-Induced Tubulointerstitial Fibrosis
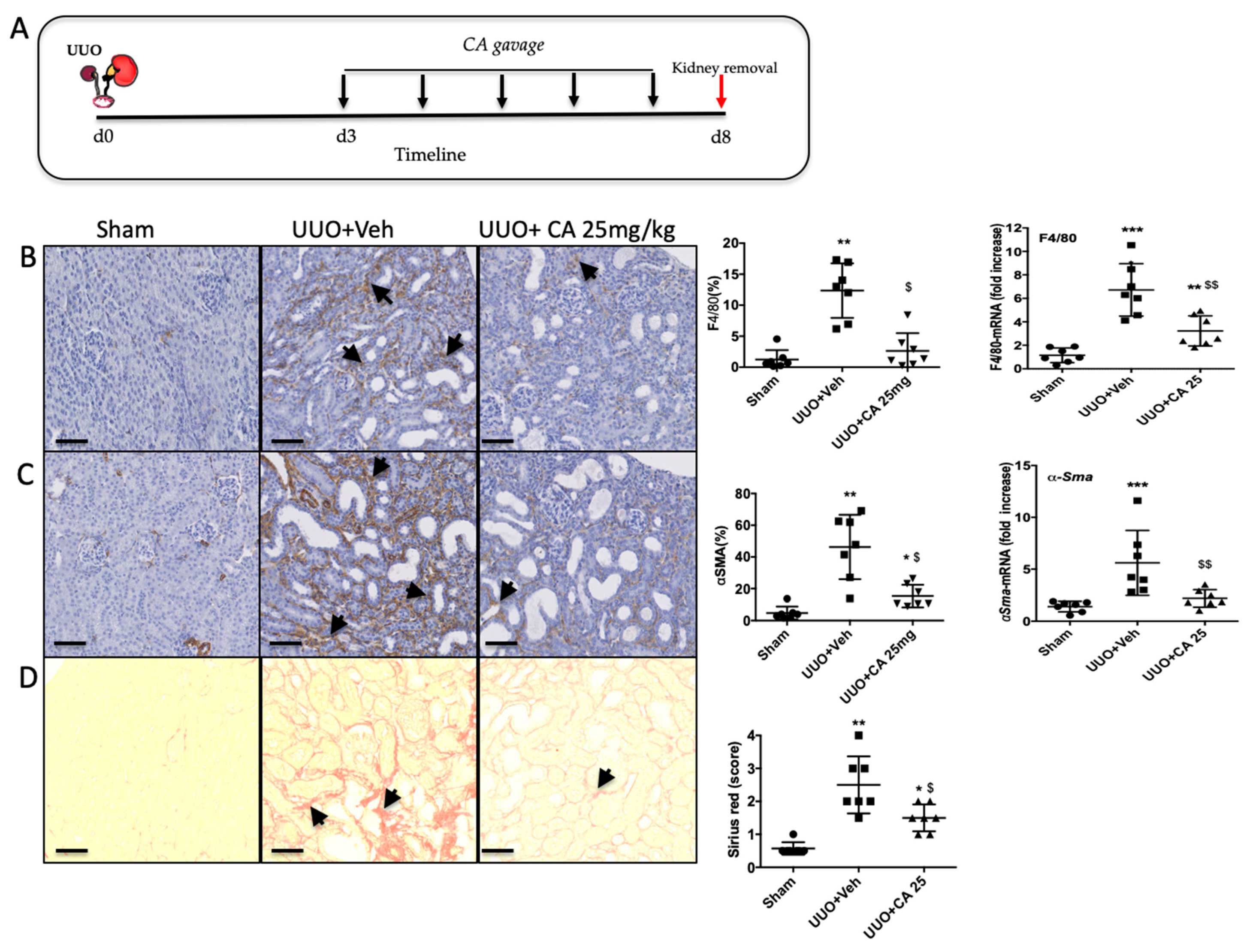
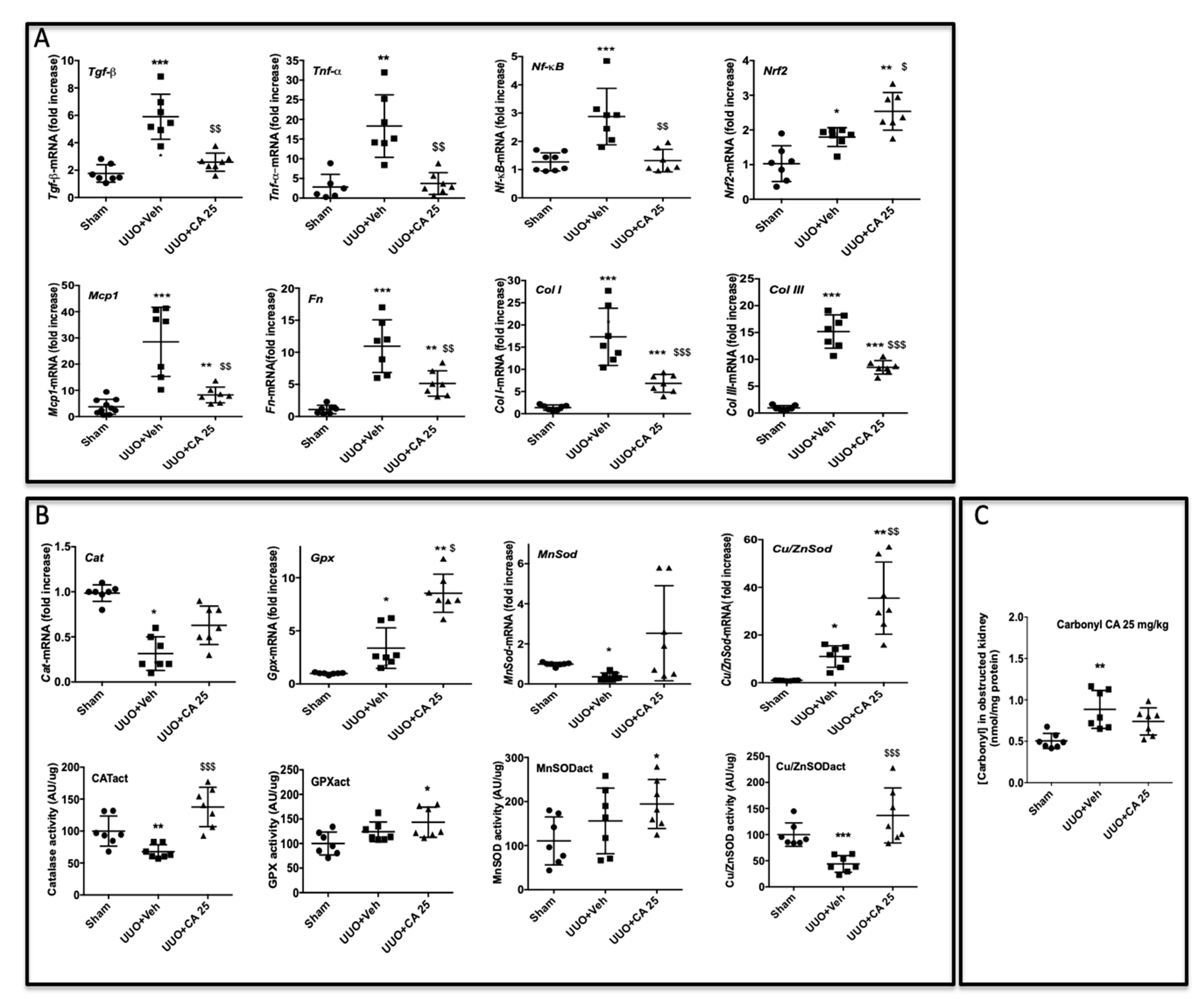
3.5. Curative Effect of Polyphenol-Rich Extract from A. borbonica in UUO-Induced Tubulointerstitial Fibrosis.
3.6. Biodistribution of Caffeic Acid and Its Metabolite 24 Hours after Administration of A. borbonica Polyphenol-Rich Extract (25 mg/kg) in Mice Kidney, Liver and Urine
3.7. Caffeic Acid (CA) Administration Mimics A. borbonica Extract’s Nephroprotective Effects
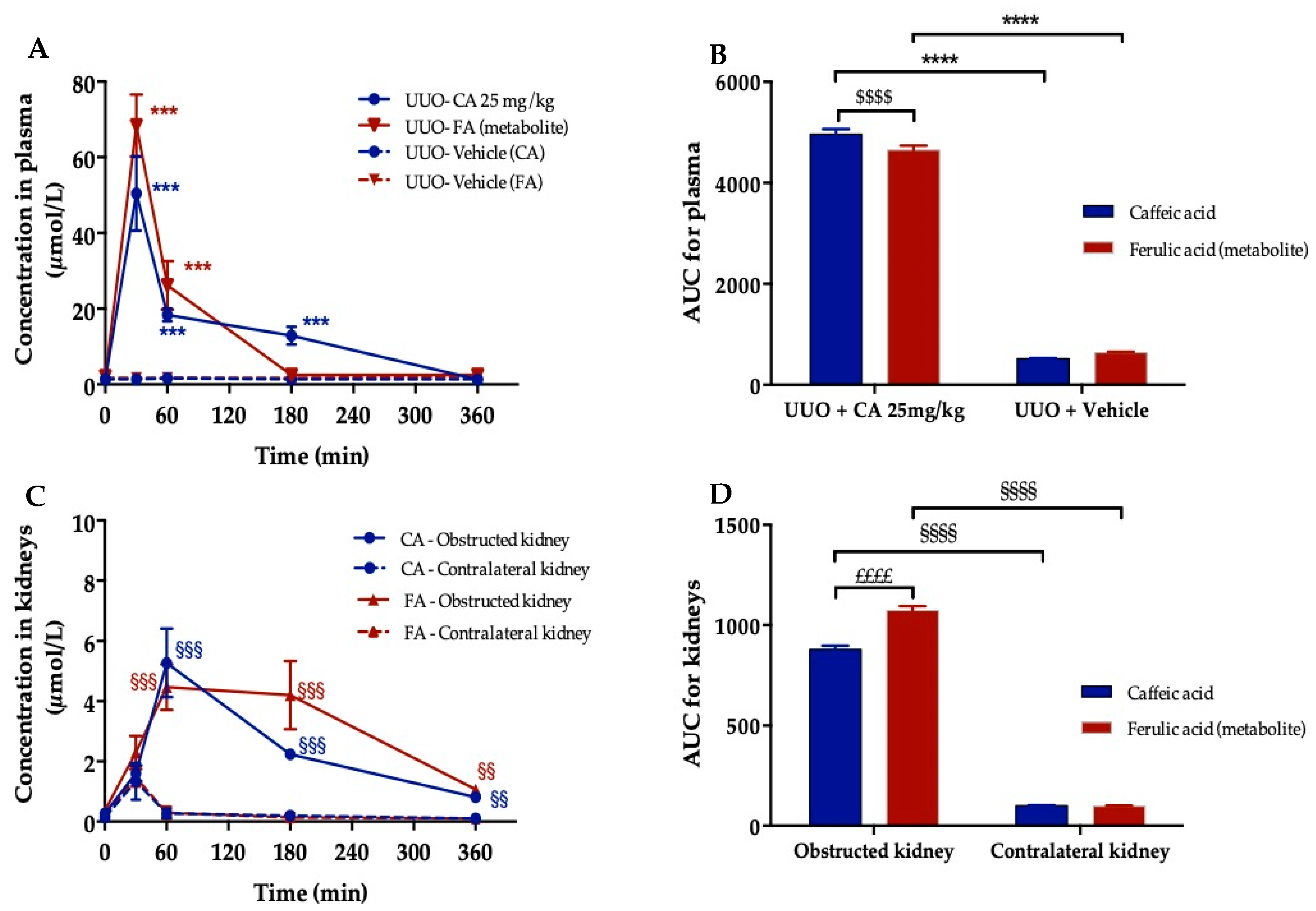
3.8. Plasma and Kidney Pharmacokinetic of Caffeic Acid
3.9. Visualization of Orally Administered Caffeic Acid (CA) in the Obstructed Kidney
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Epstein, F.H.; Klahr, S.; Schreiner, G.; Ichikawa, I. The progression of renal disease. N. Engl. J. Med. 1988, 318, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 1992, 20, 1–17. [Google Scholar] [CrossRef]
- Cohen, E. Fibrosis causes progressive kidney failure. Med. Hypotheses 1995, 45, 459–462. [Google Scholar] [CrossRef]
- Nangaku, M. Mechanisms of tubulointerstitial injury in the kidney: Final common pathways to end-stage renal failure. Intern. Med. 2004, 43, 9–17. [Google Scholar] [CrossRef]
- Hewitson, T.D. Renal tubulointerstitial fibrosis: Common but never simple. Am. J. Physiol. Physiol. 2009, 296, F1239–F1244. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet 2000, 355, 253–259. [Google Scholar] [CrossRef]
- Pitt, B.; A Poole-Wilson, P.; Segal, R.; A Martinez, F.; Dickstein, K.; Camm, A.J.; A Konstam, M.; Riegger, G.; Klinger, G.H.; Neaton, J.; et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: Randomised trial—the Losartan Heart Failure Survival Study ELITE II. Lancet 2000, 355, 1582–1587. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.-U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Köttgen, A.; Levey, A.S.; Levin, A. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef]
- Sridhar, V.S.; Rahman, H.U.; Cherney, D.Z. What have we learned about renal protection from the cardiovascular outcome trials and observational analyses with SGLT2 inhibitors? Diabetes Obes. Metab. 2020, 22, 55–68. [Google Scholar] [CrossRef]
- Schernthaner, G.; Groop, P.; Kalra, P.A.; Ronco, C.; Taal, M.W. Sodium-glucose linked transporter-2 inhibitor renal outcome modification in type 2 diabetes: Evidence from studies in patients with high or low renal risk. Diabetes Obes. Metab. 2020, 22, 1024–1034. [Google Scholar] [CrossRef]
- Hodrea, J.; Balogh, D.B.; Hosszu, A.; Lenart, L.; Besztercei, B.; Koszegi, S.; Sparding, N.; Genovese, F.; Wagner, L.J.; Szabo, A.J.; et al. Reduced O-GlcNAcylation and tubular hypoxia contribute to the antifibrotic effect of SGLT2 inhibitor dapagliflozin in the diabetic kidney. Am. J. Physiol. Physiol. 2020, 318, F1017–F1029. [Google Scholar] [CrossRef]
- Allinovi, M.; De Chiara, L.; Angelotti, M.L.; Becherucci, F.; Romagnani, P. Anti-fibrotic treatments: A review of clinical evidence. Matrix Biol. 2018, 68-69, 333–354. [Google Scholar] [CrossRef]
- Moreno, J.A.; Gomez-Guerrero, C.; Mas, S.; Sanz, A.B.; Lorenzo, O.; Ruiz-Ortega, M.; Opazo, L.; Mezzano, S.; Egido, J. Targeting inflammation in diabetic nephropathy: A tale of hope. Expert Opin. Investig. Drugs 2018, 27, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Booz, G.W.; Fan, F.; Wang, Y.; Roman, R.J. Oxidative Stress and Renal Fibrosis: Recent Insights for the Development of Novel Therapeutic Strategies. Front. Physiol. 2018, 9, 105. [Google Scholar] [CrossRef]
- Ow, C.P.C.; Ngo, J.P.; Ullah, M.; Hilliard, L.M.; Evans, R.G. Renal hypoxia in kidney disease: Cause or consequence? Acta Physiol. 2018, 222, e12999. [Google Scholar] [CrossRef] [PubMed]
- Vanherweghem, J.-L.; Tielemans, C.; Abramowicz, D.; Depierreux, M.; Vanhaelen-Fastre, R.; Vanhaelen, M.; Dratwa, M.; Richard, C.; Vandervelde, D.; Verbeelen, D.; et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet 1993, 341, 387–391. [Google Scholar] [CrossRef]
- Jadot, I.I.; Declèves, A.-E.; Nortier, J.; Caron, N. An Integrated View of Aristolochic Acid Nephropathy: Update of the Literature. Int. J. Mol. Sci. 2017, 18, 297. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Techer, S.; Amedee, J.; Girard-Valenciennes, E.; Thomas, H.; Brillant, S.; Grondin, I.; Marodon, C.; Smadja, J. Plantes medi-cinales de la Réunion inscrites à la pharmacopée française. Ethnopharmacologia 2016, 5, 7–33. [Google Scholar]
- Adsersen, A.; Adsersen, H. Plants from Réunion Island with alleged antihypertensive and diuretic effects—An experimental and ethnobotanical evaluation. J. Ethnopharmacol. 1997, 58, 189–206. [Google Scholar] [CrossRef]
- Marimoutou, M.; Le Sage, F.; Smadja, J.; D’Hellencourt, C.L.; Gonthier, M.-P.; Silva, C.R.-D. Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismutase and NF-κB genes. J. Inflamm. 2015, 12, 1–15. [Google Scholar] [CrossRef]
- Fortin, H.; Vigor, C.; Dévéhat, F.L.-L.; Robin, V.; Le Bossé, B.; Boustie, J.; Amoros, M. In vitro antiviral activity of thirty-six plants from La Réunion Island. Fitoter 2002, 73, 346–350. [Google Scholar] [CrossRef]
- LeDoux, A.; Cao, M.; Jansen, O.; Mamede, L.; Campos, P.-E.; Payet, B.; Clerc, P.; Grondin, I.; Girard-Valenciennes, E.; Hermann, T.; et al. Antiplasmodial, anti-chikungunya virus and antioxidant activities of 64 endemic plants from the Mascarene Islands. Int. J. Antimicrob. Agents 2018, 52, 622–628. [Google Scholar] [CrossRef]
- Haddad, J.G.; Koishi, A.C.; Gaudry, A.; Dos Santos, C.N.D.; Viranaicken, W.; Desprès, P.; El Kalamouni, C. Doratoxylon apetalum, an Indigenous Medicinal Plant from Mascarene Islands, Is a Potent Inhibitor of Zika and Dengue Virus Infection in Human Cells. Int. J. Mol. Sci. 2019, 20, 2382. [Google Scholar] [CrossRef]
- Lavergne, R. Tisaneurs et Plantes Médicinales Indigènes à la Réunion; Orphie: Livry Gargan, France, 2016; ISBN 979-10-298 0073-3. [Google Scholar]
- Poullain, C.; Girard-Valenciennes, E.; Smadja, J. Plants from reunion island: Evaluation of their free radical scavenging and antioxidant activities. J. Ethnopharmacol. 2004, 95, 19–26. [Google Scholar] [CrossRef]
- Taïlé, J.; Arcambal, A.; Clerc, P.; Gauvin-Bialecki, A.; Gonthier, M.-P. Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants 2020, 9, 573. [Google Scholar] [CrossRef]
- Delveaux, J.; Turpin, C.; Veeren, B.; Diotel, N.; Bravo, S.B.; Begue, F.; Álvarez, E.; Meilhac, O.; Bourdon, E.; Rondeau, P. Antirhea borbonica Aqueous Extract Protects Albumin and Erythrocytes from Glycoxidative Damages. Antioxidants 2020, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Ghaddar, B.; Veeren, B.; Rondeau, P.; Bringart, M.; D’Hellencourt, C.L.; Meilhac, O.; Bascands, J.-L.; Diotel, N. Impaired brain homeostasis and neurogenesis in diet-induced overweight zebrafish: A preventive role from A. borbonica extract. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Arcambal, A.; Taïlé, J.; Couret, D.; Planesse, C.; Veeren, B.; Diotel, N.; Gauvin-Bialecki, A.; Meilhac, O.; Gonthier, M. Protective Effects of Antioxidant Polyphenols against Hyperglycemia-Mediated Alterations in Cerebral Endothelial Cells and a Mouse Stroke Model. Mol. Nutr. Food Res. 2020, 64, e1900779. [Google Scholar] [CrossRef]
- Veeren, B.; Ghaddar, B.; Bringart, M.; Khazaal, S.; Gonthier, M.-P.; Meilhac, O.; Diotel, N.; Bascands, J.-L. Phenolic Profile of Herbal Infusion and Polyphenol-Rich Extract from Leaves of the Medicinal Plant Antirhea borbonica: Toxicity Assay Determination in Zebrafish Embryos and Larvae. Molecules 2020, 25, 4482. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in hu-mans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1299–1352. [Google Scholar] [CrossRef] [PubMed]
- Schramm, T.; Hester, Z.; Klinkert, I.; Both, J.P.; Heeren, R.M.; Brunelle, A.; Laprévote, O.; Desbenoit, N.; Robbe, M.F.; Stoeckli, M.; et al. imzML—A common data format for the flexible exchange and processing of mass spectrometry imaging data. J. Proteom. 2012, 75, 5106–5110. [Google Scholar] [CrossRef] [PubMed]
- Källback, P.; Nilsson, A.; Shariatgorji, M.; Andrén, P.E. msIQuant–Quantitation Software for Mass Spectrometry Imaging Enabling Fast Access, Visualization, and Analysis of Large Data Sets. Anal. Chem. 2016, 88, 4346–4353. [Google Scholar] [CrossRef]
- Schanstra, J.P.; Neau, E.; Drogoz, P.; Gomez, M.A.A.; Novoa, J.M.L.; Calise, D.; Pécher, C.; Bader, M.; Girolami, J.-P.; Bascands, J.-L. In vivo bradykinin B2 receptor activation reduces renal fibrosis. J. Clin. Investig. 2002, 110, 371–379. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-PhosphotungsticAcid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Klein, J.; Gonzalez, J.; Duchene, J.; Esposito, L.; Pradere, J.P.; Neau, E.; Delage, C.; Calise, D.; Ahluwalia, A.; Carayon, P.; et al. Delayed blockade of the kinin B1 receptor reduces renal inflammation and fibrosis in obstructive nephropathy. FASEB J. 2008, 23, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, P.; Navarra, G.; Cacciabaudo, F.; Leone, M.; Bourdon, E.; Militello, V. Thermal aggregation of glycated bovine serum albumin. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2010, 1804, 789–798. [Google Scholar] [CrossRef]
- De Smet, P.A.G.M. Herbal remedies. N. Engl. J. Med. 2002, 347, 2046–2056. [Google Scholar] [CrossRef]
- Colson, C.R.; De Broe, M.E. Kidney injury from alternative medicines. Adv. Chronic Kidney Dis. 2005, 12, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xie, Y.; Guo, M.; Rosner, M.H.; Yang, H.; Ronco, C. Nephrotoxicity and Chinese herbal medicine. Clin. J. Am. Soc. Nephrol. 2018, 13, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Bascands, J.-L.; Schanstra, J.P. Obstructive nephropathy: Insights from genetically engineered animals. Kidney Int. 2005, 68, 925–937. [Google Scholar] [CrossRef]
- Eddy, A.A.; López-Guisa, J.M.; Okamura, D.M.; Yamaguchi, I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr. Nephrol. 2011, 27, 1233–1247. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Kim, Y.-J.; Seo, C.-S.; Kim, H.-T.; Park, S.-R.; Lee, M.-Y.; Jung, J.-Y. Elsholtzia ciliata (Thunb.) Hylander attenuates renal inflammation and interstitial fibrosis via regulation of TGF-ß and Smad3 expression on unilateral ureteral obstruction rat model. Phytomedicine 2016, 23, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, S.; Bideskan, A.E.; Shafei, M.N.; Sadeghnia, H.R.; Soukhtanloo, M.; Shahraki, S.; Noshahr, Z.S.; Rad, A.K. Nigella sativa extract is a potent therapeutic agent for renal inflammation, apoptosis, and oxidative stress in a rat model of unilateral ureteral obstruction. Phytother. Res. 2018, 32, 2290–2298. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Xu, C.; Wang, W. Curcumin ameliorates renal fibrosis by inhibiting local fibroblast proliferation and extracellular matrix deposition. J. Pharmacol. Sci. 2014, 126, 344–350. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.-M.; Guan, S.-S.; Chiang, C.-K.; Wu, C.-T.; Liu, S.-H. The antifibrotic and anti-inflammatory effects of icariin on the kidney in a unilateral ureteral obstruction mouse model. Phytomedicine 2019, 59, 152917. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, M.; Zhou, Y.; Wang, C.; Yuan, Y.; Li, L.; Bresette, W.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Resveratrol exerts dose-dependent anti-fibrotic or pro-fibrotic effects in kidneys: A potential risk to individuals with impaired kidney function. Phytomedicine 2019, 57, 223–235. [Google Scholar] [CrossRef]
- Liu, X.; Sun, N.; Mo, N.; Lu, S.; Song, E.; Ren, C.; Li, Z. Quercetin inhibits kidney fibrosis and the epithelial to mesenchymal transition of the renal tubular system involving suppression of the Sonic Hedgehog signaling pathway. Food Funct. 2019, 10, 3782–3797. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Du, F.; Su, X.; Sun, G.; Zhou, G.; Bian, X.; Liu, N. Epigallocatechin-3-gallate attenuates unilateral ureteral obstruction-induced renal interstitial fibrosis in mice. J. Histochem. Cytochem. 2014, 63, 270–279. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef]
- Bocci, V.; Valacchi, G. Nrf2 activation as target to implement therapeutic treatments. Front. Chem. 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Zecher, M.; Guichard, C.; Velásquez, M.J.; Figueroa, G.; Rodrigo, R. Implications of oxidative stress in the pathophysiology of obstructive uropathy. Urol. Res. 2008, 37, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, M.A.; Nicholas, S.B.; Norris, K.C.; Vaziri, N.D. Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol. Dial. Transplant. 2013, 28, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway. Chem. Biodivers. 2019, 16, e1900400. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Zhang, X.-S.; Liang, C.-Z. Cryptotanshinone Attenuates Oxidative Stress and Inflammation through the Regulation of Nrf-2 and NF-κB in Mice with Unilateral Ureteral Obstruction. Basic Clin. Pharmacol. Toxicol. 2018, 123, 714–720. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Du, F.; Su, X.; Sun, G.; Zhou, G.; Bian, X.; Liu, N. Epigallocatechin-3-Gallate Attenuates Oxidative Stress and Inflammation in Obstructive Nephropathy via NF-κB and Nrf2/HO-1 Signalling Pathway Regulation. Basic Clin. Pharmacol. Toxicol. 2015, 117, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Dendooven, A.; Ishola, D.A., Jr.; Nguyen, T.Q.; Van Der Giezen, D.M.; Kok, R.J.; Goldschmeding, R.; Joles, J.A. Oxidative stress in obstructive nephropathy. Int. J. Exp. Pathol. 2010, 92, 202–210. [Google Scholar] [CrossRef]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Araújo, D.A.M.; Da Costa, J.G.M. Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy. A Review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef]
- Chuang, S.-T.; Kuo, Y.-H.; Su, M.-J. Antifibrotic effects of KS370G, a caffeamide derivative, in renal ischemia-reperfusion injured mice and renal tubular epithelial cells. Sci. Rep. 2014, 4, srep05814. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-T.; Kuo, Y.-H.; Su, M.-J. KS370G, a caffeamide derivative, attenuates unilateral ureteral obstruction-induced renal fibrosis by the reduction of inflammation and oxidative stress in mice. Eur. J. Pharmacol. 2015, 750, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Deng, Z.-Y.; Chen, X.; Zhang, B.; Fan, Y.; Li, H. Synergistic antioxidant effects of phenolic acids and carotenes on H2O2-induced H9c2 cells: Role of cell membrane transporters. Food Chem. 2021, 341, 128000. [Google Scholar] [CrossRef]
- Peng, X.; Wu, G.; Zhao, A.; Huang, K.; Chai, L.; Natarajan, B.; Yang, S.; Chen, H.; Lin, C. Synthesis of novel caffeic acid derivatives and their protective effect against hydrogen peroxide induced oxidative stress via Nrf2 pathway. Life Sci. 2020, 247, 117439. [Google Scholar] [CrossRef]
- Omar, M.H.; Mullen, W.; Stalmach, A.; Auger, C.; Rouanet, J.-M.; Teissedre, P.-L.; Caldwell, S.T.; Hartley, R.C.; Crozier, A. Absorption, disposition, metabolism, and excretion of [3-(14)C]caffeic acid in rats. J. Agric. Food Chem. 2012, 60, 5205–5214. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Williamson, G.; Clifford, M.N. Dietary hydroxycinnamates and their bioavailability. In Flavonoids and Related Compounds: Bioavailability and Function; CRC Press: Boca Raton, FL, USA, 2012; Volume 30, Available online: https://research.monash.edu/en/publications/dietary-hydroxycinnamates-and-their-bioavailability (accessed on 30 March 2021).


| Mouse Gene | Sequence | |
|---|---|---|
| Tgf-β | Forward Reverse | CCTGAGTGGCTGTCTTTTGA CGTGGAGTTTGTTATCTTTGCTG |
| Tnf-α | Forward Reverse | TCCCAGGTTCTCTTCAAGGGA ACAAGGTACAACCCATCGGC |
| Nf-κB | Forward Reverse | GTGATGGGCCTTCACACACA CATTTGAACACTGCTTTGACT |
| F4/80 | Forward Reverse | ACCACAATACCTACATGCACC AAGCAGGCGAGGAAAAGATAG |
| Nrf2 | Forward Reverse | TCCCATTTGTAGATGACCATGAG CCATGTCCTGCTCTATGCTG |
| Mcp1 | Forward Reverse | GCAGTTAACGCCCCACTCA CCAGCCTACTCATTGGGATCA |
| Fn | Forward Reverse | CTTTGGCAGTGGTCATTTCAG ATTCTCCCTTTCCATTCCCG |
| Col I | Forward Reverse | CATAAAGGGTCATCGTGGCT TTGAGTCCGTCTTTGCCAG |
| Col III | Forward Reverse | GAAGTCTCTGAAGCTGATGGG TTGCCTTGCGTGTTTGATATTC |
| α-Sma | Forward Reverse | GTGAAGAGGAAGACAGCACAG GCCCATTCCAACCATTACTCC |
| Gapdh | Forward Reverse | CTTTGTCAAGCTCATTTCCTGG TCTTGCTCAGTGTCCTTGC |
| Cat | Forward Reverse | CCTCCTCGTTCAGGATGTGGTT CGAGGGTCACGAACTGTGTCAG |
| Gpx | Forward Reverse | TGCTCATTGAGAATGTCGCGTCTC AGGCATTCCGCAGGAAGGTAAAGA |
| MnSod | Forward Reverse | ATGTTGTGTCGGGCGGCG AGGTAGTAAGCGTGCTCCCACACG |
| Cu/ZnSod | Forward Reverse | GCAGGGAACCATCCACTT TACAACCTCTGGACCCGT |
| Group | n | Body Weight (g) | Left Kidney Weight (g) | Diuresis/24 h (mL) |
|---|---|---|---|---|
| Sham | 7 | 22.02 ± 2.64 | 0.134 ± 0.01 | 1.06 ± 0.44 |
| UUO + veh | 7 | 21.58 ± 1.54 | 0.136 ± 0.01 | 1.26 ± 0.6 |
| UUO + A. b 25 mg/kg | 7 | 20.49 ± 1.20 | 0.122 ± 0.008 | 1.06 ± 0.6 |
| Concentration (mM) | Sham | UUO + Vehicle | UUO + A. b 25 mg/kg |
|---|---|---|---|
| Kidney obstructed | |||
| Caffeic acid | 0.006 ± 0.005 | 0.006 ± 0.006 | 0.144 ± 0.013 *** |
| Ferulic acid | 0.076 ± 0.060 | 0.064± 0.011 | 1.119 ± 0132 *** |
| Liver | |||
| Caffeic acid | 0.026 ± 0.006 | 0.031 ± 0.003 | 0.037 ± 0.001 |
| Ferulic acid | 0.391 ± 0.074 | 0.388 ± 0.104 | 0.426 ± 0.099 |
| Urine | |||
| Caffeic acid | 24.912 ± 6.401 | 21.161 ± 6.856 | 96.599 ± 19.704 *** |
| Ferulic acid | 10.376 ± 3.505 | 12.551 ± 3.307 | 38.002 ± 5.021 ** |
| Parameter | Oral Administration (Gavage) |
|---|---|
| Caffeic Acid | |
| AUC (0–360) | 4964 |
| Cmax (μM) | 50.5 |
| tmax (min) | 30 |
| F (%) | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veeren, B.; Bringart, M.; Turpin, C.; Rondeau, P.; Planesse, C.; Ait-Arsa, I.; Gimié, F.; Marodon, C.; Meilhac, O.; Gonthier, M.-P.; et al. Caffeic Acid, One of the Major Phenolic Acids of the Medicinal Plant Antirhea borbonica, Reduces Renal Tubulointerstitial Fibrosis. Biomedicines 2021, 9, 358. https://doi.org/10.3390/biomedicines9040358
Veeren B, Bringart M, Turpin C, Rondeau P, Planesse C, Ait-Arsa I, Gimié F, Marodon C, Meilhac O, Gonthier M-P, et al. Caffeic Acid, One of the Major Phenolic Acids of the Medicinal Plant Antirhea borbonica, Reduces Renal Tubulointerstitial Fibrosis. Biomedicines. 2021; 9(4):358. https://doi.org/10.3390/biomedicines9040358
Chicago/Turabian StyleVeeren, Bryan, Matthieu Bringart, Chloe Turpin, Philippe Rondeau, Cynthia Planesse, Imade Ait-Arsa, Fanny Gimié, Claude Marodon, Olivier Meilhac, Marie-Paule Gonthier, and et al. 2021. "Caffeic Acid, One of the Major Phenolic Acids of the Medicinal Plant Antirhea borbonica, Reduces Renal Tubulointerstitial Fibrosis" Biomedicines 9, no. 4: 358. https://doi.org/10.3390/biomedicines9040358
APA StyleVeeren, B., Bringart, M., Turpin, C., Rondeau, P., Planesse, C., Ait-Arsa, I., Gimié, F., Marodon, C., Meilhac, O., Gonthier, M.-P., Diotel, N., & Bascands, J.-L. (2021). Caffeic Acid, One of the Major Phenolic Acids of the Medicinal Plant Antirhea borbonica, Reduces Renal Tubulointerstitial Fibrosis. Biomedicines, 9(4), 358. https://doi.org/10.3390/biomedicines9040358






