Reinforcement of Colonic Anastomosis with Improved Ultrafine Nanofibrous Patch: Experiment on Pig
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation (Electrospinning Method)
2.2. Material Characterization
2.3. Experimental Design
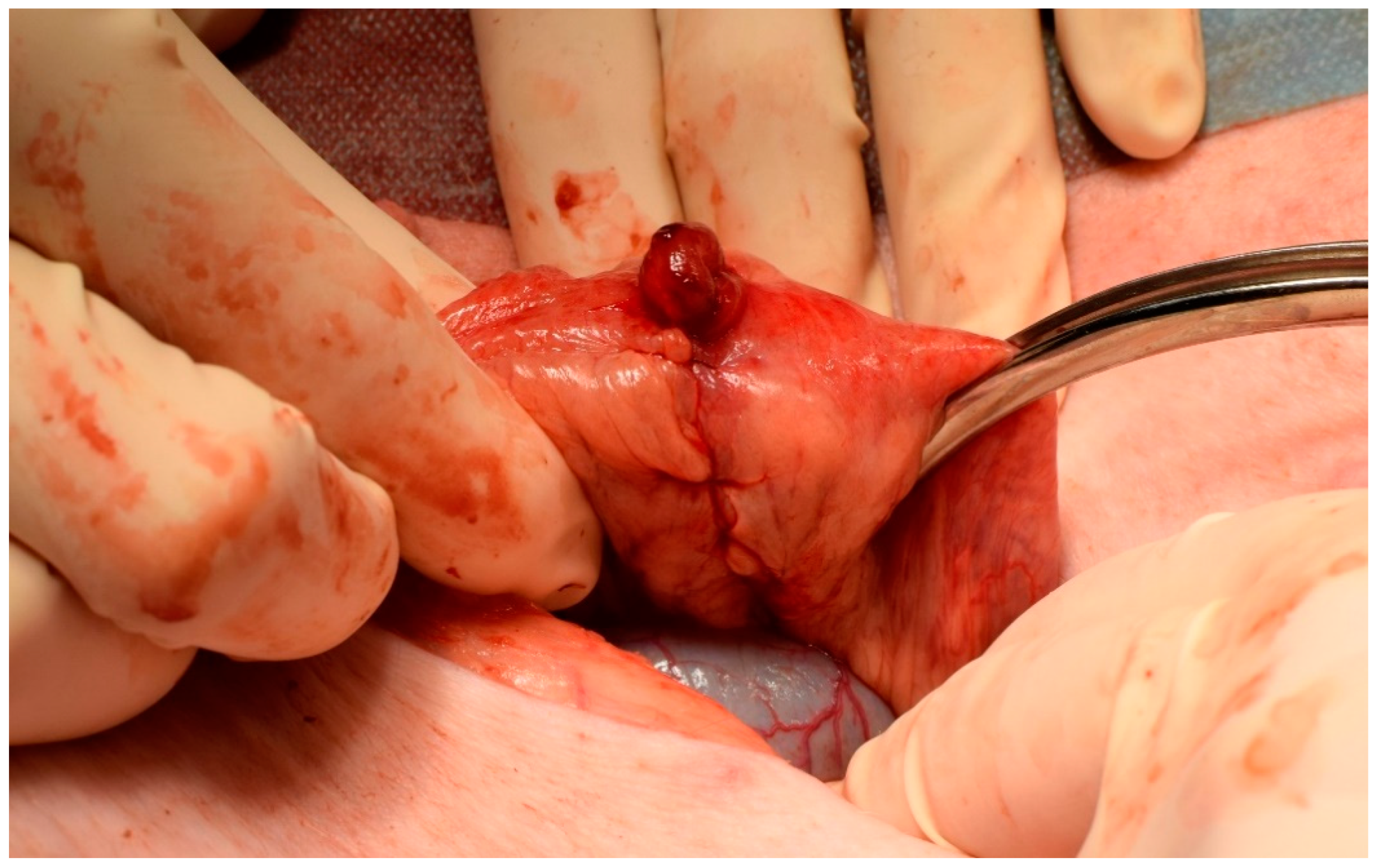
2.4. Surgical Procedure
2.5. Postoperative Observation
2.6. Macroscopic Evaluation
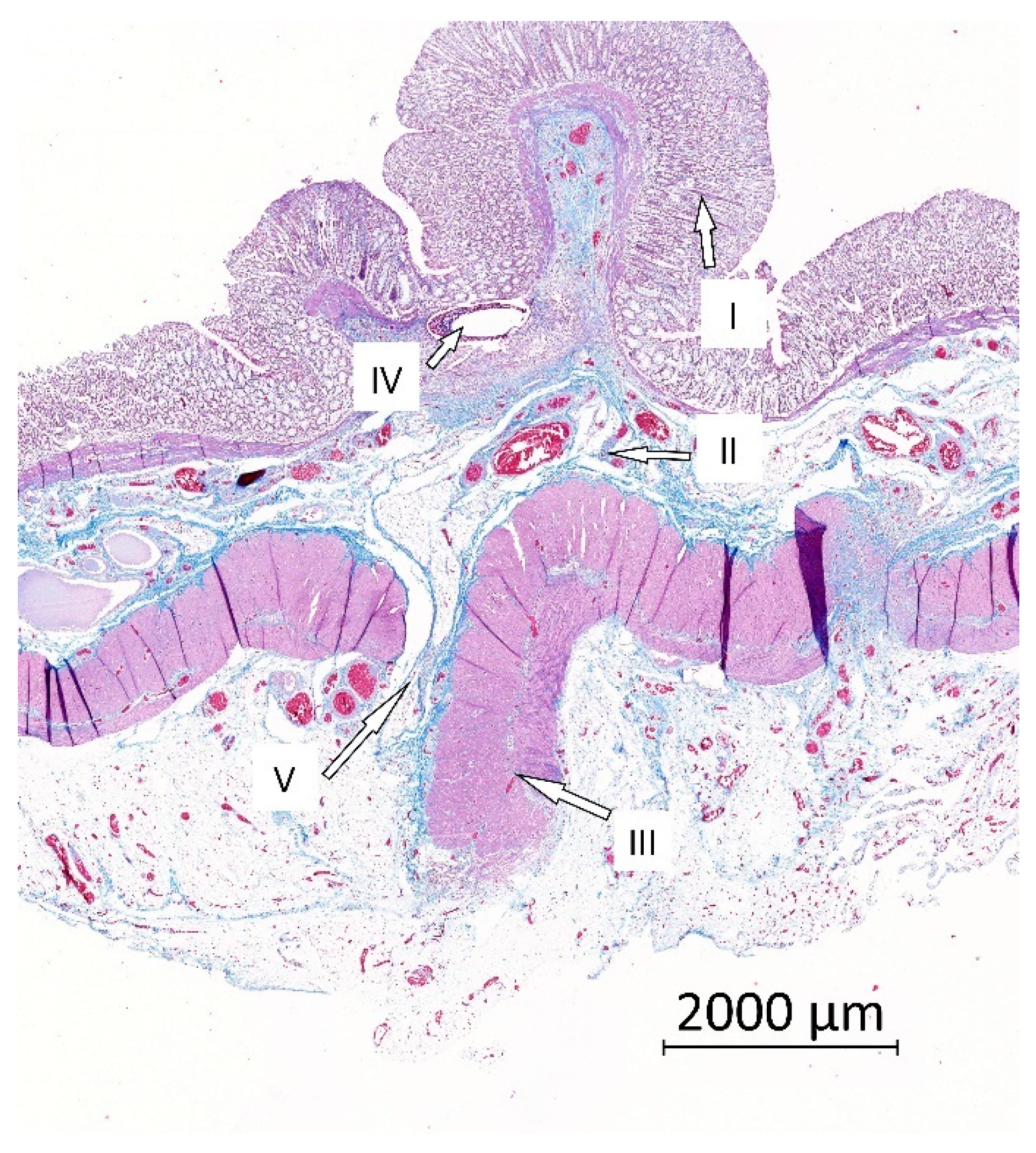
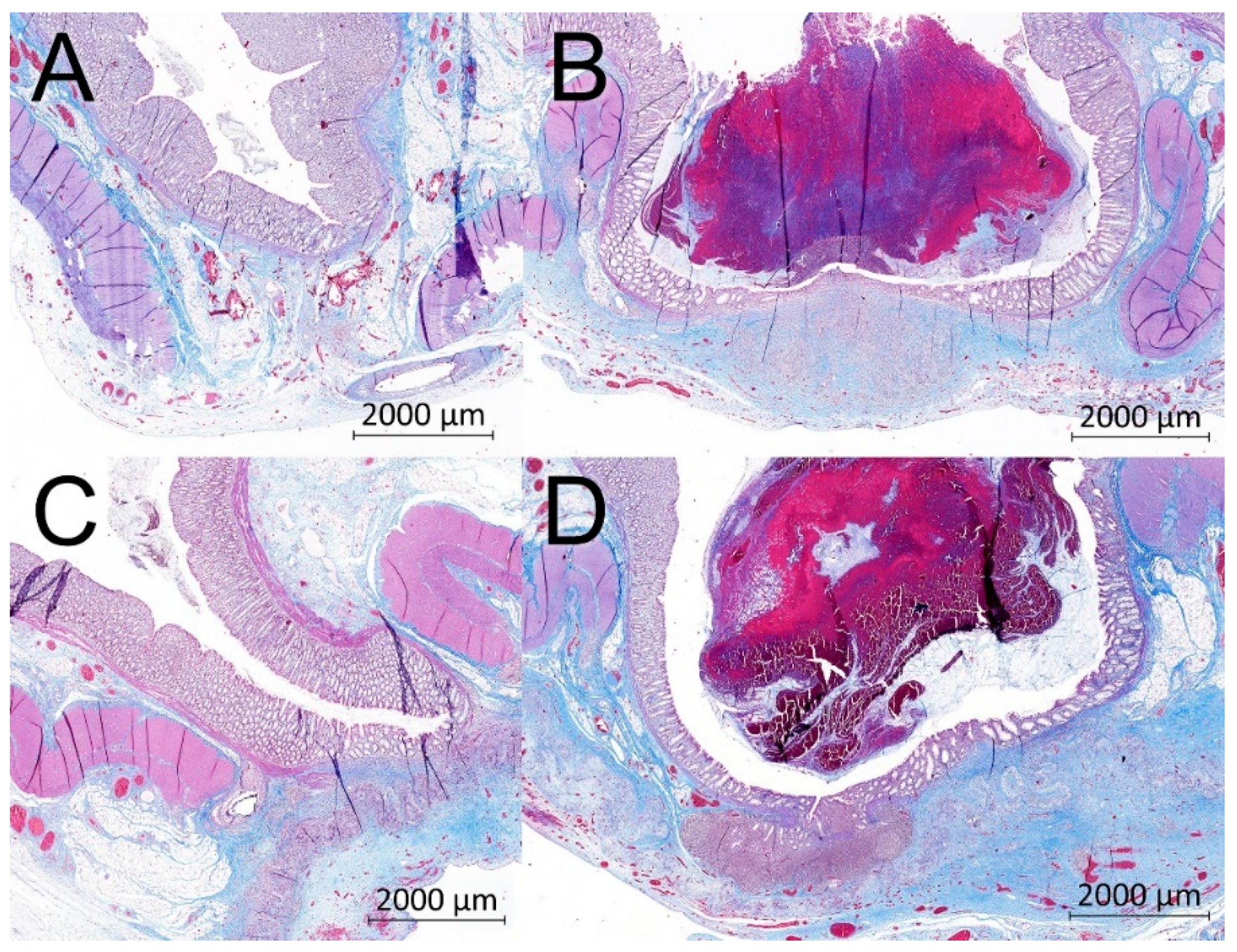
2.7. Histological Evaluation
2.8. Statistics
3. Results
3.1. Material Properties
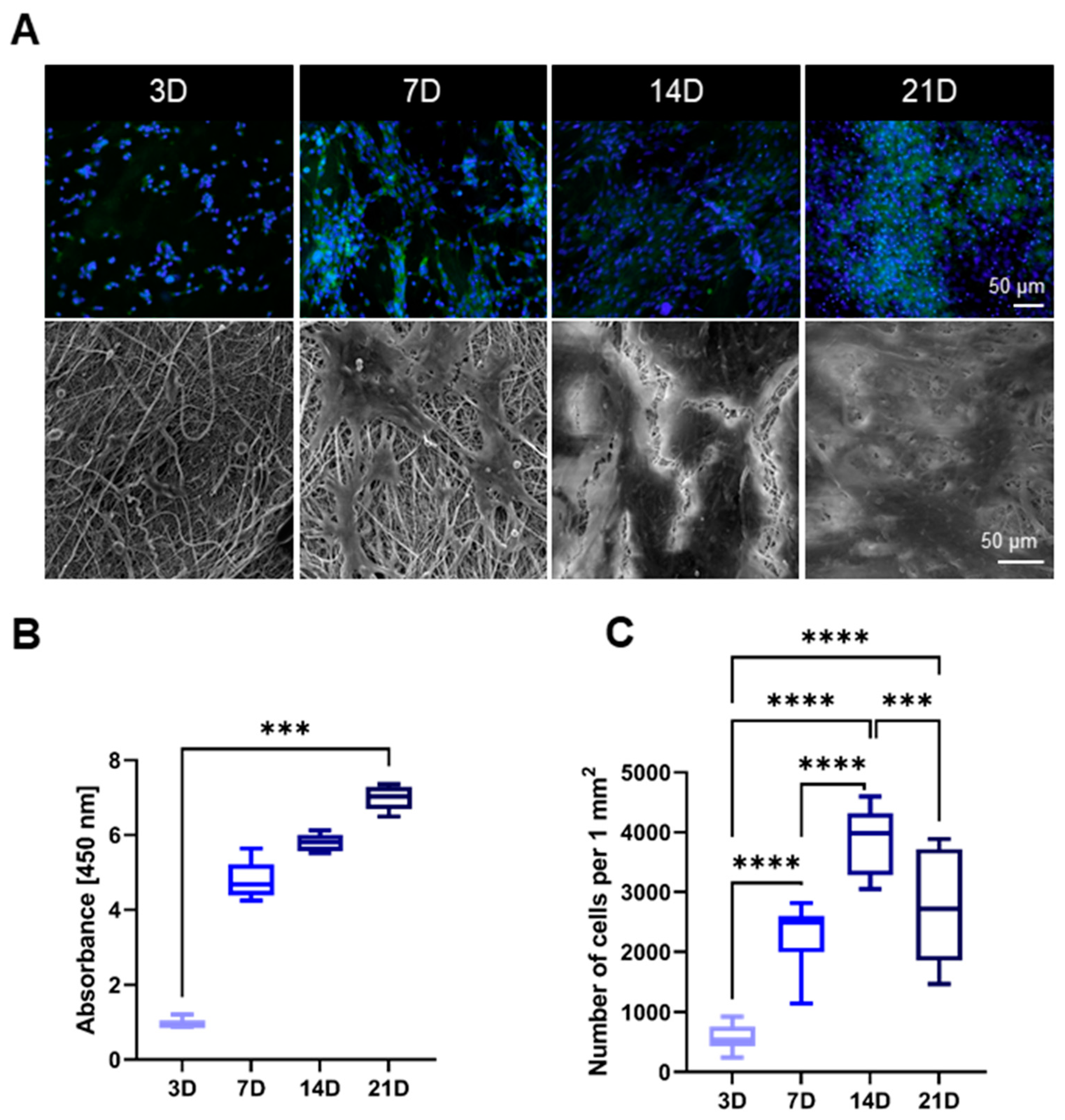
3.2. Cytocompatibility
3.3. Manipulation
3.4. Clinical Results
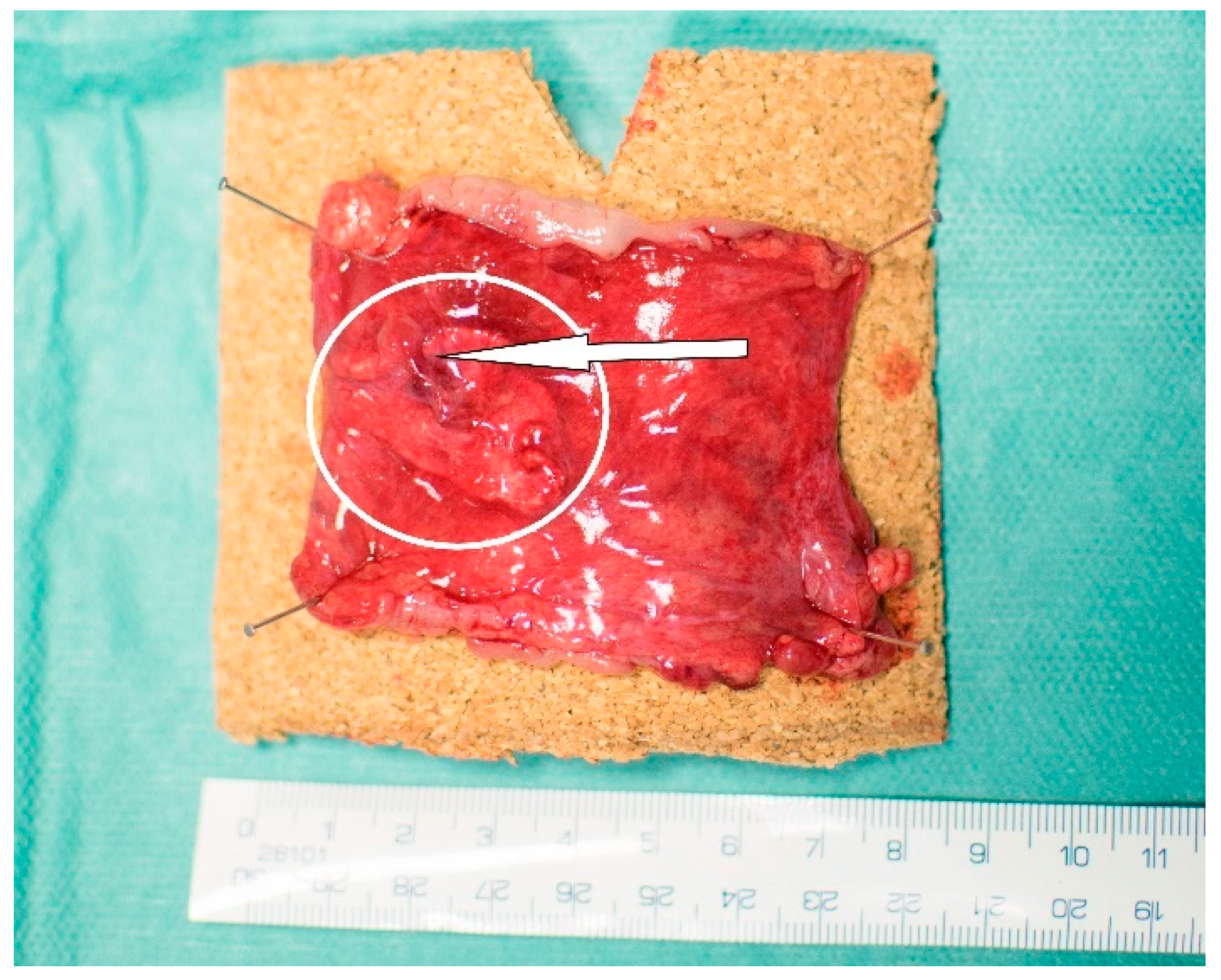
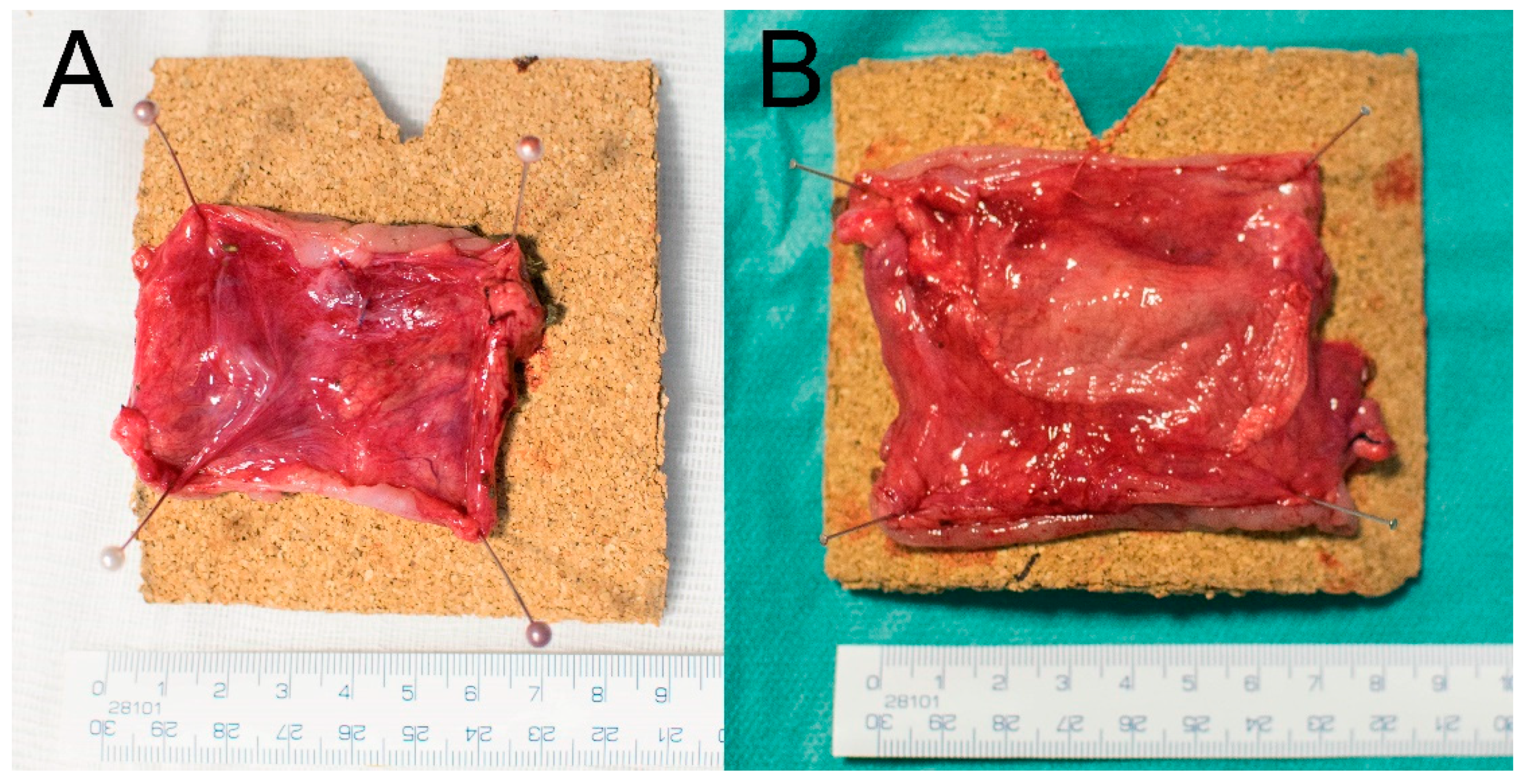
3.5. Macroscopic Results
3.6. Blood Sample Results
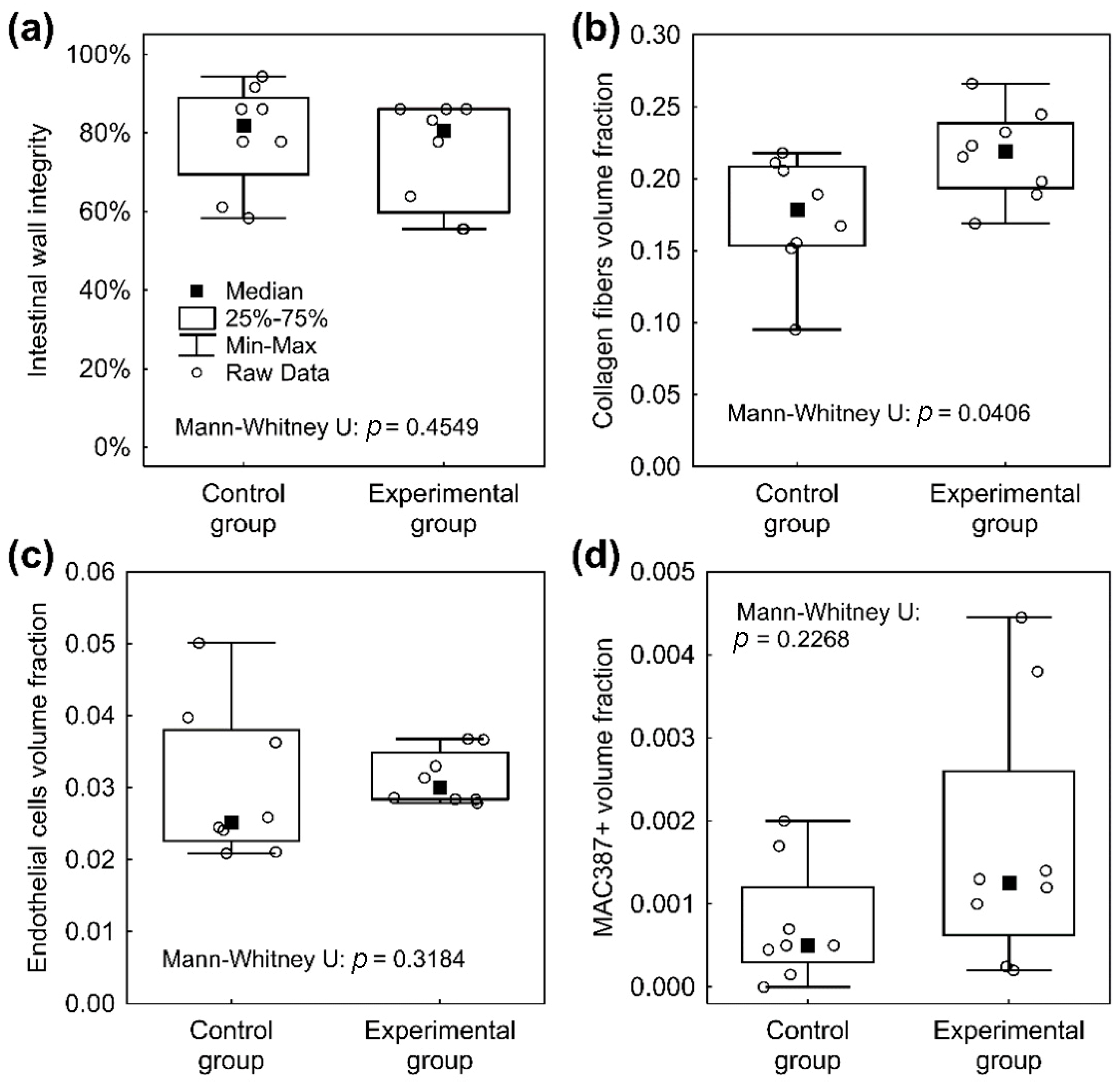
3.7. Histological Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Distance between the electrodes [mm] | 175 |
| Voltage Electrode 1 [kV] | −10 |
| Voltage Electrode 2 [kV] | 40 |
| Rewinding speed [mm/min] | 60 |
| Cartridge movement speed [mm/s] | 450–500 |
| Temperature [°C] | 22 |
| Relative humidity [%] | 50 |
Appendix B
| Layer | Points | Finding |
|---|---|---|
| Mucosa | 1/4 | Completely re-epithelized |
| 0/4 | Incompletely re-epithelized | |
| Submucosa | 1/4 | Completely healed |
| 0/4 | Purulent infiltration, necrosis | |
| Muscularis * | 3/12 | No distance (≤0.09 mm) |
| 2/12 | Distance 0.1 to 1.99 mm | |
| 1/12 | Distance 2 to 3.99 mm | |
| 0/12 | Distance over 4 mm | |
| Serosa | 3/12 | No purulent infiltration and necrosis |
| 2/12 | Purulent infiltration and/or necrosis from the muscular layer to area of nanomaterial ** | |
| 1/12 | Purulent infiltration and/or necrosis from the area of nanomaterial to the peritoneum *** | |
| 0/12 | Purulent infiltration and/or necrosis passes to the peritoneum |
Appendix C
| Animal | POD 0 Weight (kg) | POD 3 Weight (kg) | POD 7 Weight (kg) | POD 14 Weight (kg) | POD 21 Weight (kg) |
|---|---|---|---|---|---|
| Control group | |||||
| cg 01 | 34 | 31.6 | 31.4 | 33.8 | 33 |
| cg 02 | 31 | 30.1 | 29.4 | 30.7 | 30.7 |
| cg 03 | 37 | 33.8 | 33.7 | 34.5 | 34.5 |
| cg 04 | 45.5 | 41.3 | 41.1 | 42 | 42.1 |
| cg 05 | 48.4 | 43.6 | 42.8 | 44 | 43.8 |
| cg 06 | 30.6 | 30 | 30.7 | 29.5 | 32.5 |
| cg 07 | 30 | 29 | 29.8 | 33.6 | 32.1 |
| cg 08 | 27.8 | 25.9 | 27.2 | 30.5 | 30.5 |
| Experimental group | |||||
| eg 01 | 28.7 | 28.3 | 29.2 | 29.4 | 29.4 |
| eg 02 | 29.9 | 29.3 | 28.2 | 29 | 30.3 |
| eg 03 | 35.8 | 35.2 | 35.2 | 39 | 37.9 |
| eg 04 | 37.3 | 36.8 | 36 | 41 | 41 |
| eg 05 | 41.7 | 41.5 | 41.3 | 43.4 | 45.2 |
| eg 06 | 42.9 | 42.2 | 42.2 | 43.8 | 45.1 |
| eg 07 | 27.9 | 27.4 | 26.3 | 29.4 | 30 |
| eg 08 | 27.2 | 26.8 | 25.8 | 29.1 | 29 |
References
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Gessler, B.; Eriksson, O.; Angenete, E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int. J. Color. Dis. 2017, 32, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, E.C.Z.; Zarnescu, N.O.; Costea, R.; Neagu, S. Review of Risk Factors for Anastomotic Leakage in Colorectal Surgery. Chirurgia (Buchar. Rom. 1990) 2015, 110, 26305194. [Google Scholar]
- Iversen, H.; Ahlberg, M.; Lindqvist, M.; Buchli, C. Changes in Clinical Practice Reduce the Rate of Anastomotic Leakage after Colorectal Resections. World J. Surg. 2018, 42, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Kasi, P.M.; Shahjehan, F.; Cochuyt, J.J.; Li, Z.; Colibaseanu, D.T.; Merchea, A. Rising Proportion of Young Individuals with Rectal and Colon Cancer. Clin. Color. Cancer 2019, 18, e87–e95. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-Y.; Chen, W.T.-L. Management of anastomotic leakage after rectal surgery: A review article. J. Gastrointest. Oncol. 2019, 10, 1229–1237. [Google Scholar] [CrossRef]
- Fukada, M.; Matsuhashi, N.; Takahashi, T.; Imai, H.; Tanaka, Y.; Yamaguchi, K.; Yoshida, K. Risk and early predictive factors of anastomotic leakage in laparoscopic low anterior resection for rectal cancer. World J. Surg. Oncol. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Räsänen, M.; Renkonen-Sinisalo, L.; Carpelan-Holmström, M.; Lepistö, A. Low anterior resection combined with a covering stoma in the treatment of rectal cancer reduces the risk of permanent anastomotic failure. Int. J. Color. Dis. 2015, 30, 1323–1328. [Google Scholar] [CrossRef]
- Van Rooijen, S.; Huisman, D.; Stuijvenberg, M.; Stens, J.; Roumen, R.; Daams, F.; Slooter, G. Intraoperative modifiable risk factors of colorectal anastomotic leakage: Why surgeons and anesthesiologists should act together. Int. J. Surg. 2016, 36, 183–200. [Google Scholar] [CrossRef]
- Sciuto, A.; Merola, G.; De Palma, G.D.; Sodo, M.; Pirozzi, F.; Bracale, U. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J. Gastroenterol. 2018, 24, 2247–2260. [Google Scholar] [CrossRef]
- Kawada, K.; Sakai, Y. Preoperative, intraoperative and postoperative risk factors for anastomotic leakage after laparoscopic low anterior resection with double stapling technique anastomosis. World J. Gastroenterol. 2016, 22, 5718–5727. [Google Scholar] [CrossRef] [PubMed]
- La Regina, D.; Di Giuseppe, M.; Lucchelli, M.; Saporito, A.; Boni, L.; Efthymiou, C.; Cafarotti, S.; Marengo, M.; Mongelli, F. Financial Impact of Anastomotic Leakage in Colorectal Surgery. J. Gastrointest. Surg. 2018, 23, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Gregory, D.; Cool, C.L. Clinical and economic burden of colorectal and bariatric anastomotic leaks. Surg. Endosc. 2020, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ha, G.W.; Lee, M.R.; Kim, J.H. Adhesive small bowel obstruction after laparoscopic and open colorectal surgery: A systematic review and meta-analysis. Am. J. Surg. 2016, 212, 527–536. [Google Scholar] [CrossRef]
- Trotter, J.; Onos, L.; McNaught, C.; Peter, M.; Gatt, M.; Maude, K.; MacFie, J. The use of a novel adhesive tissue patch as an aid to anastomotic healing. Ann. R. Coll. Surg. Engl. 2018, 100, 230–234. [Google Scholar] [CrossRef]
- Testini, M.; Gurrado, A.; Portincasa, P.; Scacco, S.; Marzullo, A.; Piccinni, G.; Lissidini, G.; Greco, L.; De Salvia, M.A.; Bonfrate, L.; et al. Bovine Pericardium Patch Wrapping Intestinal Anastomosis Improves Healing Process and Prevents Leakage in a Pig Model. PLoS ONE 2014, 9, e86627. [Google Scholar] [CrossRef][Green Version]
- Yaita, A.; Nakamura, T.; Sugimachi, K.; Inokuchi, K. Use of free peritoneal patch in reenforcing alimentary tract anastomosis. Surg. Today 1975, 5, 56–63. [Google Scholar] [CrossRef]
- Zhong, W.; Xing, M.M.; Maibach, H.I. Nanofibrous materials for wound care. Cutan. Ocul. Toxicol. 2010, 29, 143–152. [Google Scholar] [CrossRef]
- Fu, X.; Gao, W.; Fu, X.; Shi, M.; Xie, W.; Zhang, W.; Zhao, F.; Chen, X. Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the basketweave pattern of collagen fibrils in native skin. Biomater. Sci. 2018, 6, 340–349. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Gunatillake, P.A. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; He, Y.; Chang, Q.; Xie, G.; Zhan, W.; Wang, X.; Zhou, T.; Xing, M.; Lu, F. Polycaprolactone nanofibrous mesh reduces foreign body reaction and induces adipose flap expansion in tissue engineering chamber. Int. J. Nanomed. 2016, 11, 6471–6483. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.M.; Ott, L.M.; Salash, J.R.; Fung, K.-M.; Easley, J.T.; Seim, H.B.; Johnson, J.K.; Weatherly, R.A.; Detamore, M.S. Reinforced Electrospun Polycaprolactone Nanofibers for Tracheal Repair in an In Vivo Ovine Model. Tissue Eng. Part A 2018, 24, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Mueller, M.; Daxböck, C.; Stückler, M.; Lang, I.; Leitinger, G.; Bock, E.; El-Heliebi, A.; Moser, G.; Glasmacher, B.; et al. Histological processing of un-/cellularized thermosensitive electrospun scaffolds. Histochem. Cell Biol. 2018, 151, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Rosendorf, J.; Horakova, J.; Klicova, M.; Palek, R.; Cervenkova, L.; Kural, T.; Hošek, P.; Kriz, T.; Tegl, V.; Moulisova, V.; et al. Experimental fortification of intestinal anastomoses with nanofibrous materials in a large animal model. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Horakova, J.; Klicova, M.; Erben, J.; Klapstova, A.; Novotny, V.; Behalek, L.; Chvojka, J. Impact of Various Sterilization and Disinfection Techniques on Electrospun Poly-ε-caprolactone. ACS Omega 2020, 5, 8885–8892. [Google Scholar] [CrossRef]
- Childs, D.R.; Murthy, A.S. Overview of Wound Healing and Management. Surg. Clin. N. Am. 2017, 97, 189–207. [Google Scholar] [CrossRef]
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The Current State of Peritoneal Dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef]
- Giffin, D.M.; Gow, K.W.; Warriner, C.B.; Walley, K.R.; Phang, P.T. Oxygen uptake during peritoneal ventilation in a porcine model of hypoxemia. Crit. Care Med. 1998, 26, 1564–1568. [Google Scholar] [CrossRef]
- Cai, E.Z.; Teo, E.Y.; Jing, L.; Koh, Y.P.; Qian, T.S.; Wen, F.; Lee, J.W.K.; Hing, E.C.H.; Yap, Y.L.; Lee, H.; et al. Bio-Conjugated Polycaprolactone Membranes: A Novel Wound Dressing. Arch. Plast. Surg. 2014, 41, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Asgari, S.; Shahhoseini, S.; Mahbod, M.; Atyabi, F.; Bakhshandeh, H.; Beheshtnejad, A.H. Application of polycaprolactone nanofibers as patch graft in ophthalmology. Indian J. Ophthalmol. 2018, 66, 225–228. [Google Scholar]
- García-Salinas, S.; Evangelopoulos, M.; Gámez-Herrera, E.; Arruebo, M.; Irusta, S.; Taraballi, F.; Mendoza, G.; Tasciotti, E. Electrospun anti-inflammatory patch loaded with essential oils for wound healing. Int. J. Pharm. 2020, 577, 119067. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, R.; Roberts, P.L.; Marcello, P.W.; Hall, J.F.; Read, T.E.; Schoetz, D.J. Anastomotic Leak Testing After Colorectal Resection. Arch. Surg. 2009, 144, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Bsc, C.L.S.; Van Groningen, J.T.; Lingsma, H.; Wouters, M.W.; Menon, A.G.; Kleinrensink, G.-J.; Jeekel, J.; Lange, J.F. Different Risk Factors for Early and Late Colorectal Anastomotic Leakage in a Nationwide Audit. Dis. Colon Rectum 2018, 61, 1258–1266. [Google Scholar] [CrossRef]
- Marchant-Forde, J.N.; Herskin, M.S. Pigs as laboratory animals. In Advances in Pig Welfare; Elsevier: Amsterdam, The Netherlands, 2018; pp. 445–475. [Google Scholar]
- Bertocchi, E.; Barugola, G.; Benini, M.; Bocus, P.; Rossini, R.; Ceccaroni, M.; Ruffo, G. Colorectal Anastomotic Stenosis: Lessons Learned after 1643 Colorectal Resections for Deep Infiltrating Endometriosis. J. Minim. Invasive Gynecol. 2019, 26, 100–104. [Google Scholar] [CrossRef]
- Ergul, E.; Korukluoglu, B. Peritoneal adhesions: Facing the enemy. Int. J. Surg. 2008, 6, 253–260. [Google Scholar] [CrossRef]
- Braun, K.M.; Diamond, M.P. The biology of adhesion formation in the peritoneal cavity. Semin. Pediatr. Surg. 2014, 23, 336–343. [Google Scholar] [CrossRef]
- Williams, D.L.; Browder, I.W. Murine Models of Intestinal Anastomoses. In Wound Healing: Methods and Protocols, 1st ed.; Di Pietro, L.A., Burns, A.L., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2010; pp. 133–140. [Google Scholar]
- Shogan, B.D.; Belogortseva, N.; Luong, P.M.; Zaborin, A.; Lax, S.; Bethel, C.; Ward, M.; Muldoon, J.P.; Singer, M.; Alexander, Z.; et al. Collagen degradation and MMP9 activation byEnterococcus faecaliscontribute to intestinal anastomotic leak. Sci. Transl. Med. 2015, 7, 286ra68. [Google Scholar] [CrossRef]
- Krarup, P.; Eld, M.; Jorgensen, L.; Hansen, M.B.; Ågren, M.S. Selective matrix metalloproteinase inhibition increases breaking strength and reduces anastomotic leakage in experimentally obstructed colon. Int. J. Color. Dis. 2017, 32, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.L.; Levine, Z.C.; Lowry, A.C.; Lambert, L.; Gribovskaja-Rupp, I.; Hyman, N.; Zaborina, O.; Alverdy, J.C. Identification of Collagenolytic Bacteria in Human Samples. Dis. Colon Rectum 2019, 62, 972–979. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.; Zou, Q.; Ma, J.; Jin, S.; Li, J.; Zuo, Y.; Li, Y. The long-term behaviors and differences in bone reconstruction of three polymer-based scaffolds with different degradability. J. Mater. Chem. B 2019, 7, 7690–7703. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun curcumin loaded poly(ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef]
- Wirth, U.; Rogers, S.; Haubensak, K.; Schopf, S.; Von Ahnen, T.; Schardey, H.M. Local antibiotic decontamination to prevent anastomotic leakage short-term outcome in rectal cancer surgery. Int. J. Color. Dis. 2017, 33, 53–60. [Google Scholar] [CrossRef]
- Oh, J.; Kuan, K.G.; Tiong, L.U.; Trochsler, M.; Jay, G.; Schmidt, T.A.; Barnett, H.; Maddern, G.J. Recombinant human lubricin for prevention of postoperative intra-abdominal adhesions in a rat model. J. Surg. Res. 2017, 208, 20–25. [Google Scholar] [CrossRef]
- Hirai, K.; Tabata, Y.; Hasegawa, S.; Sakai, Y. Enhanced intestinal anastomotic healing with gelatin hydrogel incorporating basic fibroblast growth factor. J. Tissue Eng. Regen. Med. 2016, 10, E433–E442. [Google Scholar] [CrossRef]
- Landes, L.C.; Drescher, D.; Tagkalos, E.; Grimminger, P.; Thieme, R.; Jansen-Winkeln, B.; Lang, H.; Gockel, I. Upregulation of VEGFR1 in a rat model of esophagogastric anastomotic healing. Acta Chir. Belg. 2017, 118, 161–166. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosendorf, J.; Klicova, M.; Cervenkova, L.; Horakova, J.; Klapstova, A.; Hosek, P.; Palek, R.; Sevcik, J.; Polak, R.; Treska, V.; et al. Reinforcement of Colonic Anastomosis with Improved Ultrafine Nanofibrous Patch: Experiment on Pig. Biomedicines 2021, 9, 102. https://doi.org/10.3390/biomedicines9020102
Rosendorf J, Klicova M, Cervenkova L, Horakova J, Klapstova A, Hosek P, Palek R, Sevcik J, Polak R, Treska V, et al. Reinforcement of Colonic Anastomosis with Improved Ultrafine Nanofibrous Patch: Experiment on Pig. Biomedicines. 2021; 9(2):102. https://doi.org/10.3390/biomedicines9020102
Chicago/Turabian StyleRosendorf, Jachym, Marketa Klicova, Lenka Cervenkova, Jana Horakova, Andrea Klapstova, Petr Hosek, Richard Palek, Jan Sevcik, Robert Polak, Vladislav Treska, and et al. 2021. "Reinforcement of Colonic Anastomosis with Improved Ultrafine Nanofibrous Patch: Experiment on Pig" Biomedicines 9, no. 2: 102. https://doi.org/10.3390/biomedicines9020102
APA StyleRosendorf, J., Klicova, M., Cervenkova, L., Horakova, J., Klapstova, A., Hosek, P., Palek, R., Sevcik, J., Polak, R., Treska, V., Chvojka, J., & Liska, V. (2021). Reinforcement of Colonic Anastomosis with Improved Ultrafine Nanofibrous Patch: Experiment on Pig. Biomedicines, 9(2), 102. https://doi.org/10.3390/biomedicines9020102






