Evaluation of the Antioxidant, Antidiabetic, and Antiplasmodial Activities of Xanthones Isolated from Garcinia forbesii and Their In Silico Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experiment Procedures
2.2. Plant Material
2.3. Extraction and Isolation
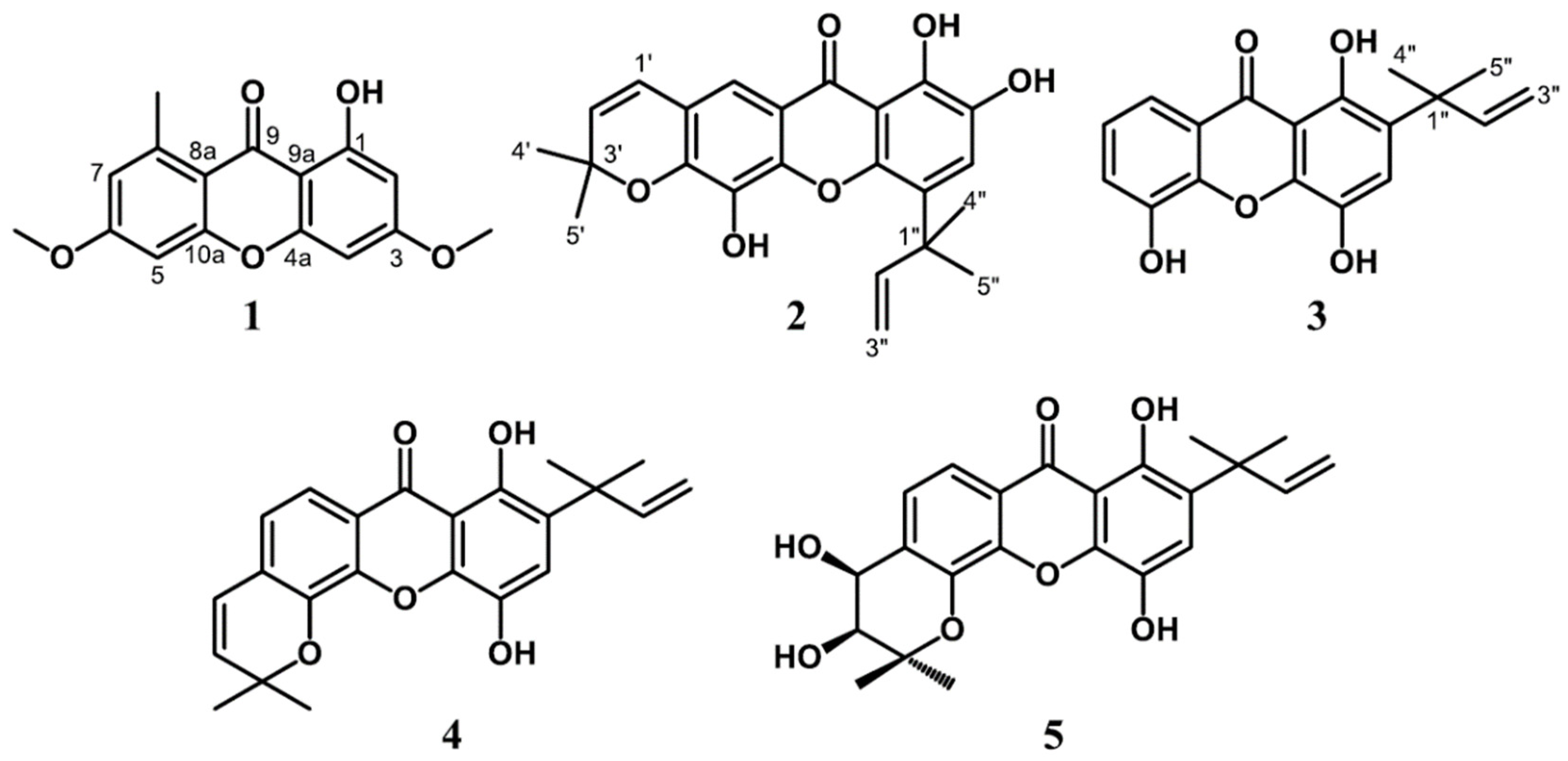
2.3.1. Lichexanthone (1)
2.3.2. Subelliptenone H (2)
2.3.3. 12b-Hydroxy-des-D-garcigerrin A (3)
2.3.4. Garciniaxanthone B (4)
2.3.5. Garcigerin A (5)
2.4. Antioxidant Assay
2.4.1. DPPH Radical Scavenging Assay
2.4.2. ABTS Radical Scavenging Assay
2.4.3. Ferric Reducing-Antioxidant Power (FRAP) Assay
2.5. In Vitro Antidiabetic Assay
2.5.1. Rat Intestinal α-glucosidase Inhibitory Activity
2.5.2. α-Amylase Inhibitory Activity
2.6. In Vitro Antiplasmodial Assay
2.7. In Silico Molecular Docking Studies and ADMET Prediction
2.8. Statistical Analysis
3. Results and Discussion
3.1. Structure Elucidation
3.2. Antioxidant Activity
3.3. In Vitro Antidiabetic Assay
3.4. In Vitro Antiplasmodial Assay
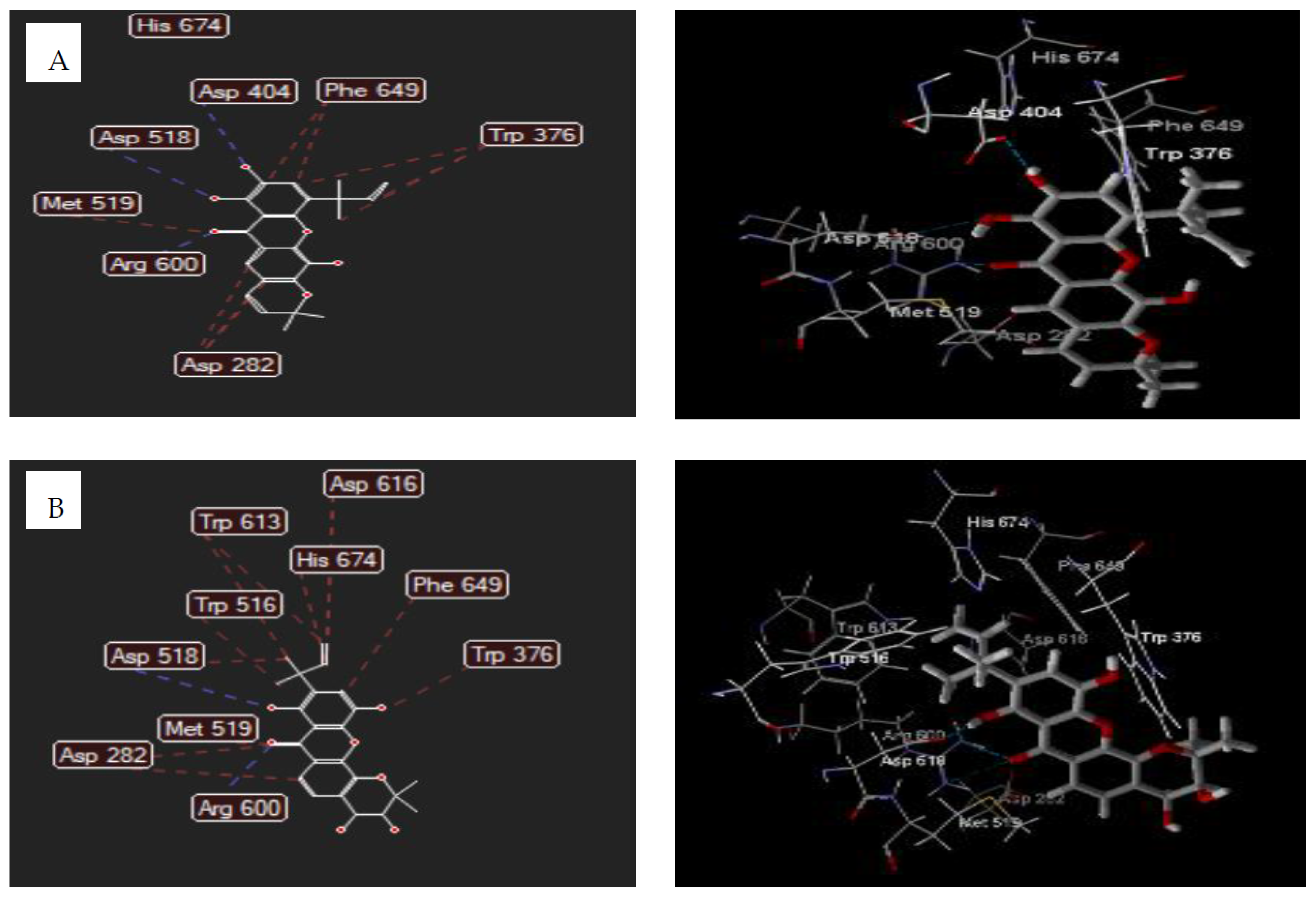
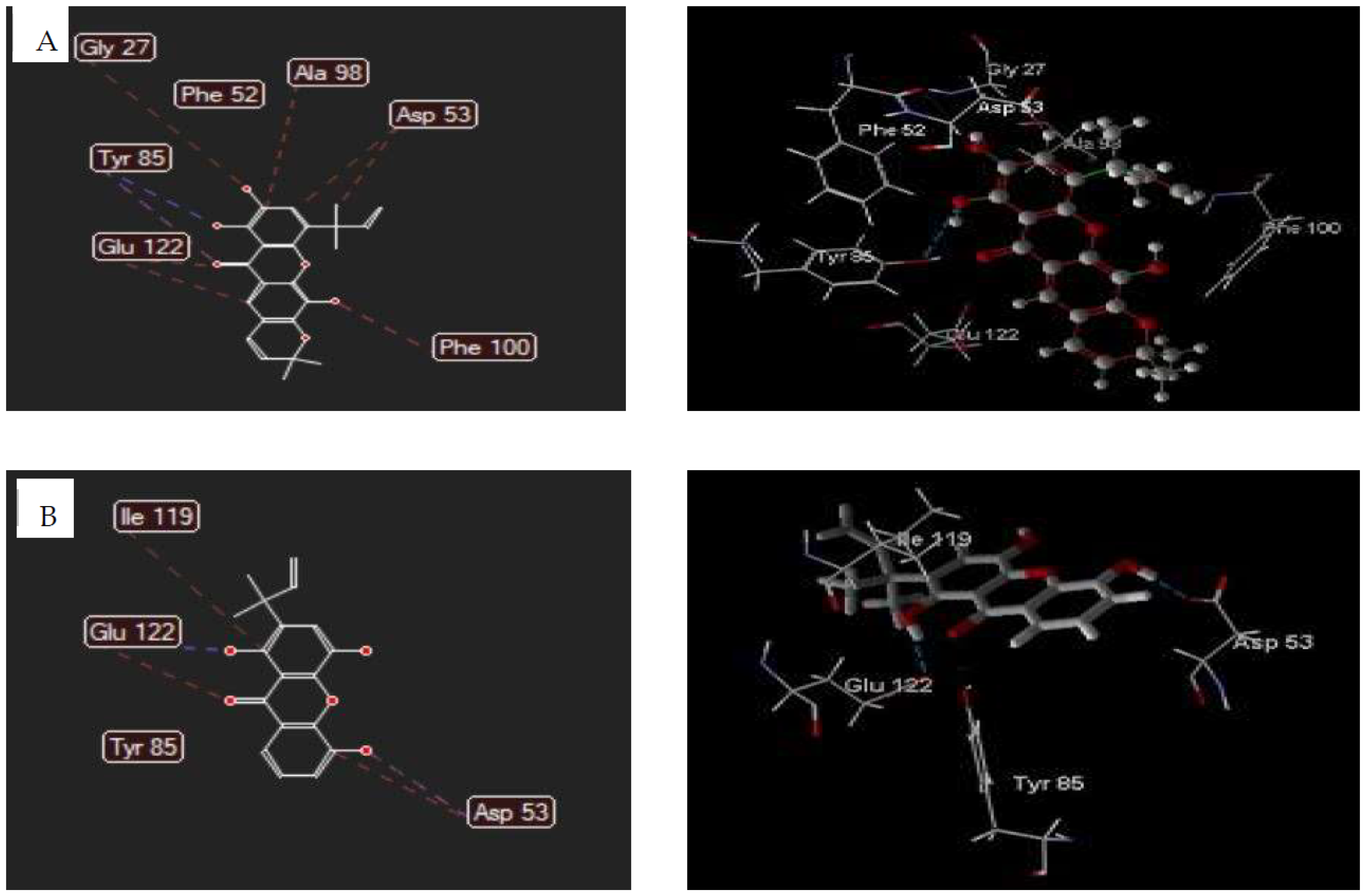
3.5. Molecular Docking Studies
3.5.1. Human Lysosomal Acid-α-glucosidase Enzyme (PDB ID: 5NN8)
3.5.2. Plasmodium falciparum Lactate Dehydrogenase Enzyme (PDB ID: 1CET)
3.5.3. ADMET Profiles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DPPH | 2,2-Diphenyl-2-picrylhydrazyl |
| ABTS | 2,2-azinobis-3-ethylbenzothia-zoline-6-sulfonic acid |
| FRAP | ferric reducing-antioxidant power |
| ADMET | absorption distribution metabolism excretion toxicity |
| TPTZ | 2,4,6 tris(2-pyridyl)-s-triazine |
| MDS | mol dock score |
| pkCSM | small-molecule pharmacokinetics prediction |
| PDB | protein data bank |
References
- Perry, L.M.; Metzger, J. Attributed properties and uses. In Medicinal Plants of East and Southeast Asia; MIT Press: Cambridge, MA, USA, 1980; 620p. [Google Scholar]
- Aravind, A.P.A.; Menon, L.N.; Rameshkumar, K.B. Structural diversity of secondary metabolites in Garcinia species. In Diversity of Garcinia Spesies in the Western Ghats: Phytochemical Perspective; Ramesshkumar, K.B., Ed.; JNTBGRI: Kerala, India, 2016; pp. 19–75. [Google Scholar]
- Subhashini, N.; Nagarajan, G.; Kavimani, S. In vitro antioxidant and anticholinesterase activities of G. combogia. Int. J. Pharm. Pharm. Sci. 2011, 3, 129–132. [Google Scholar]
- Meng, F.; Hui-Jin, F.; Yu, C.; De-Bin, W.; Guang-Zhong, Y. Antioxidant activity of Garcinia xanthochymus leaf, root and fruit extracts in vitro. Chin. J. Nat. Med. 2012, 10, 129–134. [Google Scholar]
- Hay, A.-E.; Hélesbeux, J.-J.; Duval, O.; Labaïed, M.; Grellier, P.; Richomme, P. Antimalarial xanthones from Calophyllum caledonicum and Garcinia vieillardii. Life Sci. 2004, 75, 3077–3085. [Google Scholar] [CrossRef] [Green Version]
- Monzote, L.; Cuesta-Rubio, O.; Matheeussen, A.; Van Assche, T.; Maes, L.; Cos, P. Antimicrobial Evaluation of the Polyisoprenylated Benzophenones Nemorosone and Guttiferone A: Antimicrobial evaluation of polyisoprenylated benzophenones. Phytother. Res. 2011, 25, 458–462. [Google Scholar] [CrossRef] [Green Version]
- Auranwiwat, C.; Laphookhieo, S.; Rattanajak, R.; Kamchonwongpaisan, S.; Pyne, S.G.; Ritthiwigrom, T. Antimalarial polyoxygenated and prenylated xanthones from the leaves and branches of Garcinia mckeaniana. Tetrahedron 2016, 72, 6837–6842. [Google Scholar] [CrossRef] [Green Version]
- Trinh, B.T.D.; Quach, T.T.T.; Bui, D.N.; Staerk, D.; Nguyen, L.-H.D.; Jäger, A.K. Xanthones from the twigs of Garcinia oblongifolia and their antidiabetic activity. Fitoterapia 2017, 118, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.N.; Trinh, B.T.D.; Tran, T.B.; Nguyen, L.-T.T.; Jäger, A.K.; Nguyen, L.-H.D. Anti-diabetic xanthones from the bark of Garcinia xanthochymus. Bioorg. Med. Chem. Lett. 2017, 27, 3301–3304. [Google Scholar] [CrossRef] [PubMed]
- Noor, O.D.A.; Senin, T.D. Mundar (Garcinia forbesii) Si Manggis Merah Sumber Daya Genetik Kalimantan Selatan. 2017. Available online: http://kalsel.litbang.pertanian.go.id/ (accessed on 13 September 2021).
- Harrison, L.J.; Leong, L.-S.; Sia, G.-L.; Sim, K.-Y.; Tan, H.T.-W. Xanthones from Garcinia forbesii. Phytochemistry 1993, 33, 727–728. [Google Scholar] [CrossRef]
- Arteaga-Crespo, Y.; Radice, M.; Bravo-Sanchez, L.R.; García-Quintana, Y.; Scalvenzi, L. Optimisation of ultrasound-assisted extraction of phenolic antioxidants from Ilex guayusa Loes. leaves using response surface methodology. Heliyon 2020, 6, e03043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szydłowska-Czerniak, A.; Łaszewska, A. Effect of refining process on antioxidant capacity, total phenolics and prooxidants contents in rapeseed oils. LWT Food Sci. Technol. 2015, 64, 853–859. [Google Scholar] [CrossRef]
- Worawalai, W.; Rattanangkool, E.; Vanitcha, A.; Phuwapraisirisan, P.; Wacharasindhu, S. Concise synthesis of (+)-conduritol F and inositol analogues from naturally available (+)-proto-quercitol and their glucosidase inhibitory activity. Bioorg. Med. Chem. Lett. 2012, 22, 1538–1540. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Otunola, G.A.; Afolayan, A.J. In vitro α-amylase, α-glucosidase, lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana (L.) Cogn. Heliyon 2018, 4, e00810. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Miyazaki, Y.; Inaoka, D.K.; Hartuti, E.D.; Watanabe, Y.-I.; Shiba, T.; Harada, S.; Saimoto, H.; Burrows, J.N.; Benito, F.J.G.; et al. Identification of Plasmodium falciparum Mitochondrial Malate: Quinone Oxidoreductase Inhibitors from the Pathogen Box. Genes 2019, 10, 471. [Google Scholar] [CrossRef] [Green Version]
- Rossignol, F.; Solares, M.; Balanza, E.; Coudert, J.; Clottes, E. Expression of lactate dehydrogenase A and B genes in different tissues of rats adapted to chronic hypobaric hypoxia. J. Cell. Biochem. 2003, 89, 67–79. [Google Scholar] [CrossRef]
- Díaz, A.B.; Vera, J.R.; Cote, V.; Bruno-Colmenárez, J.; de Delgado, G.D. NMR elucidation and crystal structure analysis of 1-hydroxy-3,6- dimethoxy-8-methyl-9h-xanthen-9-one (lichexanthone) isolated from Vismia baccifera (Guttiferae). Bol. Latinoam. Caribe Plantas Med. Aromát. 2010, 9, 470–474. [Google Scholar]
- Masters, K.-S.; Bras, S. Xanthones from Fungi, Lichens, and Bacteria: The Natural Products and Their Synthesis. Chem. Rev. 2012, 112, 3717–3776. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, M.; Tosa, H.; Tanaka, T.; Asai, F.; Shimano, R. Two xanthones with a 1,1-dimethylallyl group in root bark of Garcinia subelliptica. Phytochemistry 1995, 39, 945–947. [Google Scholar] [CrossRef]
- Zhong, F.; Chen, Y.; Wang, P.; Feng, H.; Yang, G. Xanthones from the Bark of Garcinia xanthochymus and their 1,1-Diphenyl-2-picrylhydrazyl Radical-Scavenging Activity. Chin. J. Chem. 2009, 27, 74–80. [Google Scholar] [CrossRef]
- Thepthong, P.; Phongpaichit, S.; Carroll, A.R.; Voravuthikunchai, S.P.; Mahabusarakam, W. Prenylated xanthones from the stem bark of Garcinia dulcis. Phytochem. Lett. 2017, 21, 32–37. [Google Scholar] [CrossRef]
- Kamiyama, A.; Mima, Y.; Kodama, M. Prenylated Xanthones From Garcinia Subelliptica. Phytochemistry 1991, 30, 3433–3436. [Google Scholar]
- Sordat-Diserens, I.; Marston, A.; Hamburger, M.; Hostettmann, K.; Rogers, C. Novel Prenylated Xanthones from Garcinia gerrardii HARVEY. Helv. Chim. Acta 1989, 72, 1001–1007. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Lulan, T.Y.K.; Fatmawati, S.; Santoso, M.; Ersam, T. α-VINIFERIN as a potential antidiabetic and antiplasmodial extracted from Dipterocarpus littoralis. Heliyon 2020, 6, e04102. [Google Scholar] [CrossRef]
- Kainama, H.; Fatmawati, S.; Santoso, M.; Papilaya, P.M.; Ersam, T. The Relationship of Free Radical Scavenging and Total Phenolic and Flavonoid Contents of Garcinia lasoar PAM. Pharm. Chem. J. 2020, 53, 1151–1157. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Nguyen, V.T.; Le, H.T.; Nguyen, H.M.; Tran, T.H.; Do Thi, T.; Nguyen, X.C.; Ha, M.T. Garcinoxanthones S–V, new xanthone derivatives from the pericarps of Garcinia mangostana together with their cytotoxic and antioxidant activities. Fitoterapia 2021, 151, 104880. [Google Scholar] [CrossRef] [PubMed]
- De Mello, R.F.A.; de Souza Pinheiro, W.B.; Benjamim, J.K.F.; de Siqueira, F.C.; Chisté, R.C.; Santos, A.S. A fast and efficient preparative method for separation and purification of main bioactive xanthones from the waste of Garcinia mangostana L. by high-speed countercurrent chromatography. Arab. J. Chem. 2021, 14, 103252. [Google Scholar] [CrossRef]
- Francik, R.; Szkaradek, N.; Zelaszczyk, D.; Marona, H. Antioxidant activity of xanthone derivatives. Acta Pol. Pharm. Drug Res. 2016, 73, 1505–1509. [Google Scholar]
- Abbas, G.; Al-Harrasi, A.S.; Hussain, H. α-Glucosidase Enzyme Inhibitors from Natural Products. In Discovery and Development of Antidiabetic Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 251–269. [Google Scholar] [CrossRef]
- Nichols, B.L.; Baker, S.S.; Quezada-Calvillo, R. Metabolic Impacts of Maltase Deficiencies. J. Pediatr. Gastroenterol. Nutr. 2018, 66, S24–S29. [Google Scholar] [CrossRef]
- Tysoe, C.; Williams, L.K.; Keyzers, R.; Nguyen, N.T.; Tarling, C.; Wicki, J.; Goddard-Borger, E.D.; Aguda, A.H.; Perry, S.; Foster, L.J.; et al. Potent Human α-Amylase Inhibition by the β-Defensin-like Protein Helianthamide. ACS Cent. Sci. 2016, 2, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.A. Amylase. Pancreapedia Exocrine Pancreas Knowledge Base; APA: Los Angeles, CA, USA, 2017. [Google Scholar] [CrossRef]
- Tahghighi, A.; Mehrizi, A.; Zakeri, S. In vitro anti-plasmodial activity of new synthetic derivatives of 1-(heteroaryl)-2- ((5-nitroheteroaryl) methylene) hydrazine. Asian Pac. J. Trop. Med. 2021, 14, 128–138. [Google Scholar]
- Malebo, H.M.; D’Alessandro, S.; Ebstie, Y.A.; Sorè, H.; Tenoh Guedoung, A.R.; Katani, S.J.; Parapini, S.; Taramelli, D.; Habluetzel, A. In vitro Multistage Malaria Transmission Blocking Activity of Selected Malaria Box Compounds. Drug Des. Dev. Ther. 2020, 14, 1593–1607. [Google Scholar] [CrossRef]
- Feroz Khan, F.; Alam, S. QSAR and docking studies on xanthone derivatives for anticancer activity targeting DNA topoisomerase IIα. Drug Des. Dev. Ther. 2014, 8, 183–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekowati, J.; Diyah, N.W.; Nofianti, K.A.; Hamid, I.S. Molecular Docking of Ferulic Acid Derivatives on P2Y12 Receptor and their ADMET Prediction. J. Math. Fundam. Sci. 2018, 50, 203–219. [Google Scholar] [CrossRef]
- Angelis, I.D.; Turco, L. Caco-2 Cells as a Model for Intestinal Absorption. Curr. Protoc. Toxicol. 2011, 47, 20–26. [Google Scholar] [CrossRef]
- Fagerholm, U. Prediction of human pharmacokinetics gastrointestinal absorption. J. Pharm. Pharmacol. 2006, 59, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Nau, R.; Sorgel, F.; Eiffert, H. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood-Brain Barrier for Treatment of Central Nervous System Infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef] [Green Version]
- Backman, J.T.; Filppula, A.M.; Niemi, M.; Neuvonen, P.J. Role of Cytochrome P450 2C8 in Drug Metabolism and Interactions. Pharmacol. Rev. 2016, 68, 168–241. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Villa, F.X.; Durán-Iturbide, N.A.; Ávila-Zárraga, J.G. Synthesis, molecular docking, and in silico ADME/Tox profiling studies of new 1-aryl-5-(3-azidopropyl) indol-4-ones: Potential inhibitors of SARS CoV-2 main protease. Bioorg. Chem. 2021, 106, 104497. [Google Scholar] [CrossRef] [PubMed]
| Position | 1 (CDCl3) | 2 (DMSO-d6) | 3 (DMSO-d6) | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 163.9 | 146.6 | 151.0 | |||
| 2 | 6.30, d (2.3) | 96.8 | 143.4 | 127.4 | ||
| 3 | 165.9 | 7.26, s | 125.4 | 7.28, s | 122.0 | |
| 4 | 6.32, d (2.3) | 92.2 | 118.8 | 136.2 | ||
| 4a | 157.1 | 133.7 | 141.2 | |||
| 10a | 159.5 | 146.4 | 144.6 | |||
| 5 | 6.65, d (2.5) | 98.6 | 121.6 | 146.2 | ||
| 6 | 163.8 | 139.4 | 7.34, d (7.0) | 120.8 | ||
| 7 | 6.68, d (2.5) | 115.6 | 112.4 | 7.30, t (7.0) | 124.2 | |
| 8 | 143.6 | 7.41, s | 113.8 | 7.60, d (7.0) | 114.8 | |
| 8a | 128.0 | 114.8 | 120.5 | |||
| 9 | 182.5 | 182.4 | 182.7 | |||
| 9a | 104.2 | 109.1 | 108.1 | |||
| 1′ | 5.91, d (10.0) | 122.6 | ||||
| 2′ | 6.59, d (10.0) | 132.5 | ||||
| 3′ | 79.5 | |||||
| 4′ | 1.58, s | 28.4 | ||||
| 5′′ | 1.58, s | 28.4 | ||||
| 1′′ | 110.7 | 98.4 | ||||
| 2′′ | 6.31, dd (17.5, 10.6) | 147.1 | 6.23, dd (17.8, 10.3) | 146.6 | ||
| 3′′ | 5.10, d (17.5) 5.01, d (10.6) | 111.2 | 5.01, d (17.8) 4.97, d (10.3) | 110.7 | ||
| 4′′ | 1.46, s | 27.4 | 1.47, s | 26.3 | ||
| 5′′ | 1.46, s | 27.4 | 1.47, s | 26.3 | ||
| 1-OH | 13.69, s | 12.84, s | 12.75, s | |||
| 2-OH | 9.41, s | |||||
| 4-OH | 10.15, s | |||||
| 5-OH | 9.16, s | 9.30, s | ||||
| 3-OMe | 4.20 s | 55.8 | ||||
| 6-OMe | 4.18 s | 55.9 | ||||
| 8-CH3 | 3.15, s | 23.6 | ||||
| Position | 4 (DMSO-d6) | 5 (DMSO-d6) | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 151.1 | 151.2 | ||
| 2 | 126.6 | 122.7 | ||
| 3 | 7.35, s | 122.6 | 7.33, s | 122.5 |
| 4 | 136.3 | 136.3 | ||
| 4a | 140.6 | 141.8 | ||
| 10a | 144.9 | 141.1 | ||
| 5 | 141.9 | 147.5 | ||
| 6 | 127.3 | 137.9 | ||
| 7 | 7.20, d (8.0) | 121.3 | 7.37, d (8.0) | 120.6 |
| 8 | 7.66, d (8.0) | 116.4 | 7.69, d (8.0) | 117.0 |
| 8a | 121.7 | 108.6 | ||
| 9 | 182.3 | 182.5 | ||
| 9a | 108.5 | 120.7 | ||
| 1′ | 6.05, d (10.0) | 120.2 | 5.38, d (4.7) | 71.8 |
| 2′ | 6.60, d (10.0) | 134.5 | 4.36, d (4.7) | 98.3 |
| 3′ | 77.7 | 69.7 | ||
| 4′ | 1.48, s | 27.7 | 1.22, s | 25.8 |
| 5′ | 1.48, s | 27.7 | 1.20, s | 25.4 |
| 1′′ | 99.5 | 85.7 | ||
| 2′′ | 6.25, dd (17.9, 10.2) | 146.7 | 6.21, dd (17.6, 10.5) | 146.7 |
| 3′′ | 5.01, d (17.9) 4.99, d (10.2) | 110.8 | 4.98, d (17.6) 4.98, d (10.5) | 110.8 |
| 4′′ | 1.58, s | 26.3 | 1.45, s | 26.3 |
| 5′′ | 1.58, s | 26.3 | 1.45, s | 26.3 |
| 1-OH | 12.91, s | 12,87, s | ||
| 4-OH | 9.49, s | 9.57, s | ||
| 1′-OH | 6.00, s | |||
| 2′-OH | 4.73, s | |||
| Compounds | Antioxidant Activity | ||||
|---|---|---|---|---|---|
| FRAP | DPPH | ABTS | |||
| µM Fe2+/g | % Inhibition (159.7 μg/mL) | IC50 (μM) | % Inhibition (99 μg/mL) | IC50 (μM) | |
| 1 | 5.7 ± 0.57 | 41.60 ± 0.02 | >1000 | 33.24 ± 0.01 | >1000 |
| 2 | 166.3 ± 1.47 | 95.29 ± 0.05 | 19.4 ± 0.15 | 97.29 ± 0.04 | 2.7 ± 0.05 |
| 3 | 203.9 ± 1.19 | 96.83 ± 0.03 | 18.9 ± 0.10 | 98.31 ± 0.05 | 0.05 ± 0.01 |
| 4 | 192.7 ± 0.77 | 95.38 ± 0.08 | 14.1 ± 0.07 | 96.15 ± 0.01 | 7.9 ± 0.01 |
| 5 | 187.8 ± 1.36 | 96.60 ± 0.05 | 20.4 ± 0.22 | 97.40 ± 0.03 | 6.5 ± 0.02 |
| Ascorbic acid | 30.6 ± 0.27 | Nt | Nt | Nt | Nt |
| Quercetin | Nt | 96.34 ± 0.01 | 4.1 ± 0.01 | 97.58 ± 0.01 | 0.17 ± 0.01 |
| Gallic acid | Nt | 97.21± 0.01 | 3.0 ± 0.01 | 96.30 ± 0.01 | 0.7 ± 0.01 |
| Compounds | α-Glucosidase | α-Amylase | |
|---|---|---|---|
| Sucrose IC50 (μM) | Maltose IC50 (μM) | Starch IC50 (μM) | |
| 1 | >1000 | >1000 | >1000 |
| 2 | 43.8 ± 1.51 | 49.3 ± 0.14 | 41.3 ± 1.24 |
| 3 | 79.6 ± 2.01 | 109.0 ± 1.31 | 28.7 ± 0.35 |
| 4 | 75.9 ± 2.11 | 139.4 ± 1.21 | 10.8 ± 0.04 |
| 5 | 37.4 ± 1.20 | 91.0 ± 1.14 | 18.7 ± 0.54 |
| Acarbose | 4.6 ± 0.51 | 3.4 ± 0.27 | 4.0 ± 0.32 |
| Compounds | % Inhibition (10 μg/mL) | IC50 (μM) |
|---|---|---|
| 1 | ˂10 | Nt |
| 2 | 86.8 ± 0.33 | 3.3 ± 0.04 |
| 3 | 87.9 ± 0.23 | 5.0 ± 0.04 |
| 4 | ˂10 | Nt |
| 5 | ˂10 | Nt |
| Chloroquine | 98.8 ± 0.25 | 0.006 ± 0.01 |
| Ligands | Total Energy (kcal/mol) | Mol Dock Score (kcal/mol) | Hydrogen Bonding | Steric Interaction |
|---|---|---|---|---|
| 2 | 108.388 | −78.81 | Arg600; Asp518; Asp404 | Arg600; Phe469(2); Asp518; Trp376 |
| 5 | 144.003 | −74.54 | Arg600(3); Asp518 | Asp282 (2); Asp518; Asp616; Trp613 (2); Trp516; Trp376; Phe649; Met519; His674 |
| Acarbose | 330.989 | −107.31 | Arg404(2); Arg600; Asp616(2); Ser676(2); Ser679; His674; Leu678; Gly651 | Asp616(3); Asp518; Leu678(3); Trp481(2); Trp618(2); Phe649; Gly651 |
| Ligand | Total Energy (kcal/mol) | Mol Dock Score (kcal/mol) | Hydrogen Bonding | Steric Interaction |
|---|---|---|---|---|
| 2 | 108.388 | −103.69 | Tyr85(2); Phe52 | Tyr85; Glu122(2); Asp53(2); Gly27; Ala98; Phe100; |
| 3 | 79.747 | −86.38 | Glu122; Asp53; Tyr85 | Glu122; Tyr85; Asp53(2); Ile119 |
| Chloroquine | 35.324 | −108.99 | Gly29; Gly99; Ser28 | Gly29; Gly99; Ala98; Asp53 |
| Ligands | Absorption | Distribution | Metabolism | Excretion | Toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caco-2 Permeability | Intestinal Abs | Skin Permeability | VDss | BBB Permeability | CNS Permeability | CYP2D6 Inhibitors | CYP3A4 Inhibitors | Total Clearance | Renal OCT2 Substrate | Oral Rat Acute Toxicity (LD50) | Oral Rat Chronic Toxicity (LOAEL) | Hepato Toxicity | |
| 1 | 1.233 | 95.93 | −2.851 | 0.039 | −0.291 | −2.098 | No | No | 0.655 | No | 2.045 | 1.449 | No |
| 2 | 0.736 | 98.018 | −2.735 | 0.046 | −1.258 | −1.93 | No | No | 0.186 | No | 1.889 | 0.696 | No |
| 3 | 1.298 | 96.82 | −2.735 | 0.068 | −1.02 | −2.029 | No | No | 0.198 | No | 2.055 | 0.633 | No |
| 4 | 0.989 | 94.818 | −2.754 | 0.143 | 0.099 | −1.679 | No | Yes | 0.176 | No | 2.057 | 0.313 | Yes |
| 5 | 0.335 | 80.007 | −2.735 | −0.014 | −1.36 | −3.040 | No | No | −0.102 | No | 2.036 | 1.695 | Yes |
| Chloroquine | 1.259 | 89.440 | −2.564 | 1.757 | 0.410 | −2.687 | Yes | No | 0.993 | Yes | 2.888 | 0.423 | Yes |
| Acarbose | −0.278 | 0 | −2.735 | −0.644 | −1.854 | −7.308 | No | No | 0.546 | No | 2.495 | 7.203 | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wairata, J.; Sukandar, E.R.; Fadlan, A.; Purnomo, A.S.; Taher, M.; Ersam, T. Evaluation of the Antioxidant, Antidiabetic, and Antiplasmodial Activities of Xanthones Isolated from Garcinia forbesii and Their In Silico Studies. Biomedicines 2021, 9, 1380. https://doi.org/10.3390/biomedicines9101380
Wairata J, Sukandar ER, Fadlan A, Purnomo AS, Taher M, Ersam T. Evaluation of the Antioxidant, Antidiabetic, and Antiplasmodial Activities of Xanthones Isolated from Garcinia forbesii and Their In Silico Studies. Biomedicines. 2021; 9(10):1380. https://doi.org/10.3390/biomedicines9101380
Chicago/Turabian StyleWairata, Johanis, Edwin Risky Sukandar, Arif Fadlan, Adi Setyo Purnomo, Muhammad Taher, and Taslim Ersam. 2021. "Evaluation of the Antioxidant, Antidiabetic, and Antiplasmodial Activities of Xanthones Isolated from Garcinia forbesii and Their In Silico Studies" Biomedicines 9, no. 10: 1380. https://doi.org/10.3390/biomedicines9101380
APA StyleWairata, J., Sukandar, E. R., Fadlan, A., Purnomo, A. S., Taher, M., & Ersam, T. (2021). Evaluation of the Antioxidant, Antidiabetic, and Antiplasmodial Activities of Xanthones Isolated from Garcinia forbesii and Their In Silico Studies. Biomedicines, 9(10), 1380. https://doi.org/10.3390/biomedicines9101380







