Machine Learning Algorithms to Predict Mortality of Neonates on Mechanical Intubation for Respiratory Failure
Abstract
:1. Introduction
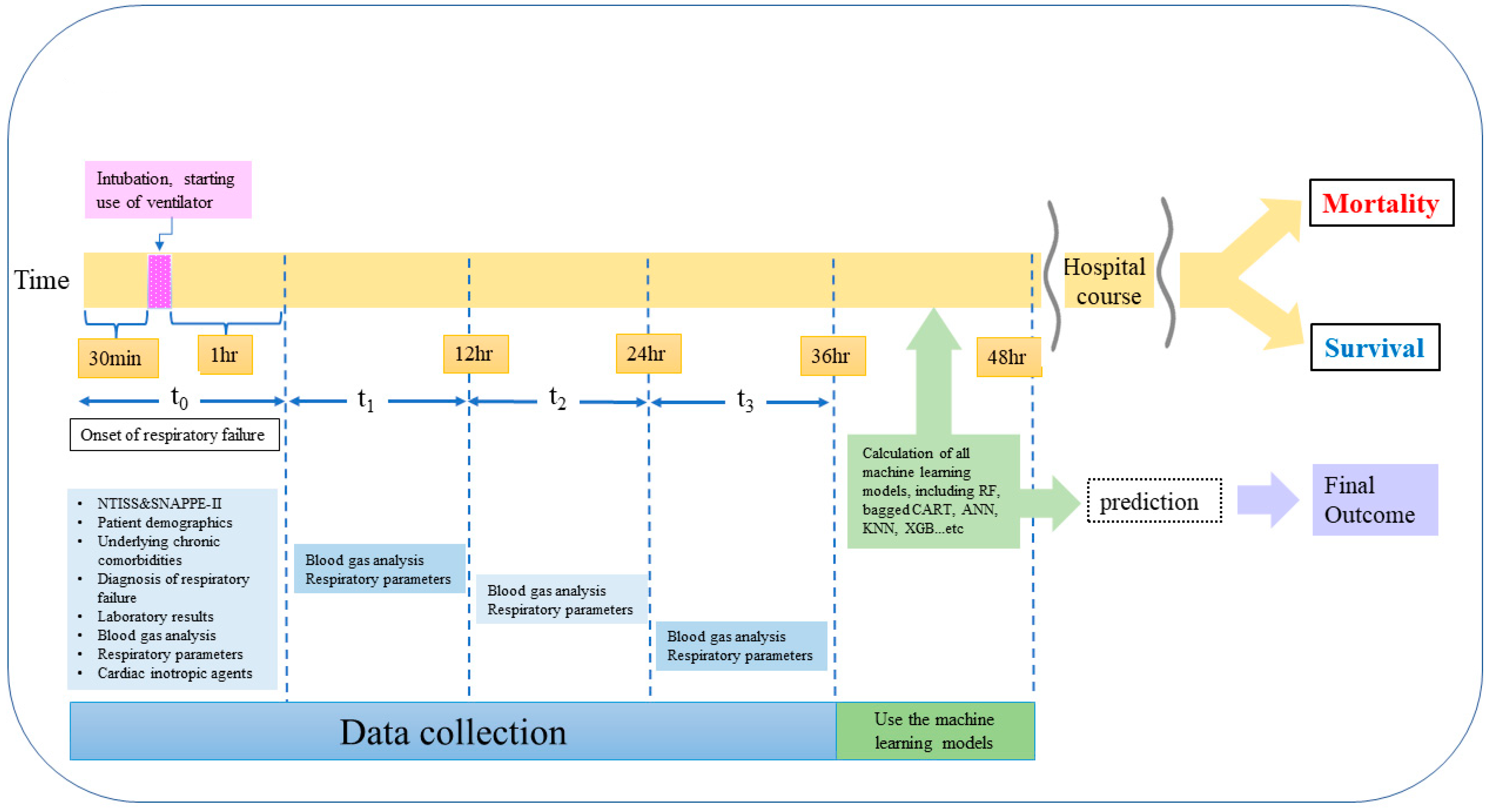
2. Methods
2.1. Patients, Setting, and Study Design
2.2. Study Variables
2.3. Statistical Analysis
3. Results
3.1. Development of Mortality Prediction Model
3.2. Rank of Predictors in the Prediction Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AaDO2 | alveolar–arterial oxygen tension difference |
| AUC | area under the receiver operating characteristic curve |
| Bagged CART | bagged classification and regression tree |
| CI | confidence interval |
| CGMH | Chang Gung Memorial Hospital |
| NICU | neonatal intensive care unit |
| NTISS | Neonatal Therapeutic Intervention Scoring System |
| OI | oxygenation index |
| OR | odds ratio |
| RF | random forest |
| SHAP | Shapley additive explanation values |
| SNAPPE-II | Score for Neonatal Acute Physiology Perinatal Extension II |
| TPN | total parenteral nutrition |
References
- Horbar, J.D.; Edwards, E.M.; Greenberg, L.T.; Morrow, K.A.; Soll, R.F.; Buus-Frank, M.E.; Buzas, J.S. Variation in Performance of Neonatal Intensive Care Units in the United States. JAMA Pediatr. 2017, 171, e164396. [Google Scholar] [CrossRef]
- Abdel-Latif, M.E.; Nowak, G.; Bajuk, B.; Glass, K.; Harley, D. Variation in hospital mortality in an Australian neonatal intensive care unit network. Arch. Dis. Child. Fetal Neonatal. Ed. 2017, 103, F331–F336. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Hsu, J.-F.; Chu, S.-M.; Lien, R.; Huang, H.-R.; Chiang, M.-C.; Fu, R.-H.; Lee, C.-W.; Huang, Y.-C. Incidence, Clinical Characteristics and Risk Factors for Adverse Outcome in Neonates with Late-onset Sepsis. Pediatr. Infect. Dis. J. 2014, 33, e7–e13. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, R.; Guenther, K.; Vach, W.; Bruder, I. Risk-adjusted mortality of VLBW infants in high-volume versus low-volume NICUs. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 104, F390–F395. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-F.; Chu, S.-M.; Huang, Y.-C.; Lien, R.; Huang, H.-R.; Lee, C.-W.; Chiang, M.-C.; Fu, R.-H.; Tsai, M.-H. Predictors of clinical and microbiological treatment failure in neonatal bloodstream infections. Clin. Microbiol. Infect. 2015, 21, 482.e9–482.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namachivayam, S.P.; Carlin, J.B.; Millar, J.; Alexander, J.; Edmunds, S.; Ganeshalingham, A.; Lew, J.; Erichson, S.; Butt, W.; Schiapbach, L.J.; et al. Gestational age and risk of mortality in term-born critically ill neonates admitted to PICUs in Australia and New Zealand. Crit Care med. 2020, 48, e648–e656. [Google Scholar] [CrossRef]
- Hsiao, H.-F.; Yang, M.-C.; Lai, M.-Y.; Chu, S.-M.; Huang, H.-R.; Chiang, M.-C.; Fu, R.-H.; Hsu, J.-F.; Tsai, M.-H. The Off-Label Use of Inhaled Nitric Oxide as a Rescue Therapy in Neonates with Refractory Hypoxemic Respiratory Failure: Therapeutic Response and Risk Factors for Mortality. J. Clin. Med. 2019, 8, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Gao, X.; Liu, C.; Yan, C.; Lin, X.; Yang, C.; Lin, Z.; Zhu, W.; Yang, Z.; Yu, F.; et al. Morbidity and Mortality of Neonatal Respiratory Failure in China: Surfactant Treatment in Very Immature Infants. Pediatrics 2012, 129, e731–e740. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Chu, S.-M.; Hsu, J.-F.; Lien, R.; Huang, H.-R.; Chiang, M.-C.; Fu, R.-H.; Lee, C.-W.; Huang, Y.-C. Risk Factors and Outcomes for Multidrug-Resistant Gram-Negative Bacteremia in the NICU. Pediatrics 2014, 133, e322–e329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhe, L.M.; McClure, E.M.; Nigussie, A.K.; Mekasha, A.; Worku, B.; Worku, A.; Demtse, A.; Eshetu, B.; Tigabu, Z.; Gizaw, M.A.; et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): A prospective, cross-sectional, observational study. Lancet Glob. Heal. 2019, 7, e1130–e1138. [Google Scholar] [CrossRef] [Green Version]
- Ellsbury, D.L.; Clark, R.; Ursprung, R.; Handler, D.L.; Dodd, E.D.; Spitzer, A.R. A Multifaceted Approach to Improving Outcomes in the NICU: The Pediatrix 100,000 Babies Campaign. Pediatrics 2016, 137, e20150389. [Google Scholar] [CrossRef] [Green Version]
- Houweling, T.A.J.; Van Klaveren, D.; Das, S.; Azad, K.; Tripathy, P.; Manandhar, D.; Neuman, M.; De Jonge, E.; Been, J.V.; Steyerberg, E.; et al. A prediction model for neonatal mortality in low- and middle-income countries: An analysis of data from population surveillance sites in India, Nepal and Bangladesh. Int. J. Epidemiol. 2019, 48, 186–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; Lee, M.H.; Chang, Y.S. The Clinical Risk Index for Babies II for Prediction of Time-Dependent Mortality and Short-Term Morbidities in Very Low Birth Weight Infants. Neonatology 2019, 116, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.S.; Field, D.J.; Manktelow, B. Neonatal disease severity scoring systems. Arch. Dis. Child Fetal Neonatal. Ed. 2005, 90, F11–F16. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.D.; Kotadia, N.; English, L.; Kissoon, N.; Ansermino, J.M.; Kabakyenga, J.; Lavoie, P.M.; Wiens, M.O. Predictors of mortality in neonates and infants hospitalized with sepsis or serious infections in developing countries: A systemic review. Front. Pediatr. 2018, 4, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harsha, S.S.; Archana, B.R. SNAPPE-II (Score for neonatal acute physiology with perinatal extension-II) in predicting mortality and morbidity in NICU. J. Clin. Diagn. Res. 2015, 9, SC10–SC12. [Google Scholar] [CrossRef] [PubMed]
- Sotodate, G.; Oyama, K.; Matsumoto, A.; Konishi, Y.; Toya, Y.; Takashimizu, N. Predictive ability of neonatal illness severity scores for early death in extremely premature infants. J. Matern. Fetal. Neonatal Med. 2020, 25, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A.; Pany, M.J.; Parikh, R.B.; Obermeyer, Z. Development and Application of a Machine Learning Approach to Assess Short-term Mortality Risk Among Patients with Cancer Starting Chemotherapy. JAMA Netw. Open 2018, 1, e180926. [Google Scholar] [CrossRef] [Green Version]
- Shillan, D.; Sterne, J.A.C.; Champneys, A.; Gibbison, B. Use of machine learning to analyze routinely collected intensive care unit data: A systemic review. Crit. Care 2019, 23, 284. [Google Scholar] [CrossRef] [Green Version]
- Ramgopal, S.; Horvat, C.M.; Yanamala, N.; Alpern, E.R. Machine Learning To Predict Serious Bacterial Infections in Young Febrile Infants. Pediatrics 2020, 146, e20194096. [Google Scholar] [CrossRef] [PubMed]
- Gleason, C.A.; Juul, S.E. Avery’s Diseases of the Newborn E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Vickers, A.J.; van Calster, B.; Steyerberg, E.W. A simple, step-by-step guide to interpreting decision curve analysis. Diagn. Progn. Res. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Rousson, V.; Zumbrunn, T. Decision curve analysis revisited: Overall net benefit, relationships to ROC curve analysis, and application to case-control studies. BMC Med. Inform. Decis. Mak. 2011, 11, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.W.; Kim, J.; Kim, D.K.; Oh, K.-H.; Joo, K.W.; Kim, Y.S.; Han, S.S. Machine learning algorithm to predict mortality in patients undergoing continuous renal replacement therapy. Crit. Care 2020, 24, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogami, C.; Tsuji, Y.; Seki, H.; Kawano, H.; To, H.; Matsumoto, Y.; Hosono, H. An artificial neural network−pharmacokinetic model and its interpretation using Shapley additive explanations. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Zverinski, D.; Pfahringer, B.; Kempfert, J.; Kuehne, T.; Sündermann, S.H.; Stamm, C.; Hofmann, T.; Falk, V.; Eickhoff, C. Machine learning for real-time prediction of complications in critical care: A retrospective study. Lancet Respir. Med. 2018, 6, 905–914. [Google Scholar] [CrossRef]
- Cole, F.S. Improving VLBW infant outcomes with big data analytics. Pediatr. Res. 2021, 90, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Kamitsuka, M.; Clark, R.H.; Kelleher, A.S.; Spitzer, A.R. Etiologies of NICU Deaths. Pediatrics 2014, 135, e59–e65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medvedev, M.M.; Brotherton, H.; Gai, A.; Tann, C.; Gale, C.; Waiswa, P.; Elbourne, D.; Lawn, J.E.; Allen, E. Development and validation of a simplified score to predict neonatal mortality risk among neonates weighing 2000 g or less (NMR-2000): An analysis using data from the UK and The Gambia. Lancet Child Adolesc. Heal. 2020, 4, 299–311. [Google Scholar] [CrossRef] [Green Version]
- Muktan, D.; Singh, R.R.; Bhatta, N.K.; Shah, D. Neonatal mortality risk assessment using SNAPPE- II score in a neonatal intensive care unit. BMC Pediatr. 2019, 19, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Dammann, O.; Shah, B.; Naples, M.; Bednarek, F.; Zupancic, J.; Allred, E.N.; Leviton, A. Interinstitutional Variation in Prediction of Death by SNAP-II and SNAPPE-II among Extremely Preterm Infants. Pediatrics 2009, 124, e1001–e1006. [Google Scholar] [CrossRef] [Green Version]
- Aluvaala, J.; Collins, G.S.; Maina, M.; Berkley, J.A.; English, M. A systemic review of neonatal treatment intensity scores and their potential application in low-resource setting hospitals for predicting mortality, morbidity and estimating resource use. Syst. Rev. 2017, 6, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dammann, O.; Shah, B.; Naples, M.; Bednarek, F.; Zupancic, J.; Allred, E.N.; Leviton, A. SNAP-II and SNAPPE-II as predictors of death among infants born before the 28th week of gestation. Inter-institutional variations. Pediatrics 2009, 124, e1001–e1006. [Google Scholar] [CrossRef] [Green Version]
- Mesquita Ramirez, M.N.; Godoy, L.E.; Alvarez Barrrientos, E. SNAP II and SNAPPE II as predictors of neonatal mortality in a pediatric intensive care unit: Does postnatal age play a role? Int. J. Pediatr. 2014, 2014, 298198. [Google Scholar] [CrossRef] [Green Version]
- Tseng, P.-Y.; Chen, Y.-T.; Wang, C.-H.; Chiu, K.-M.; Peng, Y.-S.; Hsu, S.-P.; Chen, K.-L.; Yang, C.-Y.; Lee, O.K.-S. Prediction of the development of acute kidney injury following cardiac surgery by machine learning. Crit. Care 2020, 24, 1–13. [Google Scholar] [CrossRef]
- Joshi, R.; Kommers, D.; Oosterwijk, L.; Feijs, L.; Van Pul, C.; Andriessen, P. Predicting Neonatal Sepsis Using Features of Heart Rate Variability, Respiratory Characteristics, and ECG-Derived Estimates of Infant Motion. IEEE J. Biomed. Heal. Informatics 2019, 24, 681–692. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S. A unified approach to interpreting model predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 4768–4777. [Google Scholar]
- Rodríguez-Pérez, R.; Bajorath, J. Interpretation of machine learning models using shapley values: Application to compound potency and multi-target activity predictions. J. Comput. Mol. Des. 2020, 34, 1013–1026. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Rawson, T.M.; Ahmad, R.; Buchard, A.; Georgiou, P.; Lescure, F.X.; Birgand, G.; Holmes, A.H. Machine learning for clinical decision support in infectious diseases: A narrative review of current applications. Clin. Microbiol Infect. 2020, 26, 584–595. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | All Study Subjects (Total n = 1734) | The Training Set (Total n = 1214) | The Test Set (Total n = 520) | p Values |
|---|---|---|---|---|
| Case demographics | ||||
| Gestational age (weeks), median (IQR) | 27.0 (25.0–31.3) | 27.3 (25.3–31.0) | 27.0 (25.0–31.5) | 0.324 |
| Birth weight (g), median (IQR) | 915.0 (703.5–1480.0) | 915.0 (708.0–1463.8) | 908.5 (700.0–1510.0) | 0.974 |
| Gender (male), n (%) | 1029 (59.3) | 732 (60.3) | 297 (57.1) | 0.220 |
| Birth by NSD/Cesarean section, n (%) | 548 (31.6)/1186 (68.4) | 389 (32.0)/825 (68.0) | 159 (30.6)/361 (69.4) | 0.573 |
| 5 minutes Apgar score < 7, n (%) | 566 (32.6) | 409 (33.7) | 157 (30.2) | 0.163 |
| Inborn/outborn, n (%) | 1365 (78.7)/369 (21.3) | 944 (77.8)/270 (22.2) | 421 (81.0)/99 (19.0) | 0.141 |
| Premature rupture of membrane, n (%) | 530 (30.6) | 375 (30.9) | 155 (29.8) | 0.691 |
| Maternal fever, n (%) | 214 (12.3) | 156 (12.9) | 58 (11.2) | 0.340 |
| Intrapartum antibiotic prophylaxis, n (%) | 140 (8.1) | 90 (7.4) | 50 (9.6) | 0.125 |
| Chorioamnionitis, n (%) | 32 (1.8) | 22 (1.8) | 10 (1.9) | 0.848 |
| Perinatal asphyxia, n (%) | 354 (20.4) | 255 (21.0) | 99 (19.0) | 0.363 |
| Onset of respiratory failure, day (median (IQR)) | 1.0 (1.0–3.0) | 1.0 (1.0–3.0) | 1.0 (1.0–2.0) | 0.101 |
| Diagnoses of respiratory failure, n (%) | ||||
| Respiratory distress syndrome (≥Gr II) | 1047 (60.3) | 736 (60.5) | 311 (59.8) | 0.748 |
| Transient tachypnea of newborn | 83 (4.8) | 61 (5.0) | 22 (4.2) | 0.540 |
| Complicated cardiovascular diseases | 28 (1.6) | 22 (1.8) | 6 (1.2) | 0.408 |
| Symptomatic patent ductus arteriosus | 662 (38.2) | 454 (37.4) | 208 (40.0) | 0.306 |
| Persistent pulmonary hypertension of newborn | 278 (16.0) | 187 (15.4) | 91 (17.5) | 0.284 |
| Pulmonary hemorrhage | 120 (6.9) | 91 (7.5) | 29 (5.6) | 0.179 |
| Pneumonia | 85 (4.9) | 54 (4.4) | 31 (6.0) | 0.183 |
| Air leak syndrome & | 188 (10.8) | 124 (10.2) | 64 (12.3) | 0.206 |
| Meconium aspiration syndrome | 50 (2.9) | 35 (2.9) | 15 (2.9) | 1.000 |
| Sepsis | 271 (15.6) | 190 (15.7) | 81 (15.6) | 1.000 |
| Hydrops fetalis | 34 (2.0) | 27 (2.2) | 7 (1.3) | 0.261 |
| Others # | 26 (1.5) | 20 (1.6) | 6 (1.2) | 0.523 |
| Presences of any chronic comorbidities, n (%) | 379 (21.8) | 272 (22.4) | 107 (20.6) | 0.410 |
| Presences of central venous catheter, n (%) | 522 (30.1) | 351 (28.9) | 171 (32.9) | 0.109 |
| Initial ventilator requirement *, n (%) | 0.178 | |||
| Intubation with mechanical ventilation | 1168 (67.4) | 819 (67.5) | 349 (67.1) | |
| Initial FiO2 ≤ 50 | 671 (38.7) | 464 (38.2) | 207 (39.8) | |
| Initial FiO2 > 50 | 497 (28.7) | 355 (29.2) | 142 (27.3) | |
| On high frequency oscillatory ventilation | 566 (32.6) | 395 (32.5) | 171 (32.9) | |
| High setting (FiO2 ≤ 50) | 248 (14.3) | 162 (13.3) | 86 (16.5) | |
| Low setting (FiO2 > 50) | 318 (18.3) | 233 (19.2) | 85 (16.3) | |
| Oxygenation index, median (IQR) | 11.0 (6.0–20.0) | 11.0 (6.0–20.0) | 11.0 (6.0–20.0) | 0.780 |
| AaDO2, median (IQR) | 273.0 (166.0–478.0) | 271.0 (165.8–477.0) | 280.0 (166.3–486.8) | 0.670 |
| Use of iNO | 285 (16.4) | 193 (15.9) | 92 (17.7) | 0.359 |
| Clinical features *, n (%) | ||||
| Intravascular volume expansion | 1415 (81.6) | 1001 (82.5) | 414 (79.6) | 0.188 |
| Requirement of cardiac inotropic agents | 1206 (69.6) | 842 (69.4) | 364 (70.0) | 0.820 |
| Metabolic acidosis | 677 (39.0) | 484 (39.9) | 193 (37.1) | 0.307 |
| Coagulopathy | 1226 (70.7) | 864 (71.2) | 362 (69.6) | 0.527 |
| Requirement of blood transfusion ** | 558 (32.2) | 391 (32.2) | 167 (32.1) | 1.000 |
| Laboratory data at onset of respiratory failure | ||||
| Leukocytosis or leukopenia | 446 (25.7) | 304 (25.0) | 142 (27.3) | 0.337 |
| Shift to left in WBC (immature > 20%) | 158 (9.1) | 103 (8.5) | 55 (10.6) | 0.172 |
| Anemia (hemoglobin level < 11.5 g/dL) | 317 (18.3) | 223 (18.4) | 94 (18.1) | 0.946 |
| Thrombocytopenia (platelet < 150,000/ul) | 434 (25.0) | 297 (24.5) | 137 (26.3) | 0.432 |
| C-reactive protein (mg/dL), median (IQR) | 5.0 (2.0–19.5) | 5.0 (2.0–18.0) | 6.0 (2.0–22.0) | 0.149 |
| Severity score at onset of respiratory failure | ||||
| NTISS (median (IQR)) | 23.0 (21.0–26.0) | 24.0 (21.0–27.0) | 23.0 (21.0–26.0) | 0.254 |
| SNAPPE-II (median (IQR)) | 28.0 (22.0–40.0) | 30.0 (22.0–42.0) | 28.0 (22.0–40.0) | 0.174 |
| Final in-hospital mortality, n (%) | 278 (16.0) | 198 (16.3) | 80 (15.4) | 0.379 |
| Models | AUC (95% CI) | p Value * | p Value # | Accuracy | F1 Score |
|---|---|---|---|---|---|
| NTISS | 0.836 (0.800–0.871) | 0.701 | 0.629 | ||
| SNAPPE-II | 0.805 (0.766–0.843) | 0.757 | 0.637 | ||
| Random Forest | 0.939 (0.921–0.958) | < 0.0001 | < 0.0001 | 0.877 | 0.777 |
| Bagged CART | 0.915 (0.891–0.939) | 0.0003 | < 0.0001 | 0.864 | 0.774 |
| Support Vector Machine | 0.884 (0.856–0.912) | 0.0343 | 0.0010 | 0.833 | 0.720 |
| Elastic-net Regularized Regression | 0.844 (0.888–0.934) | 0.7409 | 0.1386 | 0.849 | 0.754 |
| k-Nearest Neighbor | 0.795 (0.759–0.832) | 0.1200 | 0.7345 | 0.698 | 0.613 |
| Artificial Neural Networks | 0.782 (0.742–0.822) | 0.0487 | 0.4186 | 0.773 | 0.635 |
| eXtreme Gradient Boosting | 0.776 (0.737–0.815) | 0.0307 | 0.2981 | 0.719 | 0.582 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, J.-F.; Yang, C.; Lin, C.-Y.; Chu, S.-M.; Huang, H.-R.; Chiang, M.-C.; Wang, H.-C.; Liao, W.-C.; Fu, R.-H.; Tsai, M.-H. Machine Learning Algorithms to Predict Mortality of Neonates on Mechanical Intubation for Respiratory Failure. Biomedicines 2021, 9, 1377. https://doi.org/10.3390/biomedicines9101377
Hsu J-F, Yang C, Lin C-Y, Chu S-M, Huang H-R, Chiang M-C, Wang H-C, Liao W-C, Fu R-H, Tsai M-H. Machine Learning Algorithms to Predict Mortality of Neonates on Mechanical Intubation for Respiratory Failure. Biomedicines. 2021; 9(10):1377. https://doi.org/10.3390/biomedicines9101377
Chicago/Turabian StyleHsu, Jen-Fu, Chi Yang, Chun-Yuan Lin, Shih-Ming Chu, Hsuan-Rong Huang, Ming-Chou Chiang, Hsiao-Chin Wang, Wei-Chao Liao, Rei-Huei Fu, and Ming-Horng Tsai. 2021. "Machine Learning Algorithms to Predict Mortality of Neonates on Mechanical Intubation for Respiratory Failure" Biomedicines 9, no. 10: 1377. https://doi.org/10.3390/biomedicines9101377
APA StyleHsu, J.-F., Yang, C., Lin, C.-Y., Chu, S.-M., Huang, H.-R., Chiang, M.-C., Wang, H.-C., Liao, W.-C., Fu, R.-H., & Tsai, M.-H. (2021). Machine Learning Algorithms to Predict Mortality of Neonates on Mechanical Intubation for Respiratory Failure. Biomedicines, 9(10), 1377. https://doi.org/10.3390/biomedicines9101377








