Role of BRCA Mutation and HE4 in Predicting Chemotherapy Response in Ovarian Cancer: A Retrospective Pilot Study
Abstract
1. Introduction
2. Experimental Section
2.1. Inclusion Criteria Were
- I.
- Age between 18 and 70 years
- II.
- HE4 test positive at diagnosis
- III.
- Eastern Cooperative Oncology Group (ECOG) performance status 0–2 according to World Health Organization (WHO) criteria
- IV.
- Residual tumor after surgical debulking < 1(RT < 1)
- V.
- No previous surgical, chemotherapeutic, or radiotherapeutic treatments for this or other kinds of cancer
2.2. Exclusion Criteria Were
- I
- Altered hepatic function (transaminases > 2.5× the upper normal level –UNL-, bilirubin > 1.5× UNL)
- II
- Altered renal function (creatinine clearance < 60 mL/min and/or serum creatinine > 2.0 mg/100 mL)
- III
- Altered hematological function (absolute neutrophil count < 1.5 × 109/L or platelets < 100 × 109/L or hemoglobin < 9 g/dL)
- IV
- Severe or uncontrolled diseases, other systemic and not compensated diseases or mental illnesses at the diagnosis
- V
- Pregnancy
- I
- Platinum-resistant: Disease progression during chemotherapy treatment or within 12 months after the end of chemotherapy.
- II
- Platinum-sensitive: Recurrence after 12 months or more.
3. Results
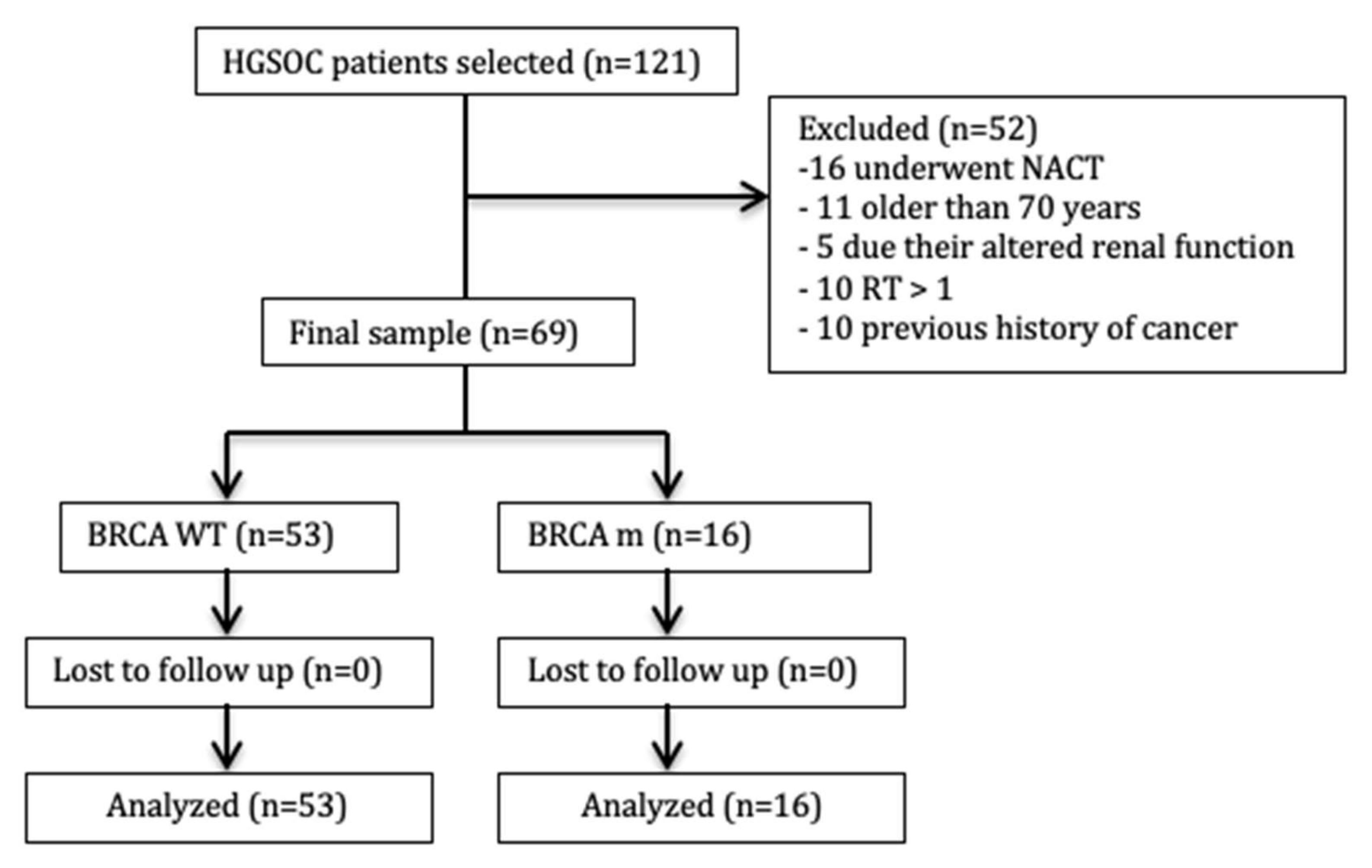
3.1. All Population
3.2. BRCA WT Group
3.3. BRCA Mutated Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, P.M.; Jordan, S. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Lisio, M.-A.; Fu, L.; Goyeneche, A.A.; Gao, Z.-H.; Telleria, C.M. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Poole, E.M.; Trabert, B.; White, E.; Arslan, A.A.; Patel, A.V.; Setiawan, V.W.; Visvanathan, K.; Weiderpass, E.; Adami, H.-O.; et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis from the Ovarian Cancer Cohort Consortium. J. Clin. Oncol. 2016, 34, 2888–2898. [Google Scholar] [CrossRef] [PubMed]
- Stratton, J.F.; Pharoah, P.; Smith, S.K.; Easton, D.; Ponder, B.A.J. A systematic review and meta-analysis of family history and risk of ovarian cancer. BJOG Int. J. Obstet. Gynaecol. 1998, 105, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Paul, S. The Breast Cancer Susceptibility Genes (BRCA) in Breast and Ovarian Cancers. Front. Biosci. 2014, 19, 605–618. [Google Scholar] [CrossRef]
- Andrews, L.; Mutch, D.G. Hereditary Ovarian Cancer and Risk Reduction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 31–48. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Moschetta, M.; George, A.; Kaye, S.B.; Banerjee, S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann. Oncol. 2016, 27, 1449–1455. [Google Scholar] [CrossRef]
- Vencken, P.M.L.H.; Kriege, M.; Hoogwerf, D.; Beugelink, S.; Van Der Burg, M.E.L.; Hooning, M.J.; Berns, E.M.; Jager, A.; Collée, M.; Burger, C.W.; et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann. Oncol. 2011, 22, 1346–1352. [Google Scholar] [CrossRef]
- Rustin, G.J.S.; Nelstrop, A.E.; Tuxen, M.K.; Lambert, H.E. Defining progression of ovarian carcinoma during follow-up according to CA 125: A North Thames Ovary Group study. Ann. Oncol. 1996, 7, 361–364. [Google Scholar] [CrossRef]
- Nustad, K.; Bast, J.R.; O’Brien, T.; Nilsson, O.; Seguin, P.; Suresh, M.R.; Saga, T.; Nozawa, S.; Børmer, O.P.; De Bruijn, H.; et al. Specificity and Affinity of 26 Monoclonal Antibodies against the CA 125 Antigen: First Report from the ISOBM TD-1 Workshop. Tumor Biol. 1996, 17, 196–219. [Google Scholar] [CrossRef] [PubMed]
- Urban, N. Specific Keynote: Ovarian Cancer Risk Assessment and the Potential for Early Detection. Gynecol. Oncol. 2003, 88, S75–S79. [Google Scholar] [CrossRef] [PubMed]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J. Ovarian Res. 2019, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Scaletta, G.; Plotti, F.; Luvero, D.; Capriglione, S.; Montera, R.; Miranda, A.; Lopez, S.; Terranova, C.; Nardone, C.D.C.; Angioli, R. The role of novel biomarker HE4 in the diagnosis, prognosis and follow-up of ovarian cancer: A systematic review. Expert Rev. Anticancer Ther. 2017, 17, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Angioli, R.; Plotti, F.; Capriglione, S.; Aloisi, A.; Montera, R.; Luvero, D.; Miranda, A.; Cafà, E.V.; Damiani, P.; Benedetti-Panici, P. Can the preoperative HE4 level predict optimal cytoreduction in patients with advanced ovarian carcino-ma? Gynecol. Oncol. 2013, 128, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Yanaranop, M.; Anakrat, V.; Siricharoenthai, S.; Nakrangsee, S.; Thinkhamrop, B. Is the risk of ovarian malignancy algorithm better than other tests for pre-dicting ovarian malignancy in women with pelvic masses? Gynecol. Obstet. Investig. 2017, 82, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hamed, E.O.; Ahmed, H.; Sedeek, O.B.; Mohammed, A.M.; Abd-Alla, A.A.; Ghaffar, H.M.A. Significance of HE4 estimation in comparison with CA125 in diagnosis of ovarian cancer and assessment of treatment response. Diagn. Pathol. 2013, 8, 11. [Google Scholar] [CrossRef]
- Steffensen, K.D.; Waldstrøm, M.; Brandslund, I.; Petzold, M.; Jakobsen, A. The Prognostic and Predictive Value of Combined HE4 and CA-125 in Ovarian Cancer Patients. Int. J. Gynecol. Cancer 2012, 22, 1474–1482. [Google Scholar] [CrossRef]
- Aarenstrup Karlsen, M.; Høgdall, C.; Nedergaard, L.; Prahm, K.P.; Karlsen, N.M.S.; Ekmann-Gade, A.W.; Schnack, T.H.; Poulsen, T.S.; Christensen, I.J.; Høgdall, E. HE4 as a predictor of adjuvant chemotherapy resistance and survival in patients with epithe-lial ovarian cancer. APMIS 2016, 124, 1038–1045. [Google Scholar] [CrossRef]
- Pelissier, A.; Roulot, A.; Guéry, B.; Bonneau, C.; Bellet, D.; Rouzier, R. Serum CA125 and HE4 levels as predictors for optimal interval surgery and platinum sensitivity after ne-oadjuvant platinum-based chemotherapy in patients with advanced epithelial ovarian cancer. J. Ovarian Res. 2016, 9, 61. [Google Scholar] [CrossRef][Green Version]
- Moore, R.G.; Hill, E.K.; Horan, T.; Yano, N.; Kim, K.; MacLaughlan, S.; Lambert-Messerlian, G.; Tseng, Y.D.; Padbury, J.F.; Miller, M.C.; et al. HE4 (WFDC2) gene overexpression promotes ovarian tumor growth. Sci. Rep. 2015, 4, 3574. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, L.; Gao, J.; Hu, Z.; Lin, B. Promotive role of recombinant HE4 protein in proliferation and carboplatin resistance in ovarian cancer cells. Oncol. Rep. 2015, 33, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-C.; Gao, J.; Hu, Z.-H.; Schwab, C.L.; Zhuang, H.-Y.; Tan, M.-Z.; Yan, L.-M.; Liu, J.-J.; Zhang, D.-Y.; Lin, B. Membranous expressions of Lewis y and CAM-DR-related markers are independent factors of chemotherapy resistance and poor prognosis in epithelial ovarian cancer. Am. J. Cancer Res. 2015, 5, 830–843. [Google Scholar] [PubMed]
- Ribeiro, J.R.; Schorl, C.; Yano, N.; Romano, N.; Kim, K.K.; Singh, R.K.; Moore, R.G. HE4 promotes collateral resistance to cisplatin and paclitaxel in ovarian cancer cells. J. Ovarian Res. 2016, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, S.; Lee, Y.; Chung, D.; Hong, S.; Park, N. Role of human epididymis protein 4 in chemoresistance and prognosis of epithelial ovarian cancer: Role of HE4 in ovarian cancer. J. Obstet. Gynaecol. Res. 2017, 43, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Plotti, F.; Martina, B.; Corrado, T.; Daniela, L.; Giuseppe, S.; Alessandra, G.; Pierluigi, B.P.; Roberto, A. Role of human epididymis protein 4 (HE4) as predictor of response to platinum based chemotherapy: A systematic review of literature. Eur. J. Gynaecol. Oncol. 2020, 41, 889–896. [Google Scholar] [CrossRef]
- Piccart, M.; Bertelsen, K.; James, K.; Cassidy, J.; Mangioni, C.; Simonsen, E.; Stuart, G.; Kaye, S.; Vergote, I.; Blom, R.; et al. Randomized Intergroup Trial of Cisplatin-Paclitaxel Versus Cisplatin-Cyclophosphamide in Women with Advanced Epithelial Ovarian Cancer: Three-Year Results. J. Natl. Cancer Inst. 2000, 92, 699–708. [Google Scholar] [CrossRef]
- Neijt, J.P.; Engelholm, S.A.; Tuxen, M.K.; Sørensen, P.G.; Hansen, M.; Sessa, C.; De Swart, C.A.M.; Hirsch, F.R.; Lund, B.; Van Houwelingen, H.C. Exploratory Phase III Study of Paclitaxel and Cisplatin Versus Paclitaxel and Carboplatin in Advanced Ovarian Cancer. J. Clin. Oncol. 2000, 18, 3084–3092. [Google Scholar] [CrossRef]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with opti-mally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef]
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. N. Engl. J. Med. 1996, 334, 1–6. [Google Scholar] [CrossRef]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Liao, J.B. Novel Therapeutics for Ovarian Cancer: The 11th Biennial Rivkin Center Ovarian Cancer Research Symposium. Int. J. Gynecol. Cancer 2017, 27, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Plotti, F.; Guzzo, F.; Schirò, T.; Terranova, C.; Nardone, C.D.C.; Montera, R.; Luvero, D.; Scaletta, G.; Lopez, S.; Capriglione, S.; et al. Role of human epididymis protein 4 (HE4) in detecting recurrence in CA125 negative ovarian cancer patients. Int. J. Gynecol. Cancer 2019, 29, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Plotti, F.; Capriglione, S.; Terranova, C.; Montera, R.; Aloisi, A.; Damiani, P.; Muzii, L.; Scaletta, G.; Benedetti-Panici, P.; Angioli, R. Does HE4 have a role as biomarker in the recurrence of ovarian cancer? Tumor Biol. 2012, 33, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Kaijser, J.; Van Belle, V.; Van Gorp, T.; Sayasneh, A.; Vergote, I.; Bourne, T.; Van Calster, B.; Timmerman, D. Prognostic Value of Serum HE4 Levels and Risk of Ovarian Malignancy Algorithm Scores at the Time of Ovarian Cancer Diagnosis. Int. J. Gynecol. Cancer 2014, 24, 1173–1180. [Google Scholar] [CrossRef]
- Steffensen, K.D.; Waldstrøm, M.; Brandslund, I.; Lund, B.; Soerensen, S.; Petzold, M.; Jakobsen, A. Identification of high-risk patients by human epididymis protein 4 levels during follow-up of ovarian cancer. Oncol. Lett. 2016, 11, 3967–3974. [Google Scholar] [CrossRef]
- Angioli, R.; Capriglione, S.; Aloisi, A.; Luvero, D.; Cafà, E.V.; Dugo, N.; Montera, R.; De Cicco Nardone, C.; Terranova, C.; Plotti, F. REM (risk of endometrial malignancy): A proposal for a new scoring system to evaluate risk of endometrial ma-lignancy. Clin. Cancer Res. 2013, 19, 5733–5739. [Google Scholar] [CrossRef]
- Li, M.; Balch, C.; Montgomery, J.S.; Jeong, M.; Chung, J.H.; Yan, P.; Huang, T.H.M.; Kim, S.; Nephew, K.P. Integrated analysis of DNA methylation and gene expression reveals specific signaling pathways associated with platinum resistance in ovarian cancer. BMC Med. Genom. 2009, 2, 34. [Google Scholar] [CrossRef]
| Variables | BRCA WT (53 pts) | BRCA MUT (16 pts) |
|---|---|---|
| AGE (years) Mean (range) | 64 (36–70) | 51 (43–70) |
| PERFORMANCE STATUS (ECOG 0–5) | ||
| 45 (85%) | 13 (81%) |
| 8 (15%) | 3 (19%) |
| FIGO stage n (%) | ||
| 7 (13%) | 2 (12.5%) |
| 9 (17%) | 2 (12.5%) |
| 34 (64%) | 9 (56%) |
| 3 (6%) | 3 (19%) |
| RESIDUAL TUMOR n (%) | ||
| 32 (60%) | 10 (63%) |
| 21 (40%) | 6 (37%) |
| HISTOLOGICAL SUBTYPE | ||
| High-Grade Serous Ovarian Cancer n (%) | 53 (100%) | 16 (100%) |
| PLATINUM-SENSITIVE n (%) | 23 (43%) | 13 (81%) |
| PLATINUM-RESISTANCE n (%) | 30 (57%) | 3 (19%) |
| All Population | Platinum-Sensitive | Platinum-Resistant | Total | |
|---|---|---|---|---|
| HE4 value at the III cycle of CT * | HE4 negative < 70 pmol/L | 35 (83%) | 7(17%) | 42 (61%) |
| HE4 positive > 70 pmol/L | 1 (4%) | 26 (96%) | 27 (39%) | |
| BRCA WT | Platinum-Sensitive | Platinum-Resistant | Total | |
|---|---|---|---|---|
| HE4 value at the III cycle of CT | HE4 negative < 70 pmol/L | 22 (76%) | 7 (24%) | 29 (100%) |
| HE4 positive > 70 pmol/L | 1 (4%) | 23 (96%) | 24 (100%) | |
| BRCA MUTATED | Platinum-Sensitive | Platinum-Resistant | Total | |
|---|---|---|---|---|
| HE4 value at the 3rd cycle of CT | HE4 negative < 70 pmol/L | 13 (100%) | 0 | 13 (100%) |
| HE4 positive > 70 pmol/L | 0 | 3 (100%) | 3 (100%) | |
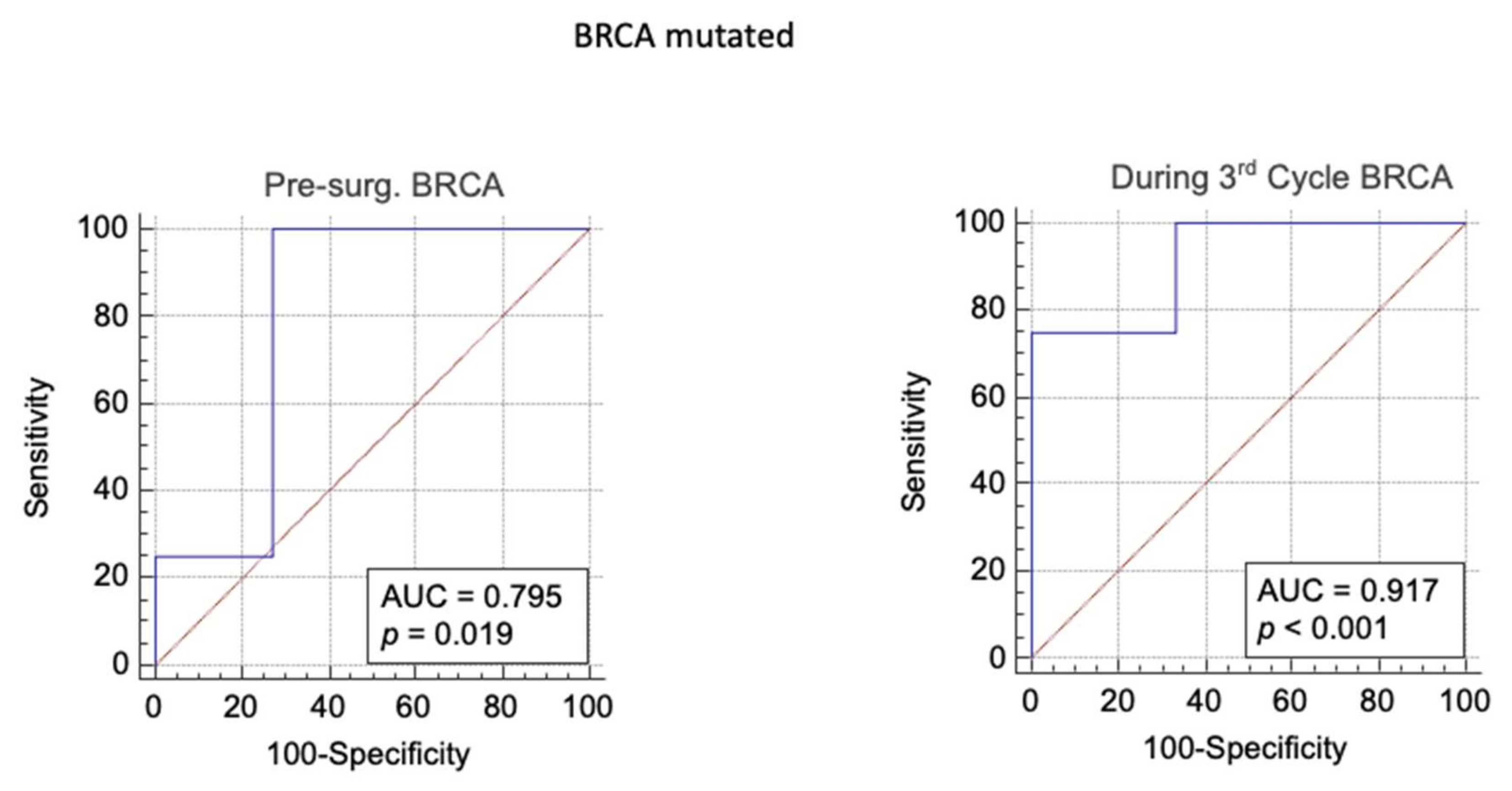
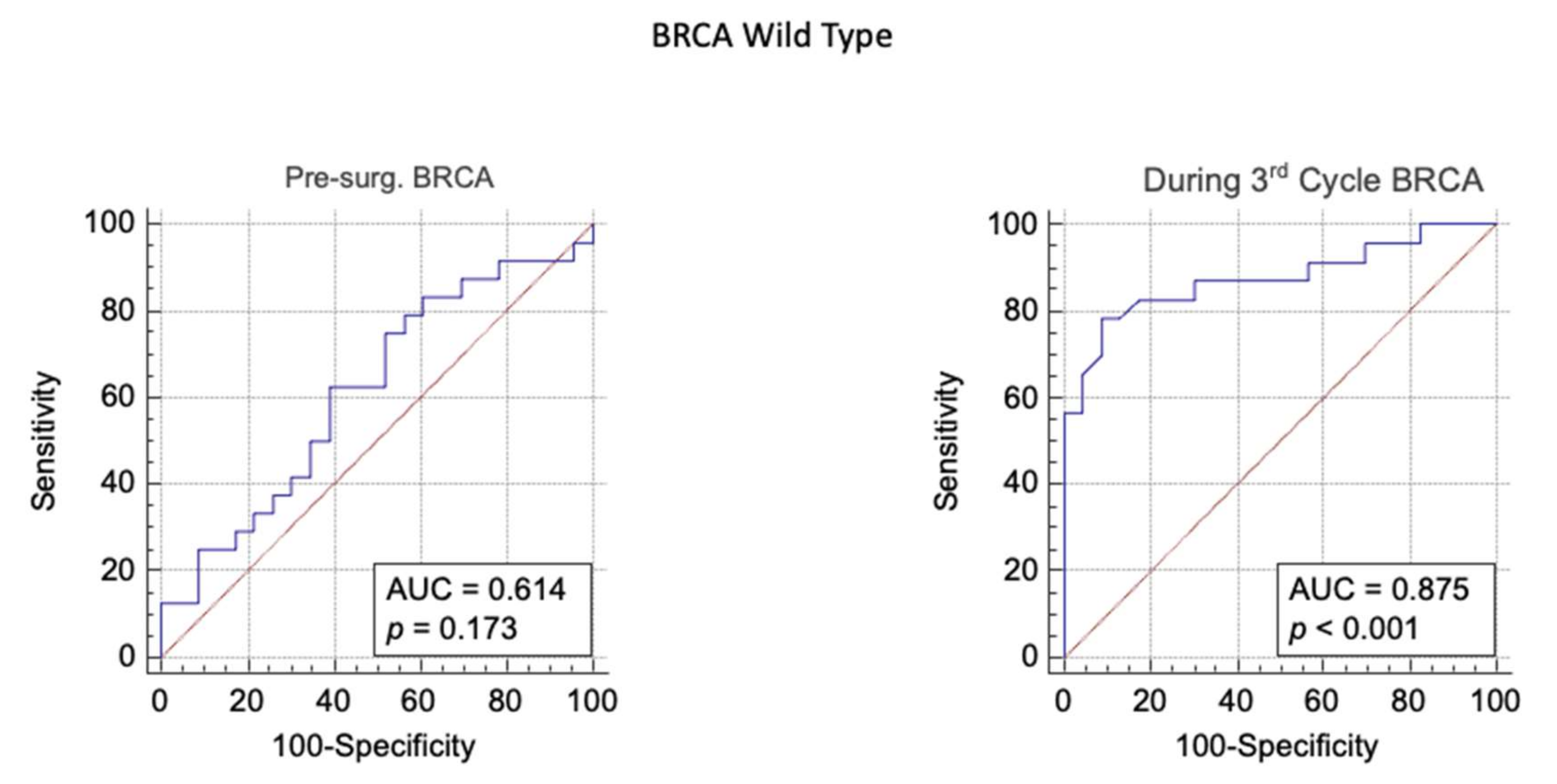
| Pre Test Probability | Area Under the Curve | Younden Index | Sensitivity | Specificity | p Value | Likehood Ratio+ | Likehood Ratio− | Post Test Odd | Post Test Probability | |
|---|---|---|---|---|---|---|---|---|---|---|
| During 3rd Cycle BRCA | 0.4 | 0.92 | > 66 | 75 | 99.9 | p < 0.001 | 750 | 0.25 | 500 | 0.99 |
| During 3rd Cycle WT | 0.4 | 0.87 | > 66 | 78 | 91 | p < 0.001 | 8.9 | 0.24 | 5.9 | 0.86 |
| Pre-Surg. BRCA | 0.4 | 0.79 | > 1767 | 99,9 | 73 | p = 0.019 | 3.7 | 0.001 | 2.4 | 0.71 |
| Pre-Surg. WT | 0.4 | 0.61 | > 231 | 63 | 61 | p = 0.17 | 1.6 | 0.62 | 1.06 | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotti, F.; Terranova, C.; Guzzo, F.; De Cicco Nardone, C.; Luvero, D.; Bartolone, M.; Dionisi, C.; Benvenuto, D.; Fabris, S.; Ciccozzi, M.; et al. Role of BRCA Mutation and HE4 in Predicting Chemotherapy Response in Ovarian Cancer: A Retrospective Pilot Study. Biomedicines 2021, 9, 55. https://doi.org/10.3390/biomedicines9010055
Plotti F, Terranova C, Guzzo F, De Cicco Nardone C, Luvero D, Bartolone M, Dionisi C, Benvenuto D, Fabris S, Ciccozzi M, et al. Role of BRCA Mutation and HE4 in Predicting Chemotherapy Response in Ovarian Cancer: A Retrospective Pilot Study. Biomedicines. 2021; 9(1):55. https://doi.org/10.3390/biomedicines9010055
Chicago/Turabian StylePlotti, Francesco, Corrado Terranova, Federica Guzzo, Carlo De Cicco Nardone, Daniela Luvero, Martina Bartolone, Camilla Dionisi, Domenico Benvenuto, Silvia Fabris, Massimo Ciccozzi, and et al. 2021. "Role of BRCA Mutation and HE4 in Predicting Chemotherapy Response in Ovarian Cancer: A Retrospective Pilot Study" Biomedicines 9, no. 1: 55. https://doi.org/10.3390/biomedicines9010055
APA StylePlotti, F., Terranova, C., Guzzo, F., De Cicco Nardone, C., Luvero, D., Bartolone, M., Dionisi, C., Benvenuto, D., Fabris, S., Ciccozzi, M., Di Donato, V., Panici, P. B., & Angioli, R. (2021). Role of BRCA Mutation and HE4 in Predicting Chemotherapy Response in Ovarian Cancer: A Retrospective Pilot Study. Biomedicines, 9(1), 55. https://doi.org/10.3390/biomedicines9010055








