Anti-HER2/neu Antibody Reduces Chemotherapy-Induced Ovarian Toxicity—From Bench to Bedside
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design in Mice
2.2. Patients
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) for AMH
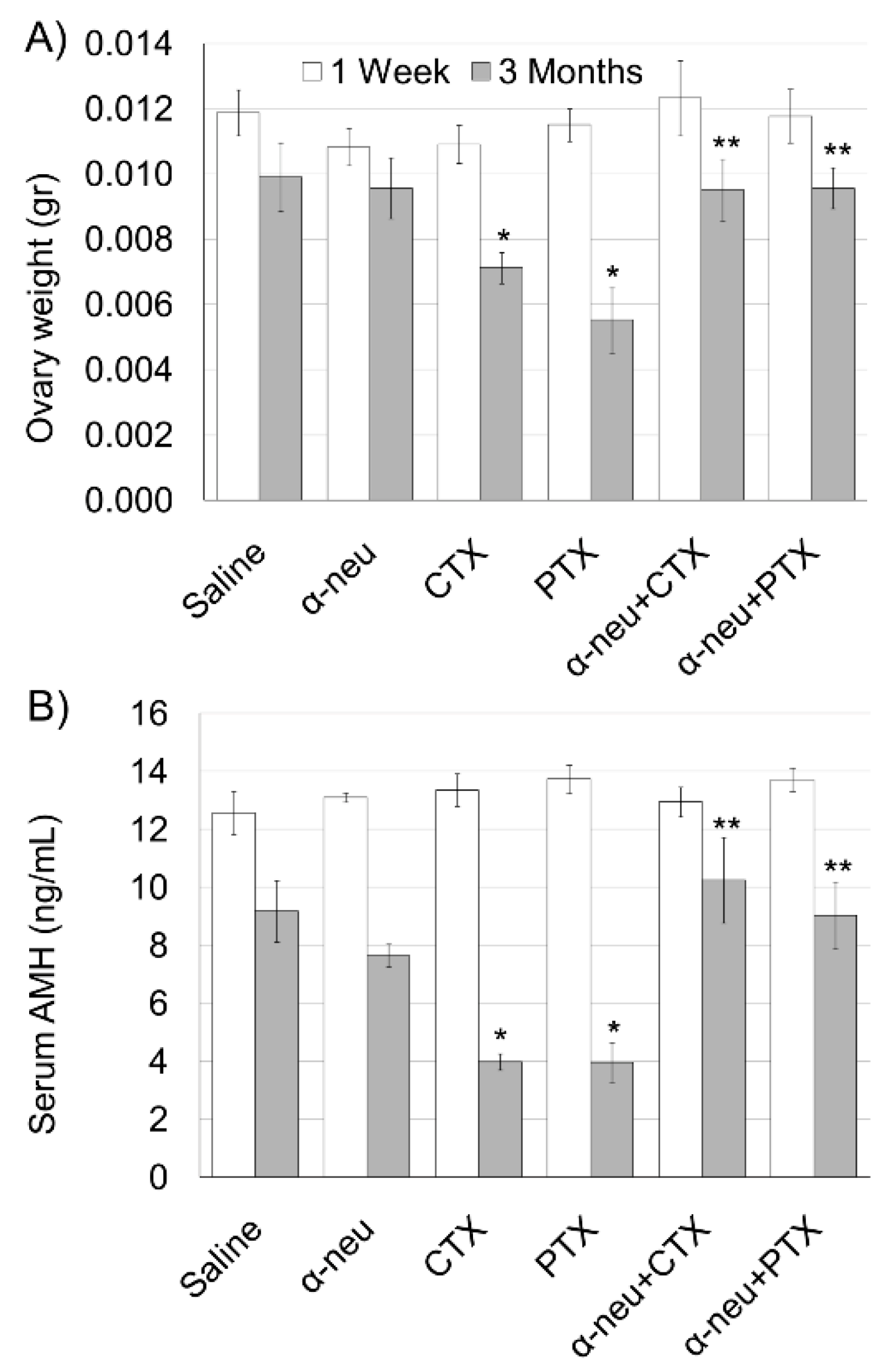
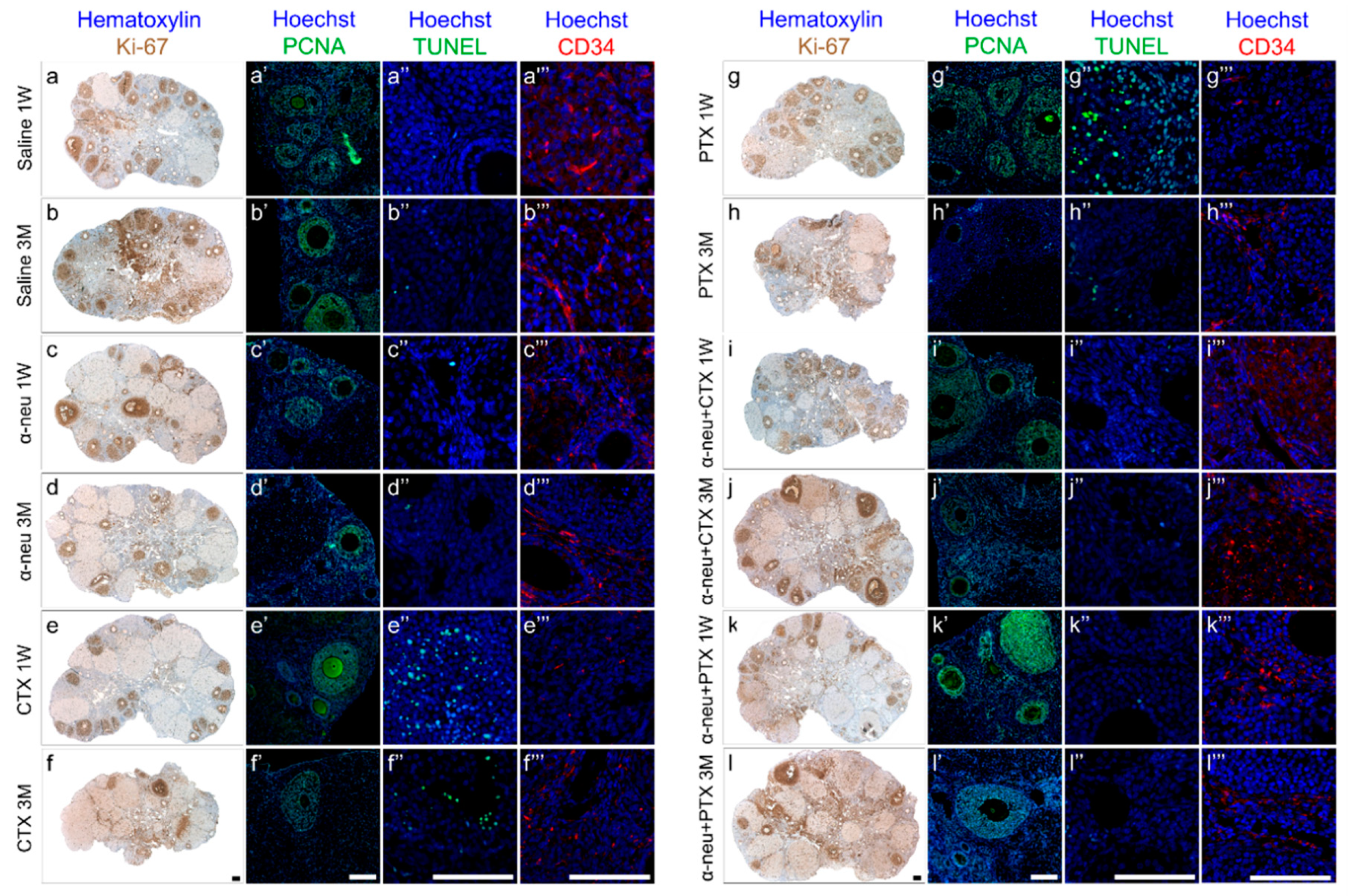
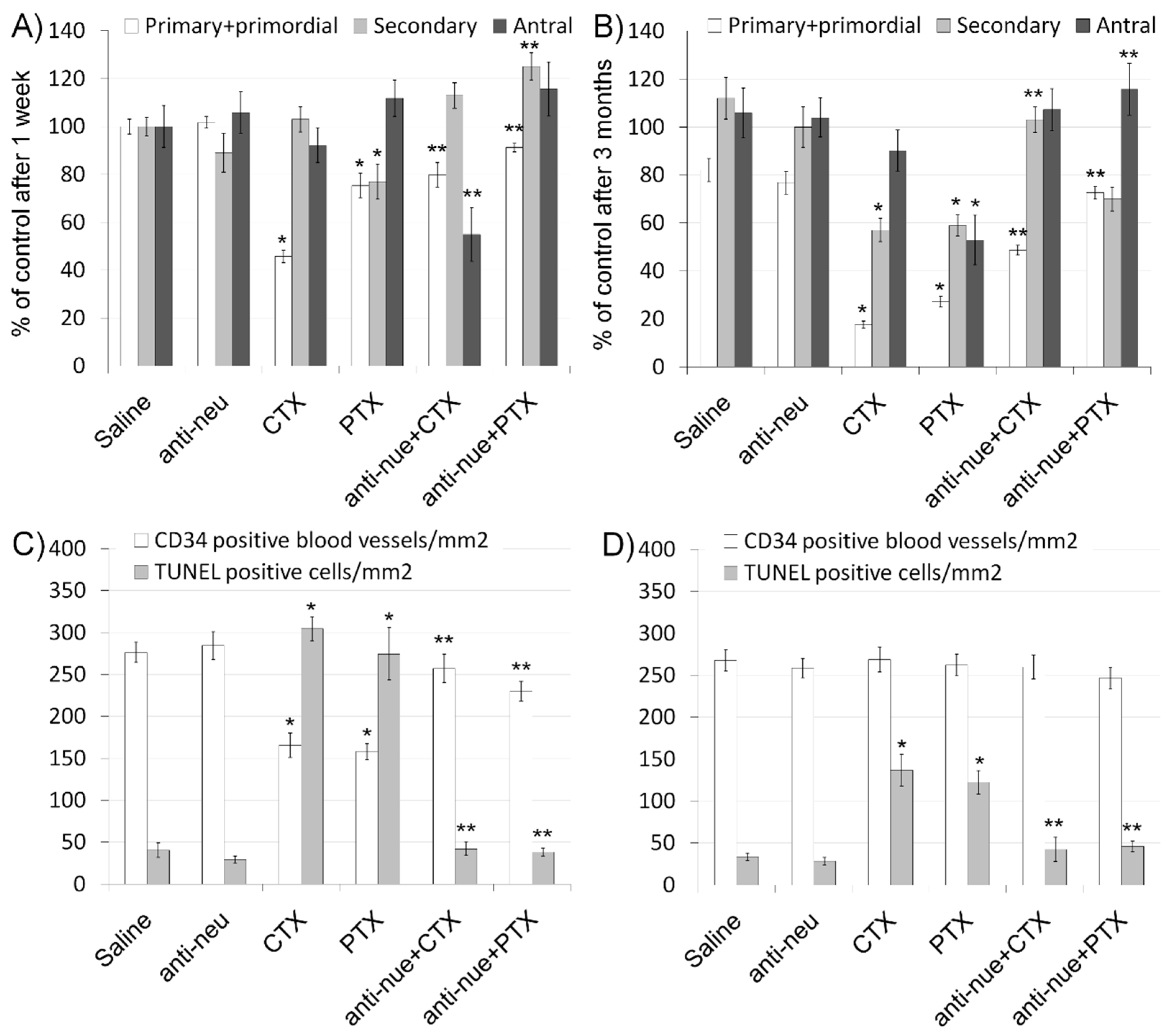
2.4. Immunohistochemistry (IHC), Terminal Transferase-Mediated Deoxyuridine 5-Triphosphate Nick-End Labeling (TUNEL) and Morphometric Analysis of Ovaries
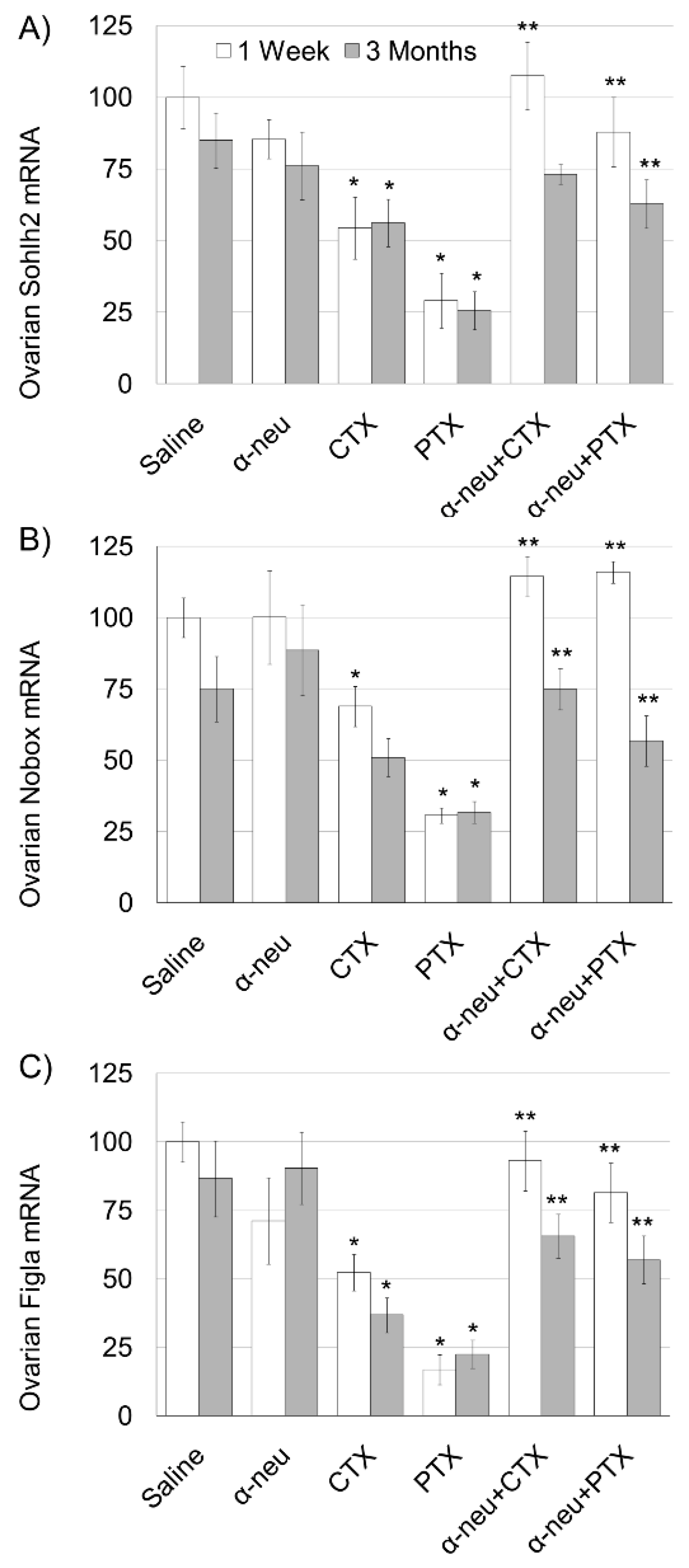
2.5. Quantitative Real-Time PCR (qPCR)
2.6. Statistical Analysis
3. Results
3.1. Anti-HER2/neu Protect from Ovarian Damage Caused by Chemotherapy in Mice
3.2. Anti-HER2/neu Lessens Chemotherapy-Induced Ovarian Toxicity in Breast Cancer Patients
3.3. HER2/neu Expression on Ovarian Vasculature
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Becker, S. A historic and scientific review of breast cancer: The next global healthcare challenge. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S1), S36–S39. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Klemp, J.; Fabian, C. Breast cancer and fertility preservation. Fertil. Steril. 2011, 95, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.S.; Stern, D.F.; Polverini, P.J.; Bender, J.R. Neuregulin activation of ErbBreceptors in vascular endothelium leads to angiogenesis. Am. J. Physiol. 1999, 277, H2205–H2211. [Google Scholar] [PubMed]
- Park, S.; Jiang, Z.; Mortenson, E.D.; Deng, L.; Radkevich-Brown, O.; Yang, X.; Sattar, H.; Wang, Y.; Brown, N.K.; Greene, M.; et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010, 18, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aharon, I.; Bar Joseph, H.; Tzabari, M.; Shenkman, B.; Farzam, N.; Levi, M.; Shalgi, R.; Stemmer, S.M.; Savion, N. Doxorubicin-induced vascular toxicity--targeting potential pathways may reduce procoagulant activity. PLoS ONE 2013, 20, e75157. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aharon, I.; Granot, T.; Meizner, I.; Hasky, N.; Tobar, A.; Rizel, S.; Yerushalmi, R.; Ben-Haroush, A.; Fisch, B.; Stemmer, S.M. Long-Term Follow-Up of Chemotherapy-Induced Ovarian Failure in Young Breast Cancer Patients: The Role of Vascular Toxicity. Oncologist 2015, 20, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Hasky, N.; Uri-Belapolsky, S.; Goldberg, K.; Miller, I.; Grossman, H.; Stemmer, S.M.; Ben-Aharon, I.; Shalgi, R. Gonadotrophin-releasing hormone agonists for fertility preservation: Unraveling the enigma? Hum. Reprod. 2015, 30, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Hasky, N.; Stemmer, S.M.; Shalgi, R.; Ben-Aharon, I. Anti-Müllerian Hormone Is a Marker for Chemotherapy-Induced Testicular Toxicity. Endocrinology 2015, 156, 3818–3827. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Shalgi, R.; Brenner, B.; Perl, G.; Purim, O.; Amit, L.; Stemmer, S.M.; Ben-Aharon, I. The impact of oxaliplatin on the gonads: From bedside to the bench. Mol. Hum. Reprod. 2015, 21, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Bar-Joseph, H.; Ben-Aharon, I.; Tzabari, M.; Tsarfaty, G.; Stemmer, S.M.; Shalgi, R. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PLoS ONE 2011, 6, e23492. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aharon, I.; Levi, M.; Margel, D.; Yerushalmi, R.; Rizel, S.; Perry, S.; Sharon, E.; Hasky, N.; Abir, R.; Fisch, B.; et al. Premature ovarian aging in BRCA carriers: A prototype for systemic precocious aging? Oncotarget 2018, 9, 15931–15941. [Google Scholar] [CrossRef] [PubMed]
- Uri-Belapolsky, S.; Shaish, A.; Eliyahu, E.; Grossman, H.; Levi, M.; Chuderland, D.; Ninio-Many, L.; Hasky, N.; Shashar, D.; Almog, T.; et al. Interleukin-1 deficiency prolongs ovarian lifespan in mice. PNAS 2014, 111, 12492–12497. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Demeestere, I.; Viglietti, G.; de Azambuja, E. Oncofertility counselling in premenopausal women with HER2-positive breast cancer. Oncotarget 2019, 10, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, K.J.; Guo, H.; Barry, W.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; Albain, K.S.; Rugo, H.S.; Ellis, M.J.; et al. Chemotherapy-related amenorrhea after adjuvant paclitaxel-trastuzumab (APT trial). Breast Cancer Res. Treat. 2015, 151, 589–596. [Google Scholar] [CrossRef]
- Guan, H.; Jia, S.F.; Zhou, Z.; Stewart, J.; Kleinerman, E.S. Herceptin down-regulates HER-2/neu and vascular endothelial growth factor expression and enhances taxol-induced cytotoxicity of human Ewing’s sarcoma cells in vitro and in vivo. Clin. Cancer Res. 2005, 11, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Hardee, M.E.; Eapen, R.J.; Rabbani, Z.N.; Dreher, M.R.; Marks, J.; Blackwell, K.L.; Dewhirst, M.W. Her2/neu signaling blockade improves tumor oxygenation in a multifactorial fashion in Her2/neu+ tumors. Cancer Chemother. Pharmacol. 2009, 63, 219–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shen, G.; Huang, H.; Zhang, A.; Zhao, T.; Hu, S.; Cheng, L.; Liu, J.; Xiao, W.; Ling, B.; Wu, Q.; et al. In vivo activity of novel anti-ErbB2 antibody chA21 alone and with Paclitaxel or Trastuzumab in breast and ovarian cancer xenograft models. Cancer Immunol. Immunother. 2011, 60, 339–348. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.R.; Walsh, A.J.; Sit, W.; Arteaga, C.L.; Chen, J.; Cook, R.S.; Skala, M.C. In vivo hyperspectral imaging of microvessel response to trastuzumab treatment in breast cancer xenografts. Biomed. Opt. Express 2014, 16, 2247–2261. [Google Scholar] [CrossRef] [PubMed]
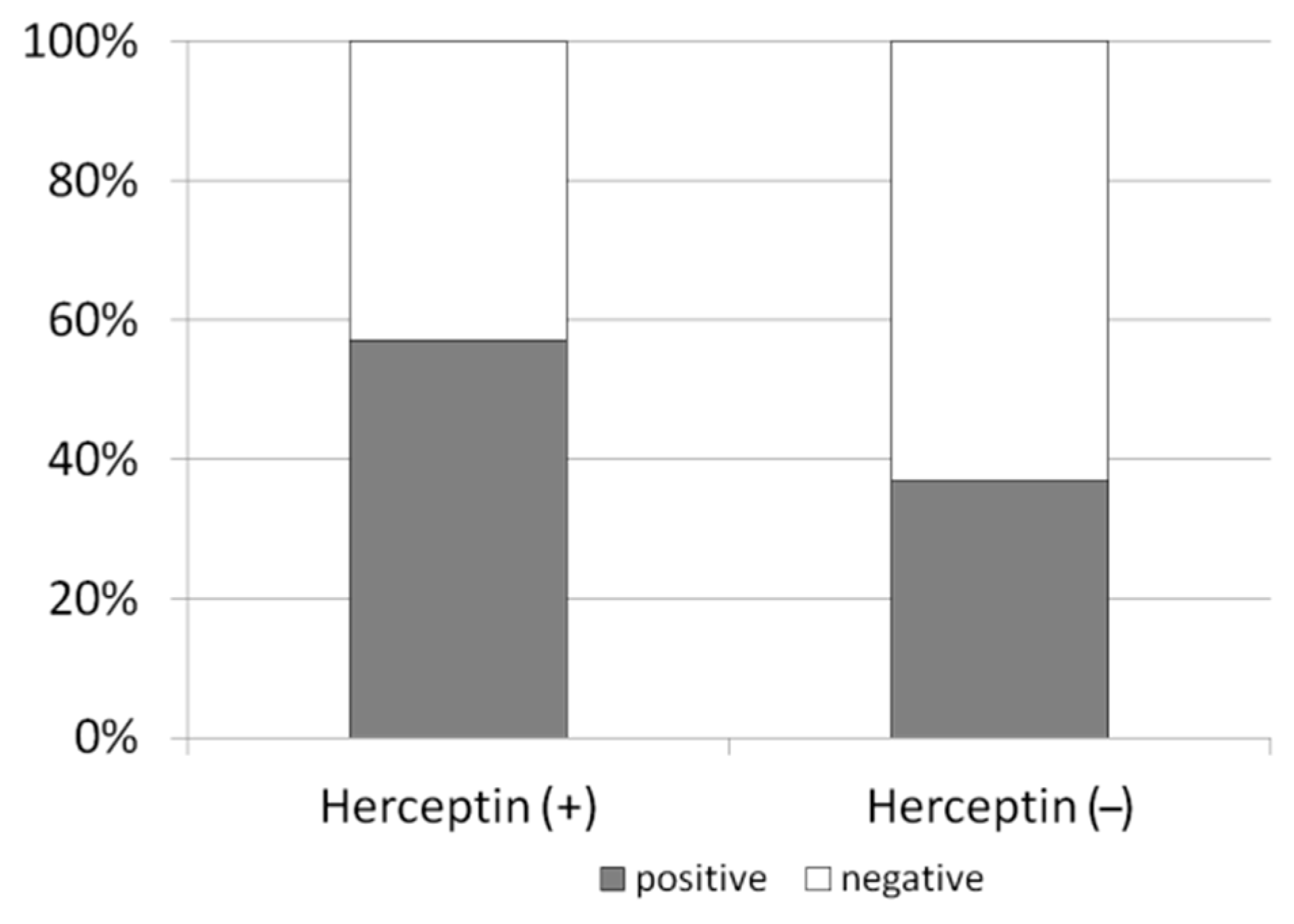
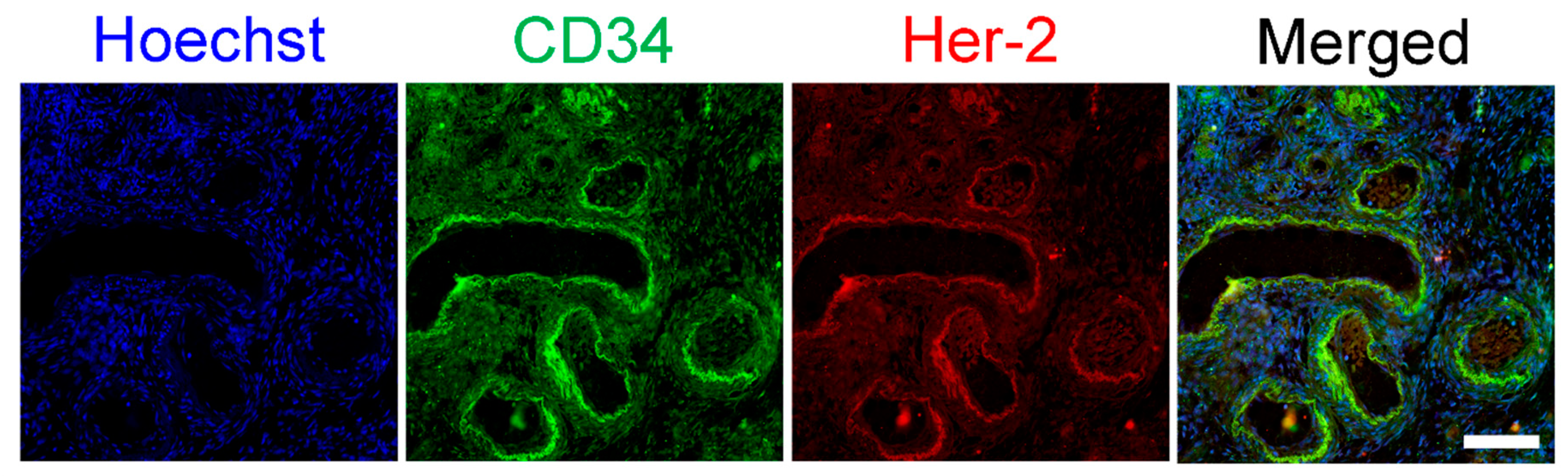
| Patient | Age | Stage | HER2 | Chemo Protocol |
|---|---|---|---|---|
| 1 | 27 | 1c | 3+ | DC-H |
| 2 | 29 | 1 | 3+ | ACT-H |
| 3 | 34 | 3 | 3+ | T-H |
| 4 | 34 | 1c | − | ACT |
| 5 | 36 | 2 | − | AT |
| 6 | 31 | 2b | − | AC |
| 7 | 39 | 2a | 3+ | T-H |
| 8 | 38 | 1a | 3+ | AC-H |
| 9 | 31 | 1c | − | AC |
| 10 | 43 | 1b | 3+ | DC-H |
| 11 | 33 | 1b | − | AT |
| 12 | 40 | 2 | − | AT |
| 13 | 37 | 2 | − | AT |
| 14 | 42 | 3 | 3+ | T-H |
| 15 | 37 | 2a | − | ACD |
| 16 | 31 | 2a | − | DC |
| 17 | 34 | 3 | 2 | A |
| 18 | 38 | 1 | 2+ | DC-H |
| 19 | 35 | 2a | 1 | DC |
| 20 | 29 | 1 | 3+ | DC-H |
| 21 | 35 | 2a | − | AC |
| 22 | 26 | 4 | 3+ | T-H |
| 23 | 38 | 2a | 3+ | T-H |
| 24 | 27 | 2b | − | AC |
| 25 | 37 | 1b | − | AC |
| 26 | 38 | 2a | − | DC |
| 27 | 29 | 2 | − | AC |
| 28 | 37 | 3 | − | A |
| 29 | 28 | 2 | − | AC |
| 30 | 38 | 2 | 3+ | ACT-H |
| 31 | 28 | 2 | 3+ | T-H |
| 32 | 36 | 4 | 3+ | DC-H |
| 33 | 26 | 2 | − | AC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levi, M.; Goshen-Lago, T.; Yerushalmi, R.; Granot, T.; Stemmer, S.M.; Shalgi, R.; Ben-Aharon, I. Anti-HER2/neu Antibody Reduces Chemotherapy-Induced Ovarian Toxicity—From Bench to Bedside. Biomedicines 2020, 8, 577. https://doi.org/10.3390/biomedicines8120577
Levi M, Goshen-Lago T, Yerushalmi R, Granot T, Stemmer SM, Shalgi R, Ben-Aharon I. Anti-HER2/neu Antibody Reduces Chemotherapy-Induced Ovarian Toxicity—From Bench to Bedside. Biomedicines. 2020; 8(12):577. https://doi.org/10.3390/biomedicines8120577
Chicago/Turabian StyleLevi, Mattan, Tal Goshen-Lago, Rinat Yerushalmi, Tal Granot, Salomon M. Stemmer, Ruth Shalgi, and Irit Ben-Aharon. 2020. "Anti-HER2/neu Antibody Reduces Chemotherapy-Induced Ovarian Toxicity—From Bench to Bedside" Biomedicines 8, no. 12: 577. https://doi.org/10.3390/biomedicines8120577
APA StyleLevi, M., Goshen-Lago, T., Yerushalmi, R., Granot, T., Stemmer, S. M., Shalgi, R., & Ben-Aharon, I. (2020). Anti-HER2/neu Antibody Reduces Chemotherapy-Induced Ovarian Toxicity—From Bench to Bedside. Biomedicines, 8(12), 577. https://doi.org/10.3390/biomedicines8120577





