Using Ex Vivo Porcine Jejunum to Identify Membrane Transporter Substrates: A Screening Tool for Early—Stage Drug Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Porcine Intestine
2.3. Experimental Protocol for Permeation Studies
2.4. Analytical Methods
2.5. Data Analysis
2.5.1. Permeability Calculations
- dc/dt: change in the acceptor concentration calculated from the slope of the concentration–time curve between 20 and 80 min
- V: volume of the buffer in the donor compartment (7 mL)
- A: exposed surface area (1.26 cm2)
- C0: initial concentration of the substrate in the donor compartment (100 µM, in case of digoxin: 50 µM)
2.5.2. Calculating Drug Deposition (QDEP) and Drug Permeation (QPERM)
- mint 2h: amount of drug in the intestinal membrane at the end of the experiment (t = 2 h)
- macc 2h: amount of drug permeated into the acceptor compartment at the end of the experiment (t = 2 h)
- mdonor 0h: amount of drug in the donor compartment at the beginning of the experiment (t = 0 h)
2.6. Statistical Analysis
3. Results and Discussion
3.1. SLC Transporters
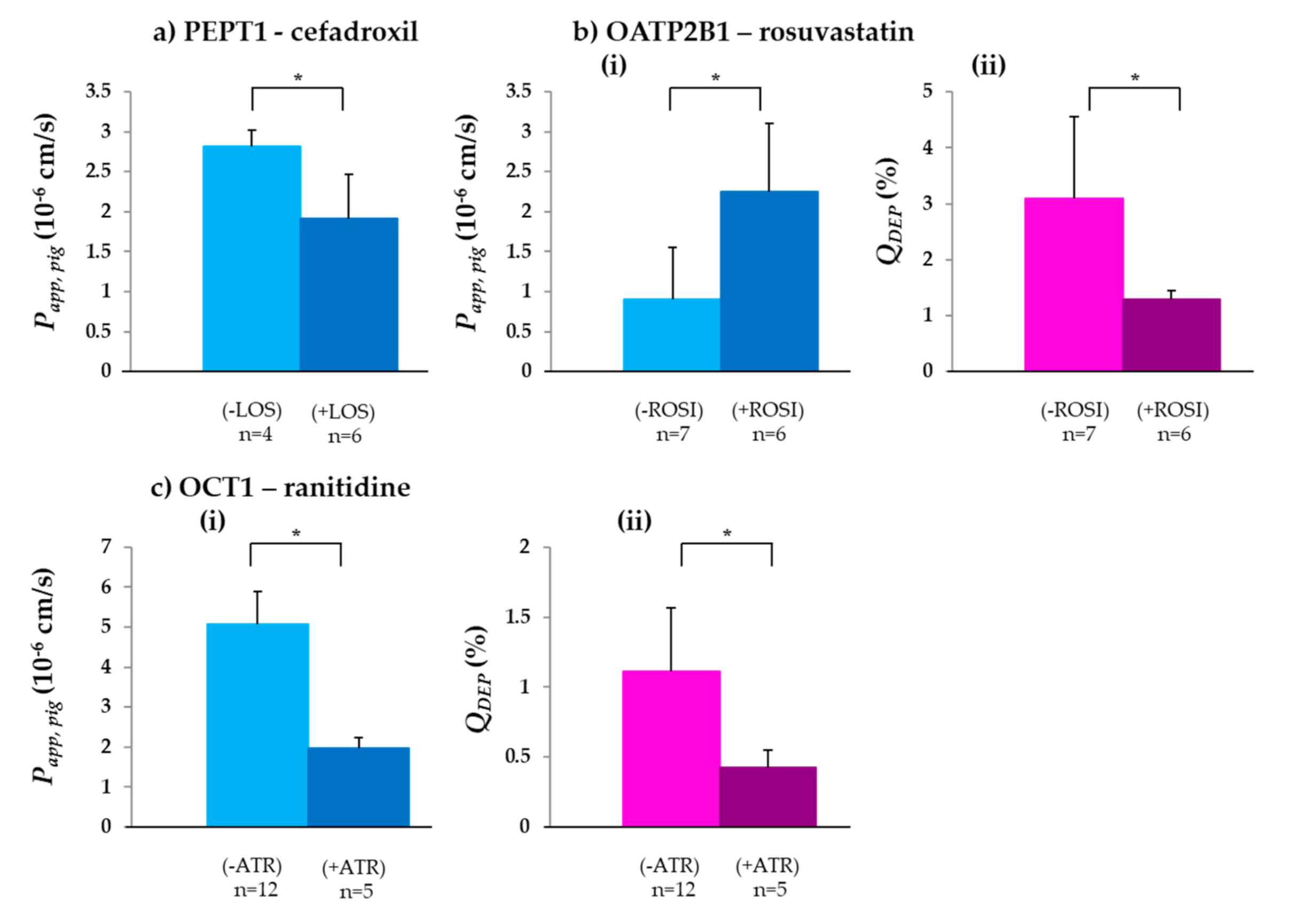
3.1.1. PEPT1
3.1.2. OATP2B1
3.1.3. OCT1
3.2. ABC Transporters
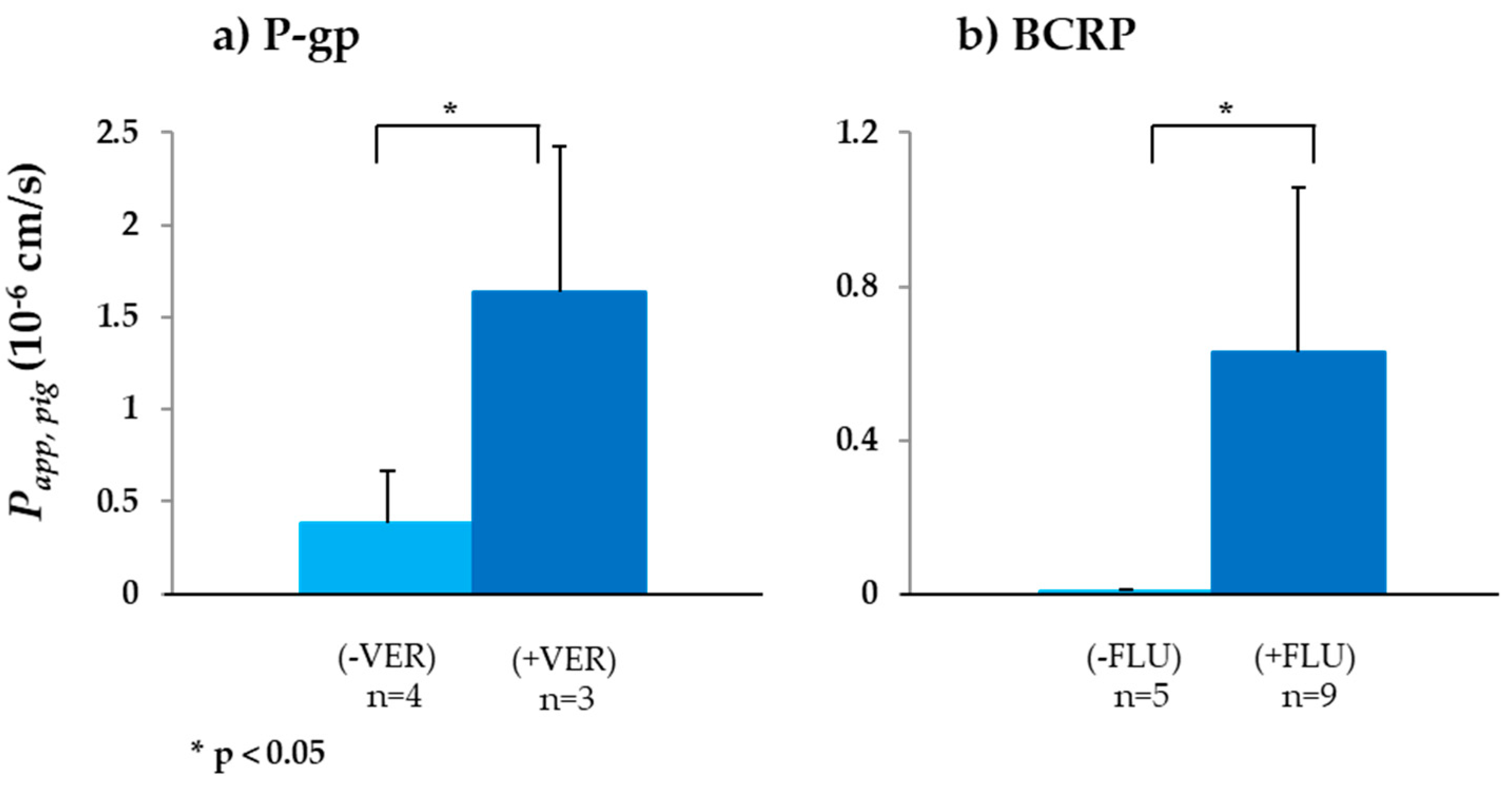
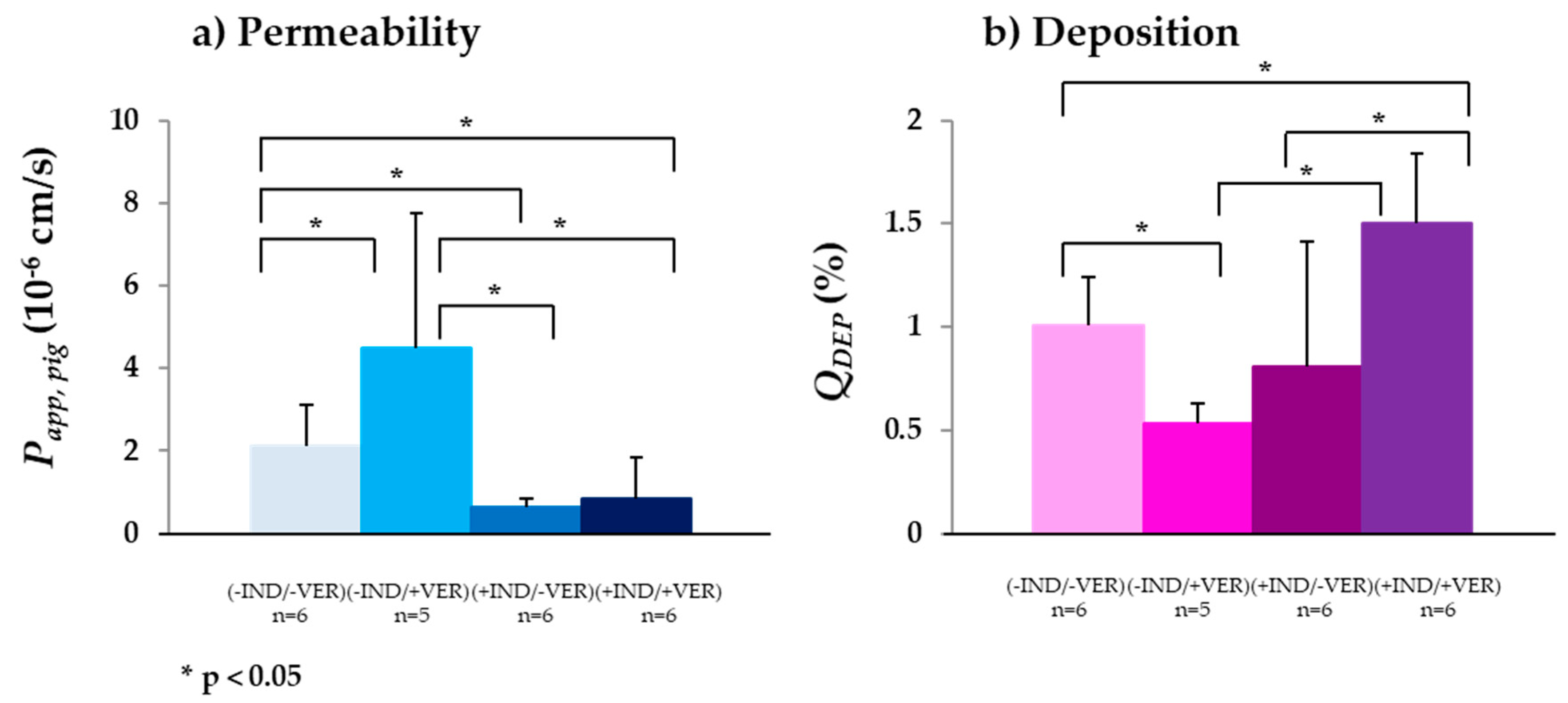
3.2.1. P-gp
3.2.2. BCRP
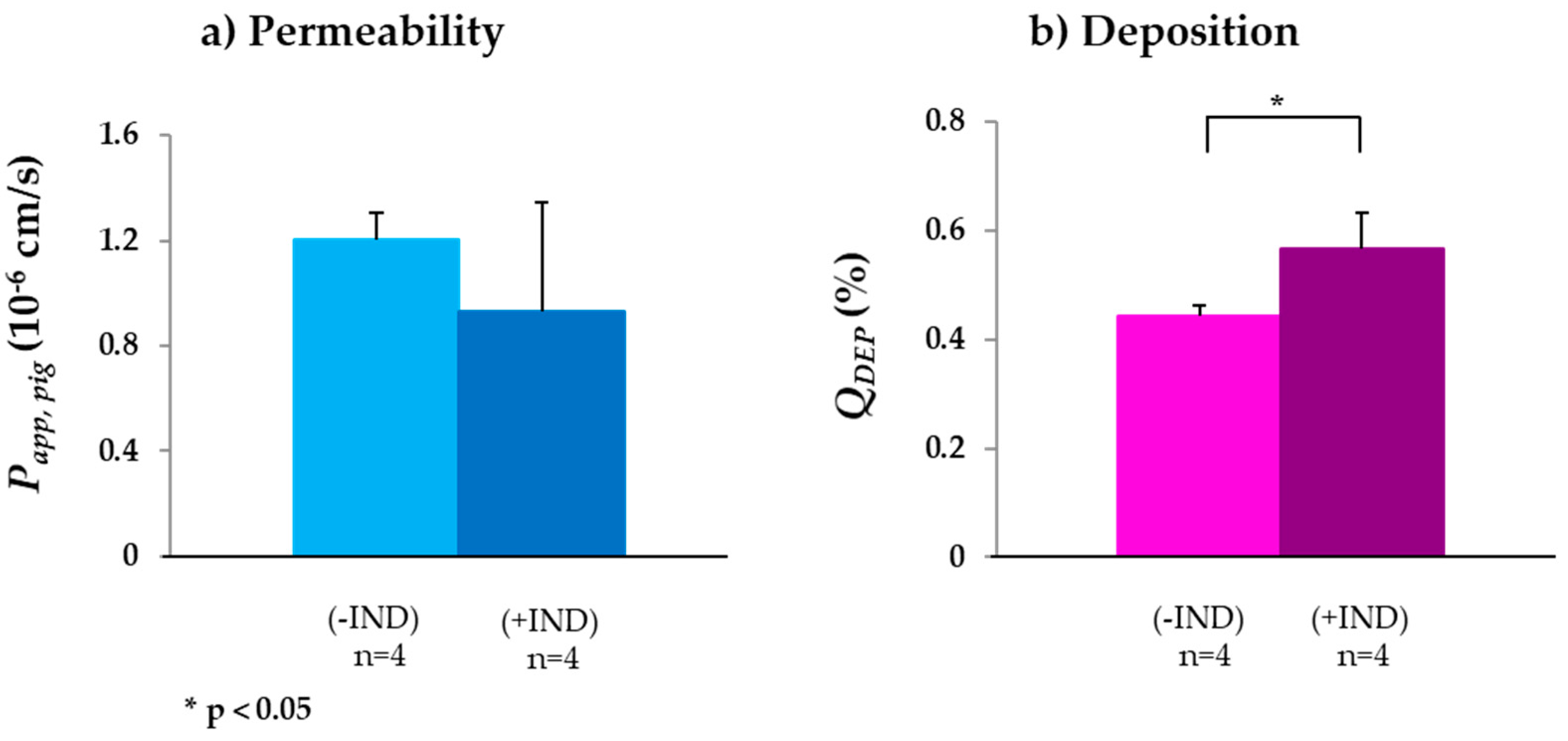
3.2.3. MRP2
3.2.4. MRP3
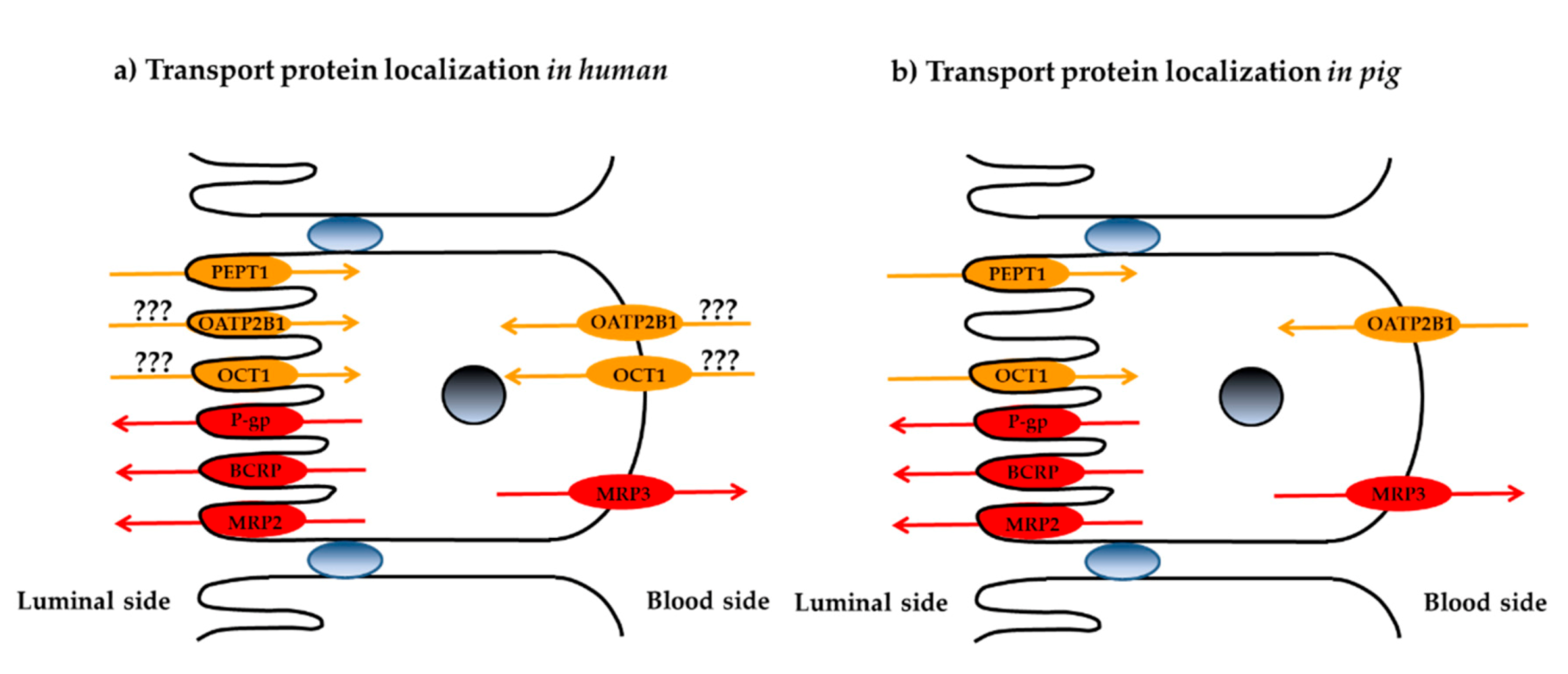
3.3. Localization of Membrane Transporters in Porcine Jejunum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005, 6, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Oswald, S. Organic anion transporting polypeptide (OATP) transporter expression, localization and function in the human intestine. Pharmacol. Ther. 2018, 195, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, K.M.; Huang, S.-M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef]
- Hillgren, K.M.; Keppler, D.; Zur, A.; Giacomini, K.M.; Stieger, B.; Cass, C.; Zhang, L. Emerging transporters of clinical importance: An update from the international transporter consortium. Clin. Pharmacol. Ther. 2013, 94, 52–63. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, S.-M.; Lesko, L.J. Transporter-mediated drug-drug interactions. Clin. Pharmacol. Ther. 2011, 89, 481–484. [Google Scholar] [CrossRef]
- Koenig, J.; Mueller, F.; Fromm, M.F. Transporters and drug-drug interactions: Important determinants of drug disposition and effects. Pharmacol. Rev. 2013, 65, 944–966. [Google Scholar] [CrossRef]
- Steffansen, B.; Nielsen, C.U.; Brodin, B.; Eriksson, A.H.; Andersen, R.; Frokjaer, S. Intestinal solute carriers: An overview of trends and strategies for improving oral drug absorption. Eur. J. Pharm. Sci. 2004, 21, 3–16. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef]
- Adibi, S.A. The oligopeptide transporter (Pept-1) in human intestine: Biology and function. Gastroenterology 1997, 113, 332–340. [Google Scholar] [CrossRef]
- Han, T.K.; Everett, R.S.; Proctor, W.R.; Ng, C.M.; Costales, C.L.; Brouwer, K.L.; Thakker, D.R. Organic cation transporter 1 (OCT1/mOCT1) is localized in the apical membrane of Caco-2 cell monolayers and enterocytes. Mol. Pharmacol. 2013, 84, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OATs abd OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012, 165, 1260–1287. [Google Scholar] [CrossRef] [PubMed]
- Estudante, M.; Morais, J.; Soveral, G.; Benet, L.Z. Intestinal drug transporter: An overview. Adv. Drug Deliv. Rev. 2013, 65, 1340. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, D.; Lennernäs, H. Intestinal permeability and drug absorption: Predictive experimental, computational and in vivo approaches. Pharmaceutics 2019, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.; Janich, S.; Roessler, J.; Hilfinger, J.; Amidon, G. H29-MTX/Caco-2 cocultures as an in vitro model for the intestinal epithelium: In vitro-in vivo correlation with permeability data from rats and humans. J. Pharm. Sci. 1996, 85, 1070–1076. [Google Scholar] [CrossRef]
- Hilgendorf, C.; Spahn-Langguth, H.; Regardh, C.; Lipka, E.; Amidon, G.; Langguth, P. Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: Permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef]
- Araujo, F.; Sarmento, B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int. J. Pharm. 2013, 458, 128–134. [Google Scholar] [CrossRef]
- Lozoya-Agullo, I.; Araujo, F.; Gonzalez-Alvarez, I.; Merino-Sanjuan, M.; Gonzalez-Alvarez, M.; Bermejo, M.; Sarmento, B. Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B coculture models to predict intestinal and colonic permeability compared to Caco-2 monoculture. Mol. Pharm. 2017, 14, 1264–1270. [Google Scholar] [CrossRef]
- Li, X.; Mu, P.; Wen, J.; Deng, Y. Carrier-mediated and energy dependent uptake and efflux of deoxynivalenol in mammalian cells. Sci. Rep. 2017, 7, 5889. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Drozdzik, M.; Gröer, C.; Penski, J.; Lapczuk, J.; Ostrowski, M.; Lai, Y.; Prasad, B. Protein abundance of clinically relevant multidrug transporters along the entire length of the human intestine. Mol. Pharm. 2014, 11, 3547–3555. [Google Scholar] [CrossRef] [PubMed]
- Maubon, N.; Le Vee, M.; Fossati, L.; Audry, M.; Le Ferrec, E.; Bolze, S.; Fardel, O. Analysis of drug transporter expression in human intestinal Caco-2 cells by real-time PCR. Fundam. Clin. Pharmacol. 2007, 21, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Ingels, F.; Deferme, S.; Destexhe, E.; Oth, M.; Van den Mooter, G.; Augustijns, P. Simulated intestinal fluid as transport medium in the Caco-2 cell culture model. Int. J. Pharm. 2002, 232, 183–192. [Google Scholar] [CrossRef]
- Sjögren, E.; Eriksson, J.; Vedin, C.; Breitholtz, K.; Hilgendorf, C. Excised segments of rat small intestine in Ussing chamber studies: A comparison of native and stripped tissue viability and permeability to drugs. Int. J. Pharm. 2016, 505, 361–368. [Google Scholar] [CrossRef]
- Ungell, A.-L.; Nylander, S.; Bergstrand, S.; Sjöberg, A.; Lennernäs, H. Membrane transport of drugs in different regions of the intestinal tract of the rat. J. Pharm. Sci. 1998, 87, 360–366. [Google Scholar] [CrossRef]
- Lennernäs, H. Animal data: The contributions of the Ussing chamber and perfusion systems to predicting human oral drug delivery in vivo. Adv. Drug Deliv. Rev. 2007, 59, 1103–1120. [Google Scholar] [CrossRef]
- Neirinckx, E.; Vervaet, C.; Michiels, J.; De Smet, S.; Van den Broeck, W.; Remon, J.P.; De Backer, P.; Croubels, S. Feasibility of the Ussing chamber technique for the determination of in vitro jejunal permeability of passively absorbed compounds in different animal species. J. Vet. Pharmacol. Ther. 2010, 34, 290–297. [Google Scholar] [CrossRef]
- Escribano, E.; Sala, X.G.; Salamanca, J.; Navarro, C.R.; Regué, J.Q. Single-pass intestinal perfusion to establish the intestinal permeability of model drugs in mouse. Int. J. Pharm. 2012, 436, 472–477. [Google Scholar] [CrossRef]
- Sjöberg, A.; Lutz, M.; Tannergren, C.; Wingolf, C.; Borde, A.; Ungell, A.-L. Comprehensive study on regional human intestinal permeability and prediction of fraction absorbed of drugs using the Ussing chamber technique. Eur. J. Pharm. Sci. 2013, 48, 166–180. [Google Scholar] [CrossRef]
- Rozehnal, V.; Nakai, D.; Hoepner, U.; Fischer, T.; Kamiyama, E.; Takahashi, M.; Mueller, J. Human small intestinal and colonic tissue mounted in the Ussing chamber as a tool for characterizing the intestinal absorption of drugs. Eur. J. Pharm. Sci. 2012, 46, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Toguchi, H.; Nishibayashi, T.; Higaki, K.; Sugita, A.; Koganei, K.; Kamada, N.; Kitazume, M.T.; Hisamatsu, T.; Sato, T.; et al. Establishment of novel prediction system of intestinal absorption in humans using human intestinal tissue. J. Pharm. Sci. 2013, 102, 2564–2571. [Google Scholar] [CrossRef]
- Deglaire, A.; Moughan, P.J. Animal models for determining amino acid digestibility in humans—A review. Br. J. Nutr. 2012, 108, S273–S281. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.K.; Lei, X.G.; Miller, D.D. The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Exp. Biol. Med. 2008, 233, 651–664. [Google Scholar] [CrossRef]
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef]
- Sjögren, E.; Abrahamsson, B.; Augustijns, P.; Becker, D.; Bolger, M.B.; Brewster, M.; Brouwers, J.; Flanagan, T.; Harwood, M.; Heinen, C.; et al. In vivo methods for drug absorption—Comparative physiologies, model selection, correlation with in vitro methods (IVIC), and applications for formulation/API/excipient characterization including food effects. Eur. J. Pharm. Sci. 2014, 57, 99–151. [Google Scholar] [CrossRef]
- Westerhout, J.; van de Steeg, A.; Grossouw, D.; Zeijdner, E.E.; Krul, C.A.M.; Verwei, M.; Wortelboer, H.M. A new approach to predict human intestinal absorption using porcine intestinal tissue and biorelevant matrices. Eur. J. Pharm. Sci. 2014, 63, 167–177. [Google Scholar] [CrossRef]
- Shikanga, E.A.; Hamman, J.H.; Chen, W.; Combrinck, S.; Gericke, N.; Viljoen, A.M. In vitro permeation of mesembrine alkaloids from Sceletium tortuosum across porcine buccal, sublingual, and intestinal mucosa. Planta Med. 2012, 78, 260–268. [Google Scholar] [CrossRef]
- Erk, T.; Hauser, J.; Williamson, G.; Renouf, M.; Steiling, H.; Dionisi, F.; Richling, E. Structure- and dose-absorption relationships of coffee polyphenols. Biofactors 2013, 40, 103–112. [Google Scholar] [CrossRef]
- Deusser, H.; Rogoll, D.; Scheppach, W.; Volk, A.; Melcher, R.; Richling, E. Gastrointestinal absorption and metabolism of apple polyphenols ex vivo by the pig intestinal mucosa in the Ussing chamber. Biotechnol. J. 2013, 8, 363–370. [Google Scholar] [CrossRef]
- Herrmann, J.; Hermes, R.; Breves, G. Transepithelial transport and intraepithelial metabolism of short-chain fatty acids (SCFA) in the porcine proximal colon are influenced by SCFA concentration and luminal pH. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 158, 169–176. [Google Scholar] [CrossRef]
- Gerber, W.; Hamman, J.H.; Steyn, J.D. Excipient-drug pharmacokinetic interactions: Effect of disintegrants on efflux across excised pig intestinal tissues. J. Food Drug Anal. 2018, 26, S115–S124. [Google Scholar] [CrossRef]
- Atlabachew, M.; Combrinck, S.; Viljoen, A.M.; Hamman, J.H.; Gouws, C. Isolation and in vitro permeation of phenylpropylamino alkaloids from Khat (Catha edulis) across oral and intestinal mucosal tissues. J. Ethnopharmacol. 2016, 194, 307–315. [Google Scholar] [CrossRef]
- Aucamp, M.; Odendaal, R.; Wilna, L.; Hamman, J. Amorphous azithromycin with improved aqueous solubility and intestinal membrane permeability. Drug Dev. Ind. Pharm. 2015, 41, 1100–1108. [Google Scholar] [CrossRef]
- Arnold, Y.E.; Thorens, J.; Bernard, S.; Kalia, Y.N. Drug transport across porcine intestine using an Ussing chamber system: Regional differences and the effect of P-glycoprotein and CYP3A4 activity on drug absorption. Pharmaceutics 2019, 11, 139. [Google Scholar] [CrossRef]
- Hoegman, M.; Moerk, A.-C.; Roomans, G.M. Hypertonic saline increases tight junction permeability in airway epithelium. Eur. Respir. J. 2002, 20, 1444–1448. [Google Scholar] [CrossRef]
- Clark, L.L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1151–G1166. [Google Scholar] [CrossRef]
- UCSF-FDA TransPortal. Available online: http://transportal.compbio.ucsf.edu (accessed on 1 August 2018).
- Available online: https://www.drugbank.ca/drugs (accessed on 1 June 2019).
- Benet, L.Z.; Broccatelli, F.; Oprea, T.I. BDDCS applied to over 900 drugs. AAPS J. 2011, 13, 519–547. [Google Scholar] [CrossRef]
- Knütter, I.; Kottra, G.; Fischer, W.; Daniel, H.; Brandsch, M. High-affinity interaction of sartans with H+/peptide transporters. Drug Metab. Dispos. 2009, 37, 143–149. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report EMA/633696/2016; European Medicines Agency: London, UK, 2016. [Google Scholar]
- Ho, R.H.; Tirona, R.G.; Leake, B.F.; Glaeser, H.; Lee, W.; Lemke, C.J.; Wang, Y.-H.; Kim, R.B. Basic-liver, pancreas, and biliary tract. Gastroenterology 2006, 130, 1793–1806. [Google Scholar] [CrossRef]
- Bachmakov, I.; Glaeser, H.; Fromm, M.F.; König, J. Interaction of oral antidiabetic drugs with hepatic uptake transporters: Focus on organic anion transporting polypeptides and organic cation transporter 1. Diabetes 2008, 57, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Benet, L.Z. Predicting drug disposition via application of BCS: Transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 2005, 22, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Bourdet, D.L.; Pritchard, J.B.; Thakker, D.R. Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3). J. Pharmacol. Exp. Ther. 2005, 315, 1288–1297. [Google Scholar] [CrossRef]
- Müller, J.; Lips, K.S.; Metzner, L.; Neubert, R.H.H. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT). Biochem. Pharmacol. 2005, 70, 1851–1860. [Google Scholar] [CrossRef]
- Collet, A.; Tanianis-Hughes, J.; Hallifax, D.; Warhurst, G. Predicting P-glycoprotein effects on oral absorption: Correlation of transport in Caco-2 with drug pharmacokinetics in wild-type and mdr1a(-/-) mice in vivo. Pharm. Res. 2004, 21, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Humphreys, J.E.; Webster, L.O.; Balakrishnan, A.; Keogh, J.P.; Kunta, J.R.; Serabjit-Singh, C.; Polli, J.W. In vitro p-glycoprotein inhibition assays for assessment of clinical drug interaction potential of new drug candidates: A recommendation for probe substrates. Drug Metab. Dispos. 2006, 34, 786–792. [Google Scholar] [CrossRef]
- Dahan, A.; Amidon, G.L. Small intestinal efflux mediated by MRP2 and BCRP shifts sulfasalazine intestinal permeability from high to low, enabling its colonic targeting. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G371–G377. [Google Scholar] [CrossRef]
- Jani, M.; Szabo, P.; Kis, E.; Molnar, E.; Glavinas, H.; Krajcsi, P. Kinetic characterization of sulfasalazine transport by human ATP-binding cassette G2. Biol. Pharm. Bull. 2009, 32, 497–499. [Google Scholar] [CrossRef]
- Hirano, M.; Maeda, K.; Matsushima, S.; Nozaki, Y.; Kusuhara, H.; Sugiyama, Y. Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol. Pharmacol. 2005, 68, 800–807. [Google Scholar] [CrossRef]
- Yamashiro, W.; Maeda, K.; Hirouchi, M.; Adachi, Y.; Zhuohan, H.; Sugiyama, Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug Metab. Dispos. 2006, 34, 1247–1254. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.K.; van den Heuvel, J.J.M.W.; Koenderink, J.B.; Russel, F.G.M. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J. Pharm. Exp. Ther. 2007, 320, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Kondo, S.; Koga, T.; Yoda, N.; Nakazato, S.; Emoto, C.; Mukai, T.; Toguchi, H. Evaluation of intestinal metabolism and absorption using Ussing chamber system, equipped with intestinal tissue from rats and dogs. Eur. J. Pharm. Biopharm. 2018, 122, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Vaessen, S.F.C.; van Lipzig, M.M.H.; Pieters, R.H.H.; Krul, C.A.M.; Wortelboer, H.M.; van de Steeg, A. Regional expression levels of drug transporters and metabolizing enzymes along the pig and human intestinal tract and comparison with Caco-2 cells. Drug Metab. Dispos. 2017, 45, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.-J.; Kanal, Y.; Nussberger, S.; Ganapathy, V.; Leibach, F.H.; Romero, M.F.; Singh, S.; Boron, W.F.; Hediger, M.A. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature 1994, 368, 563–566. [Google Scholar] [CrossRef]
- Shen, H.; Smith, D.E.; Yang, T.; Huang, Y.G.; Schnermann, J.B.; Brosius, F.C., III. Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am. J. Physiol. Ren. Physiol. 1999, 276, F658–F665. [Google Scholar] [CrossRef] [PubMed]
- Posada, M.M.; Smith, D.E. In vivo absorption and disposition of cefadroxil after escalating oral doses in wild-type and Pept I knockout mice. Pharm. Res. 2013, 30, 2931–2939. [Google Scholar] [CrossRef]
- Naruhashi, K.; Sai, Y.; Tamai, I.; Suzuki, N.; Tsuji, A. PepT1 mRNA expression is induced by starvation and its level correlates with absorptive transport of cefadroxil longitudinally in the rat intestine. Pharm. Res. 2002, 19, 1417–1423. [Google Scholar] [CrossRef]
- Cao, X.; Gibbs, S.T.; Fang, L.; Miller, H.A.; Landowski, C.P.; Shin, H.-C.; Lennernas, H.; Zhing, Y.; Amidon, G.L.; Yu, L.X.; et al. Why is it challenging to predict intestinal drug absorption and oral bioavailability in human using rat model. Pharm. Res. 2006, 28, 1675–1686. [Google Scholar] [CrossRef]
- Haslam, I.S.; O’Reilly, D.A.; Sherlock, D.J.; Kauser, A.; Womack, C.; Coleman, T. Pancreatoduodenectomy as a source of human small intestine for Ussing chamber investigations and comparative studies with rat tissue. Biopharm. Drug Dispos. 2011, 32, 210–221. [Google Scholar] [CrossRef]
- Watanabe, E.; Takahashi, M.; Hayashi, M. A possibility to predict the absorbability of poorly water-soluble drugs in humans based on rat intestinal permeability assessed by an in vitro chamber method. Eur. J. Pharm. Sci. 2004, 58, 659–665. [Google Scholar] [CrossRef]
- Müller, J.; Keiser, M.; Drozdzik, M.; Oswald, S. Expression, regulation and function of intestinal drug transporters: An update. Biol. Chem. 2017, 398, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, Z.; Tay-Sontheimer, J.; Levy, R.H.; Raguenuea-Majlessi, I. Intestinal drug interactions mediated by OATPs: A systematic review of preclinical and clinical findings. J. Pharm. Sci. 2017, 106, 2312–2325. [Google Scholar] [CrossRef]
- Tamai, I. Oral drug delivery utilizing intestinal OATP transporters. Adv. Drug Deliv. Rev. 2012, 64, 508–514. [Google Scholar] [CrossRef]
- Meddings, J.B.; Swain, M.G. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology 2000, 119, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Bistritz, L.; Meddings, J.B. Alterations in intestinal permeability. Gut 2006, 55, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Keiser, M.; Kaltheuner, L.; Wildberg, C.; Müller, J.; Grube, M.; Partecke, L.I.; Heidecke, C.-D.; Oswald, S. The organic anion-transporting peptide 2B1 is localized in the basolateral membrane of the human jejunum and Caco-2 monolayers. J. Pharm. Sci. 2017, 106, 2657–2663. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Nozawa, T.; Imai, K.; Nezu, J.-I.; Tsui, A.; Tamai, I. Involvement of human organic anion transporting polypeptide OATP-B in pH-dependent transport across intestinal apical membrane. J. Pharmacol. Exp. Ther. 2003, 306, 703–708. [Google Scholar] [CrossRef]
- Takamatsu, N.; Kim, O.-N.; Welage, L.S.; Idkaidek, N.M.; Hayashi, Y.; Barnett, J.; Yamamoto, R.; Lipka, E.; Lennernäs, H.; Hussain, A.; et al. Human jejunal permeability of two polar drugs: Cimetidine and ranitidine. Pharm. Res. 2001, 18, 742–744. [Google Scholar] [CrossRef]
- Jonker, J.W.; Wagenaar, E.; Mol, C.A.A.M.; Buitelaar, M.; Koepsell, H.; Smit, J.W.; Schinkel, A.H. Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol. Cell. Biol. 2001, 21, 5471–5477. [Google Scholar] [CrossRef]
- Wang, D.-S.; Jonker, J.W.; Kato, Y.; Kusuhara, H.; Schinkel, A.H.; Sugiyama, Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 2002, 203, 510–515. [Google Scholar] [CrossRef]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Takano, M. Intestinal efflux transporters and drug absorption. Expert Opin. Drug Metab. Toxicol. 2008, 4, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Locher, K.P. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 2016, 23, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Hamon, Y.; Giovanna, C. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef]
- Marzolini, C.; Paus, E.; Buclin, T.; Kim, R.B. Polymorphisms in human MDR1 (P-glycoprotein): Recent advances and clinical relevance. Clin. Pharmacol. Ther. 2004, 75, 13–33. [Google Scholar] [CrossRef]
- Fenner, K.; Troutman, M.; Kempshall, S.; Cook, J.; Ware, J.; Smith, D.; Lee, C. Drug-drug interactions mediated through P-glycoprotein: Clinical relevance and in vitro—In vivo correlation using digoxin as a probe drug. Clin. Pharmacol. Ther. 2009, 85, 173–181. [Google Scholar] [CrossRef]
- Kim, R.B. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab. Rev. 2002, 34, 47–54. [Google Scholar] [CrossRef]
- Kim, Y.; Chen, J. Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing conformation. Science 2018, 359, 915–919. [Google Scholar] [CrossRef]
- Bourdet, D.L.; Thakker, D.R. Saturable absorptive transport of the hydrophilic organic cation ranitidine in Caco-2 cells: Role of pH-dependent organic cation uptake system and P-glycoprotein. Pharm. Res. 2006, 23, 1165–1177. [Google Scholar] [CrossRef]
- Guo, T.; Huang, J.; Zhang, H.; Dong, L.; Guo, D.; Guo, L.; He, F.; Bhutto, Z.A.; Wang, L. Abcb1 in pigs: Molecular cloning, tissues distribution, functional analysis, and its effect on pharmacokinetics of enrofloxacin. Sci. Rep. 2016, 6, 32244. [Google Scholar] [CrossRef]
- de Lannoy, I.A.; Silverman, M. The MDR1 gene product, P-glycoprotein, mediates the transport of the cardiac glycoside, digoxin. Biochem. Biophys. Res. Commun. 1992, 189, 551–557. [Google Scholar] [CrossRef]
- Dahlgren, D.; Roos, C.; Sjögren, E.; Lennernäs, H. Direct in vivo human intestinal permeability (Peff) determined with different clinical perfusion and intubation methods. J. Pharm. Sci. 2015, 104, 2702–2726. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Morales, A.; Lennernäs, H.; Aarons, L.; Rostami-Hodjegan, A. Translating human effective jejunal intestinal permeability to surface-dependent intrinsic permeability: A pragmatic method for a more mechanistic prediction of regional oral drug absorption. AAPS J. 2015, 17, 1177–1192. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Pavek, P. Breast cancer resistance protein (BCRP/ABCG2). Int. J. Biochem. Cell Biol. 2005, 37, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Lozoya-Agullo, I.; Gonzalez-Alvarez, I.; Gonzalez-Avarez, M.; Merino-Sanjuan, M.; Bermejo, M. In situ perfusion model in rat colon for drug absorption studies: Comparison with small intestine and Caco-2 cell model. J. Pharm. Sci. 2015, 104, 3136–3145. [Google Scholar] [CrossRef]
- Nekkanti, V.; Wang, Z.; Betageri, G.V. Pharmacokinetic evaluation of improved oral bioavailability of valsartan: Proliposomes versus self-nanoemulsifying drug delivery system. AAPS PharmSciTech 2016, 17, 851–862. [Google Scholar] [CrossRef]
- Dahan, A.; Amidon, G.L. MRP2 mediated drug-drug interaction: Indomethacin increases sulfasalazine absorption in the small intestine, potentially decreasing its colonic targeting. Int. J. Pharm. 2010, 386, 216–220. [Google Scholar] [CrossRef]
- Ming, X.; Knight, B.M.; Thakker, D.R. Vectorial transport of fexofenadine across Caco-2 cells: Involvement of apical uptake and basolateral efflux transporters. Mol. Pharm. 2011, 8, 1677–1686. [Google Scholar] [CrossRef]
| Transport Protein | Substrate (BCS/BDDCS) | Km (µM) | MW (g/mol) | log P [49] | log D a Octanol/H2O, pH 7.0 | Solubility in KBR (n) (mg/mL) | Inhibitor (Ki/IC50) (µM) |
|---|---|---|---|---|---|---|---|
| SLC Transport Proteins | |||||||
| PEPT1 | Cefadroxil (-/III [50]) C16H17N3O5S | n.d. | 363.39 | −0.4 | −3.15 | 45.03 ± 1.10 (4) | Losartan (24/52) [51] |
| OATP2B1 | Rosuvastatin (III [52]/III [50]) C22H28FN3O6S | 2.4 [53] | 481.54 | 0.13 | −1.91 | 0.42 ± 0.16 (4) | Rosiglitazone (-/5.2) [54] |
| OCT1 | Ranitidine (III [55]/III [50]) C14H22N2O3 | 70 [56] | 314.40 | 0.27 | −1.44 | 19.64 ± 2.78 (3) | Atropine (-/1.2) [57] |
| ABC Transport Proteins | |||||||
| P−gp | Digoxin (II [55]/IV [50]) C41H64O14 | 73 [58] | 780.94 | 1.26 | 1.29 | 0.04 ± 0.01 (3) | Verapamil (-/10.7) [59] |
| Fexofenadine (III [55]/III [50]) C32H39NO4 | n.d. | 501.66 | 5.6 | 1.23 | 0.42 ± 0.16 (4) | ||
| BCRP | Sulfasalazine (IV [60]/II [50]) C18H14N4O5S | 0.7 [61] | 398.39 | 0.4 | −0.10 | 3.31 ± 0.14 (5) | Fluvastatin (5.43/-) [62] |
| MRP2 | Valsartan (III [55]/III [50]) C24H29N5O3 | 30.4 [63] | 435.5 | 3.68 | −0.68 | 3.71 ± 0.68 (5) | Indomethacin (-/0.06) [64] |
| MRP3 | Fexofenadine (III [55]/III [50]) C32H39NO4 | n.d. | 501.66 | 5.6 | 1.23 | 0.42 ± 0.16 (4) | Indomethacin (-/-) |
| Drug | Transporter | Papp,pig (10−6 cm/s) | QDEP (%) | QPERM (n)(%) | QDEP (%) | QPERM (n)(%) | Papp,rat (10−6 cm/s) | Papp,human (10−6 cm/s) | |
|---|---|---|---|---|---|---|---|---|---|
| (−INH) (n) | (+INH) (n) | (−INH) | (+INH) | (−INH) | (−INH) | ||||
| Cefadroxil | PEPT1 | 2.82 ± 0.20 (4) | 1.91 ± 0.55 (6) | 1.31 ± 0.40 | 0.50 ± 0.18 (4) | 1.47 ± 0.25 | 0.31 ± 0.12 (6) | 4.99 ± 0.50 [70] | - |
| Rosuvastatin | OATP2B1 | 0.91 ± 0.64 (7) | 2.25 ± 0.85 (6) | 3.10 ± 1.46 | 0.17 ± 0.09 (7) | 1.30 ± 0.14 | 0.29 ± 0.16 (6) | - | 6.95 ± 1.50 [30] |
| Ranitidine | OCT1 | 5.07 ± 0.83 (12) | 1.96 ± 0.28 (5) | 1.11 ± 0.46 | 0.62 ± 0.17 (12) | 0.43 ± 0.12 | 0.21 ± 0.03 (5) | 4.00 [72] | 5.50 [72] |
| Digoxin | P-gp | 0.38 ± 0.23 (4) | 1.64 ± 0.79 (3) | 0.62 ± 0.17 | 0.21 ± 0.12 (4) | 0.40 ± 0.19 | 0.12 ± 0.04 (3) | 6.4 ± 1.9 [14] | 1.44 ± 0.72 [30] |
| Sulfasalazine | BCRP | 0.01 ± 0.00 (5) | 0.63 ± 0.43 (9) | 3.23 ± 0.56 | 0.00 ± 0.00 (5) | 2.47 ± 1.24 | 0.12 ± 0.07 (9) | 2.76 ± 0.19 [73] | 0.09 ± 0.06 [30] |
| Valsartan | MRP2 | 1.20 ± 0.10 (4) | 0.93 ± 0.41 (4) | 0.44 ± 0.02 | 0.17 ± 0.02 (4) | 0.57 ± 0.07 | 0.24 ± 0.10 (4) | - | - |
| (−IND/−VER) (n) | (−IND/+VER) (n) | (+IND/−VER) (n) | (+IND/+VER) (n) | |
|---|---|---|---|---|
| Papp,pig (10−6 cm/s) (n) a | 2.11 ± 0.73 (6) | 4.48 ± 3.29 (5) | 0.64 ± 0.20 (6) | 0.84 ± 0.52 (6) |
| QDEP (%) (n) | 1.00 ± 0.24 (6) | 0.54 ± 0.09 (5) | 0.81 ± 0.61 (6) | 1.50 ± 0.33 (6) |
| QPERM (%) (n) | 0.06 ± 0.01 (6) | 0.36 ± 0.20 (5) | 0.27 ± 0.05 (6) | 0.22 ± 0.10 (6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnold, Y.E.; Kalia, Y.N. Using Ex Vivo Porcine Jejunum to Identify Membrane Transporter Substrates: A Screening Tool for Early—Stage Drug Development. Biomedicines 2020, 8, 340. https://doi.org/10.3390/biomedicines8090340
Arnold YE, Kalia YN. Using Ex Vivo Porcine Jejunum to Identify Membrane Transporter Substrates: A Screening Tool for Early—Stage Drug Development. Biomedicines. 2020; 8(9):340. https://doi.org/10.3390/biomedicines8090340
Chicago/Turabian StyleArnold, Yvonne E., and Yogeshvar N. Kalia. 2020. "Using Ex Vivo Porcine Jejunum to Identify Membrane Transporter Substrates: A Screening Tool for Early—Stage Drug Development" Biomedicines 8, no. 9: 340. https://doi.org/10.3390/biomedicines8090340
APA StyleArnold, Y. E., & Kalia, Y. N. (2020). Using Ex Vivo Porcine Jejunum to Identify Membrane Transporter Substrates: A Screening Tool for Early—Stage Drug Development. Biomedicines, 8(9), 340. https://doi.org/10.3390/biomedicines8090340







