Potential for Novel Biomarkers in Diabetes-Associated Chronic Kidney Disease: Epigenome, Metabolome, and Gut Microbiome
Abstract
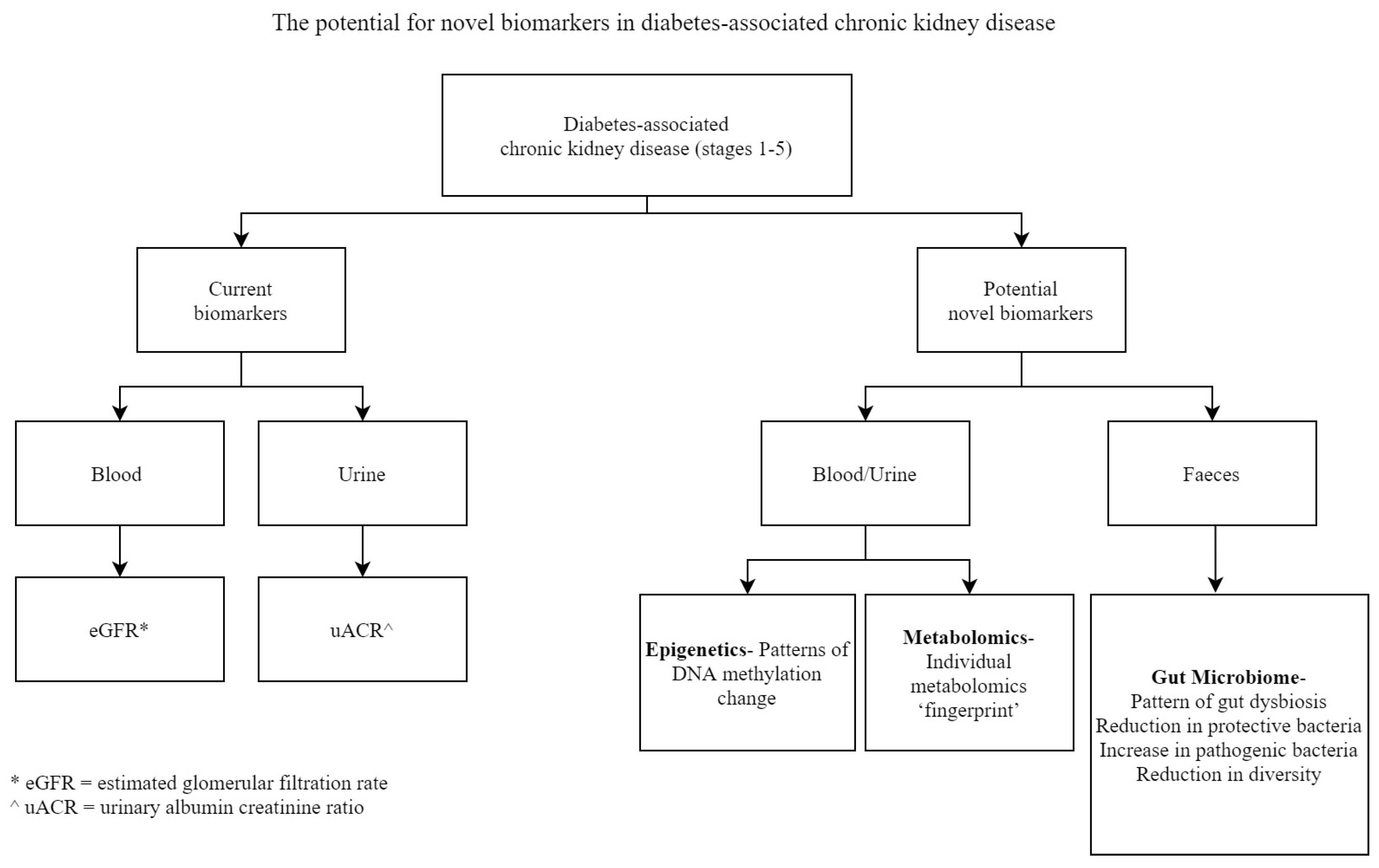
1. The Need for Improved Biomarkers in Diabetes-Associated Chronic Kidney Disease
2. Epigenetic Biomarkers in Diabetes-Associated Chronic Kidney Disease
2.1. DNA Methylation as a Biomarker
2.2. Emerging Trends in Epigenetic Modification Analysis
2.3. DNA Methylation in Diabetes-Associated Kidney Disease
3. Metabolomic Biomarkers in Diabetes-Associated Chronic Kidney Disease
4. Gut Microbiome Biomarkers in Diabetes-Associated Chronic Kidney Disease
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- World Health Organization; International Programme on Chemical Safety. Biomarkers and Risk Assessment: Concepts and Principles/Published under the Joint Sponsorship of the United Nations environment Programme, the International Labour Organisation, and the World Health Organization; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- How Kit, A.; Nielsen, H.M.; Tost, J. DNA methylation based biomarkers: Practical considerations and applications. Biochimie 2012, 94, 2314–2337. [Google Scholar] [CrossRef]
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The Global Epidemiology of Diabetes and Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Badrick, T.; Turner, P. The Uncertainty of the eGFR. Indian J. Clin. Biochem. 2013, 28, 242–247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gross, J.L.; de Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic Nephropathy: Diagnosis, Prevention, and Treatment. Diabetes Care 2005, 28, 164. [Google Scholar] [CrossRef] [PubMed]
- Rheinberger, M.; Boger, C.A. Diabetic nephropathy: New insights into diagnosis, prevention and treatment. Dtsch. Med. Wochenschr. 2014, 139, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Foundation, N.K. ACR. Available online: https://www.kidney.org/kidneydisease/siemens_hcp_acr (accessed on 29 July 2020).
- Krolewski, A.S.; Niewczas, M.A.; Skupien, J.; Gohda, T.; Smiles, A.; Eckfeldt, J.H.; Doria, A.; Warram, J.H. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care 2014, 37, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, B.P.; Al-Kassab, A.S.; Ilag, L.L.; Zawacki, C.M.; Herman, W.H. Does microalbuminuria predict diabetic nephropathy? Diabetes Care 2001, 24, 1560–1566. [Google Scholar] [CrossRef]
- Ninomiya, T.; Perkovic, V.; de Galan, B.E.; Zoungas, S.; Pillai, A.; Jardine, M.; Patel, A.; Cass, A.; Neal, B.; Poulter, N.; et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. JASN 2009, 20, 1813–1821. [Google Scholar] [CrossRef]
- Chu, A.Y.; Tin, A.; Schlosser, P.; Ko, Y.-A.; Qiu, C.; Yao, C.; Joehanes, R.; Grams, M.E.; Liang, L.; Gluck, C.A.; et al. Epigenome-wide association studies identify DNA methylation associated with kidney function. Nat. Commun. 2017, 8, 1286. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.Y.; Kwon, Y.K.; Lee, D.; Jung, H.Y.; Ryu, H.M.; Cho, J.H.; Ryu, D.H.; Kim, Y.L.; Hwang, G.S. Changes in serum metabolites with the stage of chronic kidney disease: Comparison of diabetes and non-diabetes. Clin. Chim. Acta 2016, 459, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.W.; Lin, C.-Y.; Chang, L.-C.; Lee, C.-C.; Chiu, C.-Y.; Hsu, H.-J.; Sun, C.-Y.; Chen, Y.-C.; Kuo, Y.-L.; Yang, C.-W.; et al. Gut Microbiota as Diagnostic Tools for Mirroring Disease Progression and Circulating Nephrotoxin Levels in Chronic Kidney Disease: Discovery and Validation Study. Int. J. Biol. Sci. 2020, 16, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Cooper, M.E.; Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 2016, 12, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396. [Google Scholar] [CrossRef]
- Cañadas-Garre, M.; Anderson, K.; McGoldrick, J.; Maxwell, A.P.; McKnight, A.J. Genomic approaches in the search for molecular biomarkers in chronic kidney disease. J. Transl. Med. 2018, 16, 292. [Google Scholar] [CrossRef]
- Bechtel, W.; McGoohan, S.; Zeisberg, E.M.; Muller, G.A.; Kalbacher, H.; Salant, D.J.; Muller, C.A.; Kalluri, R.; Zeisberg, M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 2010, 16, 544–550. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Lecamwasam, A.; Sexton-Oates, A.; Carmody, J.; Ekinci, E.I.; Dwyer, K.M.; Saffery, R. DNA methylation profiling of genomic DNA isolated from urine in diabetic chronic kidney disease: A pilot study. PLoS ONE 2018, 13, e0190280. [Google Scholar] [CrossRef]
- Bell, C.G.; Teschendorff, A.E.; Rakyan, V.K.; Maxwell, A.P.; Beck, S.; Savage, D.A. Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med. Genom. 2010, 3, 33. [Google Scholar] [CrossRef]
- Sapienza, C.; Lee, J.; Powell, J.; Erinle, O.; Yafai, F.; Reichert, J.; Siraj, E.S.; Madaio, M. DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics 2011, 6. [Google Scholar] [CrossRef]
- Ko, Y.-A.; Mohtat, D.; Suzuki, M.; Park, A.S.D.; Izquierdo, M.C.; Han, S.Y.; Kang, H.M.; Si, H.; Hostetter, T.; Pullman, J.M.; et al. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol. 2013, 14, R108. [Google Scholar] [CrossRef] [PubMed]
- Mikeska, T.; Candiloro, I.L.; Dobrovic, A. The implications of heterogeneous DNA methylation for the accurate quantification of methylation. Epigenomics 2010, 2, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.S.; Herman, J.G.; McCaffrey, T.A.; Raj, D.S. Beyond genetics: Epigenetic code in chronic kidney disease. Kidney Int. 2011, 79, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, K.; Brinckmann, A.; Toliat, M.R.; Ritter, H.; Nurnberg, P. Evaluation of a potential epigenetic biomarker by quantitative methyl-single nucleotide polymorphism analysis. Electrophoresis 2002, 23, 4072–4079. [Google Scholar] [CrossRef] [PubMed]
- Bibikova, M.; Le, J.; Barnes, B.; Saedinia-Melnyk, S.; Zhou, L.; Shen, R.; Gunderson, K. Genome-wide DNA methylation profiling using Infinium assay. Epigenomics 2009, 1. [Google Scholar] [CrossRef]
- Walter, K.; Holcomb, T.; Januario, T.; Du, P.; Evangelista, M.; Kartha, N.; Iniguez, L.; Soriano, R.; Huw, L.; Stern, H.; et al. DNA methylation profiling defines clinically relevant biological subsets of non-small cell lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 2360–2373. [Google Scholar] [CrossRef]
- Cross, S.H.; Charlton, J.A.; Nan, X.; Bird, A.P. Purification of CpG islands using a methylated DNA binding column. Nat. Genet. 1994, 6, 236–244. [Google Scholar] [CrossRef]
- Bentley, D.R. Whole-genome re-sequencing. Curr. Opin. Genet. Dev. 2006, 16, 545–552. [Google Scholar] [CrossRef]
- Reddy, M.A.; Natarajan, R. Recent developments in epigenetics of acute and chronic kidney diseases. Kidney Int. 2015, 88, 250–261. [Google Scholar] [CrossRef]
- Ko, Y.A.; Susztak, K. Epigenomics: The science of no-longer-junk DNA. Why study it in chronic kidney disease? Semin. Nephrol. 2013, 33, 354–362. [Google Scholar] [CrossRef][Green Version]
- Toperoff, G.; Aran, D.; Kark, J.D.; Rosenberg, M.; Dubnikov, T.; Nissan, B.; Wainstein, J.; Friedlander, Y.; Levy-Lahad, E.; Glaser, B.; et al. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum. Mol. Genet. 2012, 21, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.J.; McKay, G.J.; Maxwell, A.P.; McKnight, A.J. DNA hypermethylation and DNA hypomethylation is present at different loci in chronic kidney disease. Epigenetics 2014, 9, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Wing, M.R.; Devaney, J.M.; Joffe, M.M.; Xie, D.; Feldman, H.I.; Dominic, E.A.; Guzman, N.J.; Ramezani, A.; Susztak, K.; Herman, J.G.; et al. DNA methylation profile associated with rapid decline in kidney function: Findings from the CRIC study. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2014, 29, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.J.; Liu, Y.; Zhang, J.; Goswami, C.; Lin, H.; Dominguez, J.H. Comprehensive genomic profiling in diabetic nephropathy reveals the predominance of proinflammatory pathways. Physiol. Genom. 2013, 45, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Mimura, I.; Shoji, K.; Tanaka, T.; Nangaku, M. Hypoxia and fibrosis in chronic kidney disease: Crossing at pericytes. Kidney Int. Suppl. 2014, 4, 107–112. [Google Scholar] [CrossRef]
- Swan, E.J.; Maxwell, A.P.; McKnight, A.J. Distinct methylation patterns in genes that affect mitochondrial function are associated with kidney disease in blood-derived DNA from individuals with Type 1 diabetes. Diabet. Med. J. Br. Diabet. Assoc. 2015, 32, 1110–1115. [Google Scholar] [CrossRef]
- Ghattas, M.; El-Shaarawy, F.; Mesbah, N.; Abo-Elmatty, D. DNA methylation status of the methylenetetrahydrofolate reductase gene promoter in peripheral blood of end-stage renal disease patients. Mol. Biol. Rep. 2014, 41, 683–688. [Google Scholar] [CrossRef]
- Lecamwasam, A.; Novakovic, B.; Myer, B.; Ekinci, E.I.; Dwyer, K.M.; Saffery, R. DNA methylation profiling identifies epigenetic differences between early versus late stages of diabetic chronic kidney disease. Nephrol. Dial. Transplant. 2020. Accepted for publication. [Google Scholar]
- Hocher, B.; Adamski, J. Metabolomics for clinical use and research in chronic kidney disease. Nat. Rev. Nephrol. 2017. [Google Scholar] [CrossRef]
- Beger, R.D.; Dunn, W.; Schmidt, M.A.; Gross, S.S.; Kirwan, J.A.; Cascante, M.; Brennan, L.; Wishart, D.S.; Oresic, M.; Hankemeier, T.; et al. Metabolomics enables precision medicine: “A White Paper, Community Perspective”. Metab. Off. J. Metab. Soc. 2016, 12, 149. [Google Scholar] [CrossRef]
- Darshi, M.; Van Espen, B.; Sharma, K. Metabolomics in Diabetic Kidney Disease: Unraveling the Biochemistry of a Silent Killer. Am. J. Nephrol. 2016, 44, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Ruttkies, C.; Treutler, H.; Neumann, S. Bioinformatics can boost metabolomics research. J. Biotechnol. 2017, 261, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Budde, K.; Gök, Ö.-N.; Pietzner, M.; Meisinger, C.; Leitzmann, M.; Nauck, M.; Köttgen, A.; Friedrich, N. Quality assurance in the pre-analytical phase of human urine samples by 1H NMR spectroscopy. Arch. Biochem. Biophys. 2016, 589, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Ryan, D.; Brennan, L.; Tenori, L.; Luchinat, C.; Gao, X.; Zeri, A.C.; Gowda, G.A.N.; et al. Recommendations and Standardization of Biomarker Quantification Using NMR-Based Metabolomics with Particular Focus on Urinary Analysis. J. Proteome Res. 2016, 15, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Davies, R. The metabolomic quest for a biomarker in chronic kidney disease. Clin. Kidney J. 2018, 11, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Ravani, P.; Parfrey, P.S.; Dicks, E.; Barrett, B.J. Clinical research of kidney diseases II: Problems of study design. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2007, 22, 2785–2794. [Google Scholar] [CrossRef][Green Version]
- Grams, M.E.; Shafi, T.; Rhee, E.P. Metabolomics Research in Chronic Kidney Disease. J. Soc. Nephrol. 2018, 29, 1588. [Google Scholar] [CrossRef]
- Coresh, J.; Inker, L.A.; Sang, Y.; Chen, J.; Shafi, T.; Post, W.S.; Shlipak, M.G.; Ford, L.; Goodman, K.; Perichon, R.; et al. Metabolomic profiling to improve glomerular filtration rate estimation: A proof-of-concept study. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2019, 34, 825–833. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; Alexander, D.; Couper, D.J.; Boerwinkle, E. Medium-term variability of the human serum metabolome in the Atherosclerosis Risk in Communities (ARIC) study. Omics J. Integr. Biol. 2014, 18, 364–373. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef]
- Pena, M.J.; de Zeeuw, D.; Mischak, H.; Jankowski, J.; Oberbauer, R.; Woloszczuk, W.; Benner, J.; Dallmann, G.; Mayer, B.; Mayer, G.; et al. Prognostic clinical and molecular biomarkers of renal disease in type 2 diabetes. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2015, 30 (Suppl. S4), iv86–iv95. [Google Scholar] [CrossRef]
- Sharma, K.; Karl, B.; Mathew, A.V.; Gangoiti, J.A.; Wassel, C.L.; Saito, R.; Pu, M.; Sharma, S.; You, Y.H.; Wang, L.; et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J. Am. Soc. Nephrol. 2013, 24, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Clish, C.B.; Wenger, J.; Roy, J.; Elmariah, S.; Pierce, K.A.; Bullock, K.; Anderson, A.H.; Gerszten, R.E.; Feldman, H.I. Metabolomics of Chronic Kidney Disease Progression: A Case-Control Analysis in the Chronic Renal Insufficiency Cohort Study. Am. J. Nephrol. 2016, 43, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Breit, M.; Weinberger, K.M. Metabolic biomarkers for chronic kidney disease. Arch. Biochem. Biophys. 2016, 589, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002, 22, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Lin, C.J.; Chen, H.H.; Pan, C.F.; Chuang, C.K.; Wang, T.J.; Sun, F.J.; Wu, C.J. p-Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J. Clin. Lab. Anal. 2011, 25, 191–197. [Google Scholar] [CrossRef]
- Vaziri, N.; Wong, J.; Pahl, M.; Piceno, Y.; Yuan, J.; DeSantis, T.; Ni, Z.; Nguyen, T.; Andersen, G. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Hida, M.; Aiba, Y.; Sawamura, S.; Suzuki, N.; Satoh, T.; Koga, Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 1996, 74, 349–355. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Simões-Silva, L.; Pestana, M.; Araujo, R.; Soares-Silva, I.J. Chapter Three—The Role of the Gut Microbiome on Chronic Kidney Disease. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: New York, NY, USA, 2016; Volume 96, pp. 65–94. [Google Scholar]
- Lun, H.; Yang, W.; Zhao, S.; Jiang, M.; Xu, M.; Liu, F.; Wang, Y. Altered gut microbiota and microbial biomarkers associated with chronic kidney disease. Microbiologyopen 2019, 8, e00678. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xie, S.; Lv, D.; Wang, P.; He, H.; Zhang, T.; Zhou, Y.; Lin, Q.; Zhou, H.; Jiang, J.; et al. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci. Rep. 2017, 7, 2870. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Quinn, R.A.; Debelius, J.; Xu, Z.Z.; Morton, J.; Garg, N.; Jansson, J.K.; Dorrestein, P.C.; Knight, R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 2016, 535, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Mao, G.; Bennet, W.L.; Hourigan, S.K.; Dominguez-Bello, M.G.; Appel, L.J.; Wang, X. Does vaginal delivery mitigate or strengthen the intergenerational association of overweight and obesity? Findings from the Boston Birth Cohort. Int. J. Obes. 2017, 41, 497–501. [Google Scholar] [CrossRef]
- Rintala, A.; Pietilä, S.; Munukka, E.; Eerola, E.; Pursiheimo, J.-P.; Laiho, A.; Pekkala, S.; Huovinen, P. Gut Microbiota Analysis Results Are Highly Dependent on the 16S rRNA Gene Target Region, Whereas the Impact of DNA Extraction Is Minor. J. Biomol. Tech. JBT 2017, 28, 19–30. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chen, D.-Q.; Chen, L.; Liu, J.-R.; Vaziri, N.D.; Guo, Y.; Zhao, Y.-Y. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J. Transl. Med. 2019, 17, 5. [Google Scholar] [CrossRef]
- Versalovic, J.; Dore, J.; Guarner, F.; Luna, R.A.; Ringel, Y. Microbiome-Based Diagnostics: Ready for Applications in Laboratory Medicine? Clin. Chem. 2017, 63, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Vieira-Silva, S.; Raes, J. Richness and ecosystem development across faecal snapshots of the gut microbiota. Nat. Microbiol. 2018, 3, 526–528. [Google Scholar] [CrossRef] [PubMed]
| Epigenetics | Metabolomics | Gut Microbiome |
|---|---|---|
| Differentially methylated genes with potential as biomarkers in diabetes-associated CKD. | Metabolites as potential biomarkers of diabetes-associated CKD prognosis. | Gut dysbiosis and gut-derived metabolites as potential biomarkers in diabetes-associated CKD. |
| PTPN6, CEBPB, EBF1, EP300 [12] | 3-hydroxyisovalerate, aconitate, citrate, 2-ethyl,3-hydroxypropionate, glycolate, 2-methylacetoacetate and uracil [53] | Reduced Lactobacillaceae and Prevotellaceae [60] Increased Enterobacteria and Enterococci [60] |
| RUNX3 [35] | Tyrosine, formate [13] | Increased Indoxyl Sulphate (IS), p-Cresyl Sulphate (p-CS) [14] |
| PHB [22,37] | Arginine, methionine, threonine [54] | |
| MTHFR [21,22,38] | ||
| CRISP2 [39] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecamwasam, A.; Ekinci, E.I.; Saffery, R.; Dwyer, K.M. Potential for Novel Biomarkers in Diabetes-Associated Chronic Kidney Disease: Epigenome, Metabolome, and Gut Microbiome. Biomedicines 2020, 8, 341. https://doi.org/10.3390/biomedicines8090341
Lecamwasam A, Ekinci EI, Saffery R, Dwyer KM. Potential for Novel Biomarkers in Diabetes-Associated Chronic Kidney Disease: Epigenome, Metabolome, and Gut Microbiome. Biomedicines. 2020; 8(9):341. https://doi.org/10.3390/biomedicines8090341
Chicago/Turabian StyleLecamwasam, Ashani, Elif I. Ekinci, Richard Saffery, and Karen M. Dwyer. 2020. "Potential for Novel Biomarkers in Diabetes-Associated Chronic Kidney Disease: Epigenome, Metabolome, and Gut Microbiome" Biomedicines 8, no. 9: 341. https://doi.org/10.3390/biomedicines8090341
APA StyleLecamwasam, A., Ekinci, E. I., Saffery, R., & Dwyer, K. M. (2020). Potential for Novel Biomarkers in Diabetes-Associated Chronic Kidney Disease: Epigenome, Metabolome, and Gut Microbiome. Biomedicines, 8(9), 341. https://doi.org/10.3390/biomedicines8090341





