Co-Microencapsulation of Islets and MSC CellSaics, Mosaic-Like Aggregates of MSCs and Recombinant Peptide Pieces, and Therapeutic Effects of Their Subcutaneous Transplantation on Diabetes
Abstract
1. Introduction
2. Experimental Section
2.1. Animals
2.2. Materials
2.3. Isolation of Rat Islets
2.4. Preparation of MSC CellSaics
2.5. Preparation of Alginate Microcapsules
2.6. Preparation of MSC CellSaic(in) and MSC CellSaic(None)
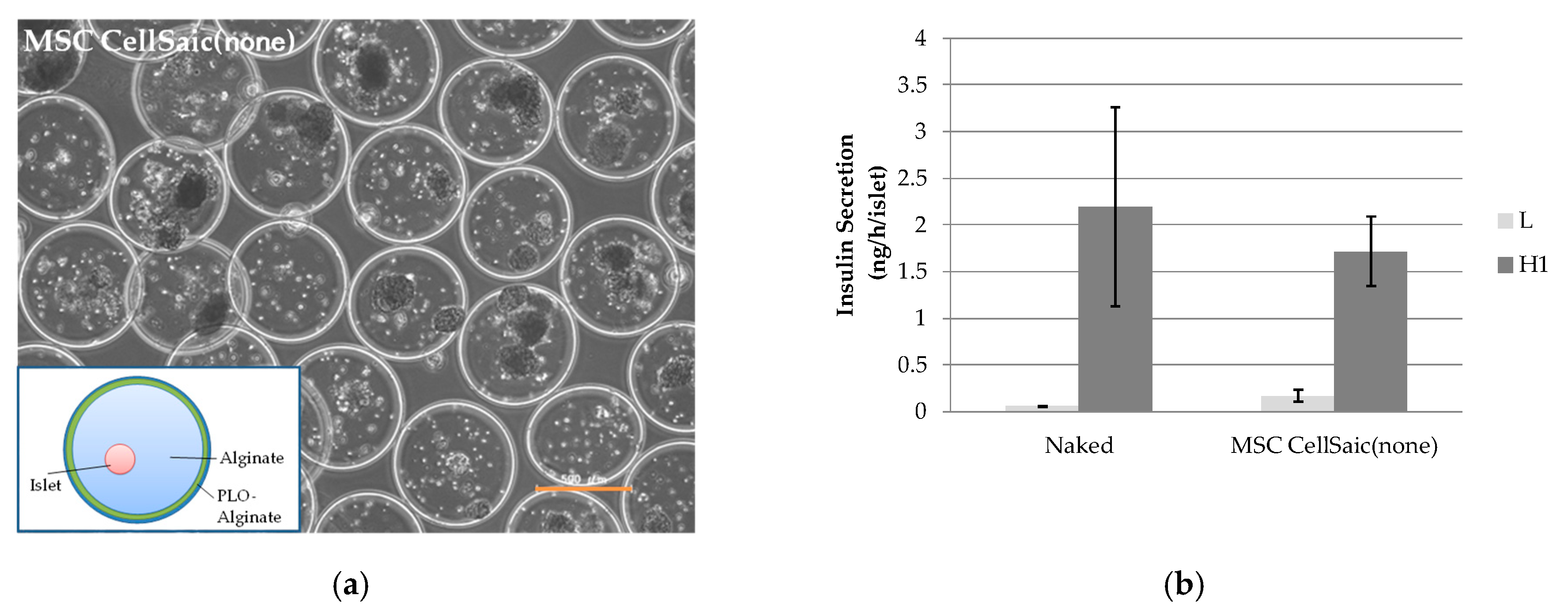
2.7. In Vitro Evaluations of MSC CellSaic(in) and MSC CellSaic(None)
2.8. In Vivo Evaluations of MSC CellSaic(In), MSC CellSaic(None), and MSC CellSaic(Out)
2.9. Statistical Analyses
3. Results
3.1. Preparation of Immuno-Isolating Microcapsules
3.1.1. Selection of Cross-Linking Agents for Alginate Microcapsules
3.1.2. In Vivo Evaluation of MSC CellSaic (None)
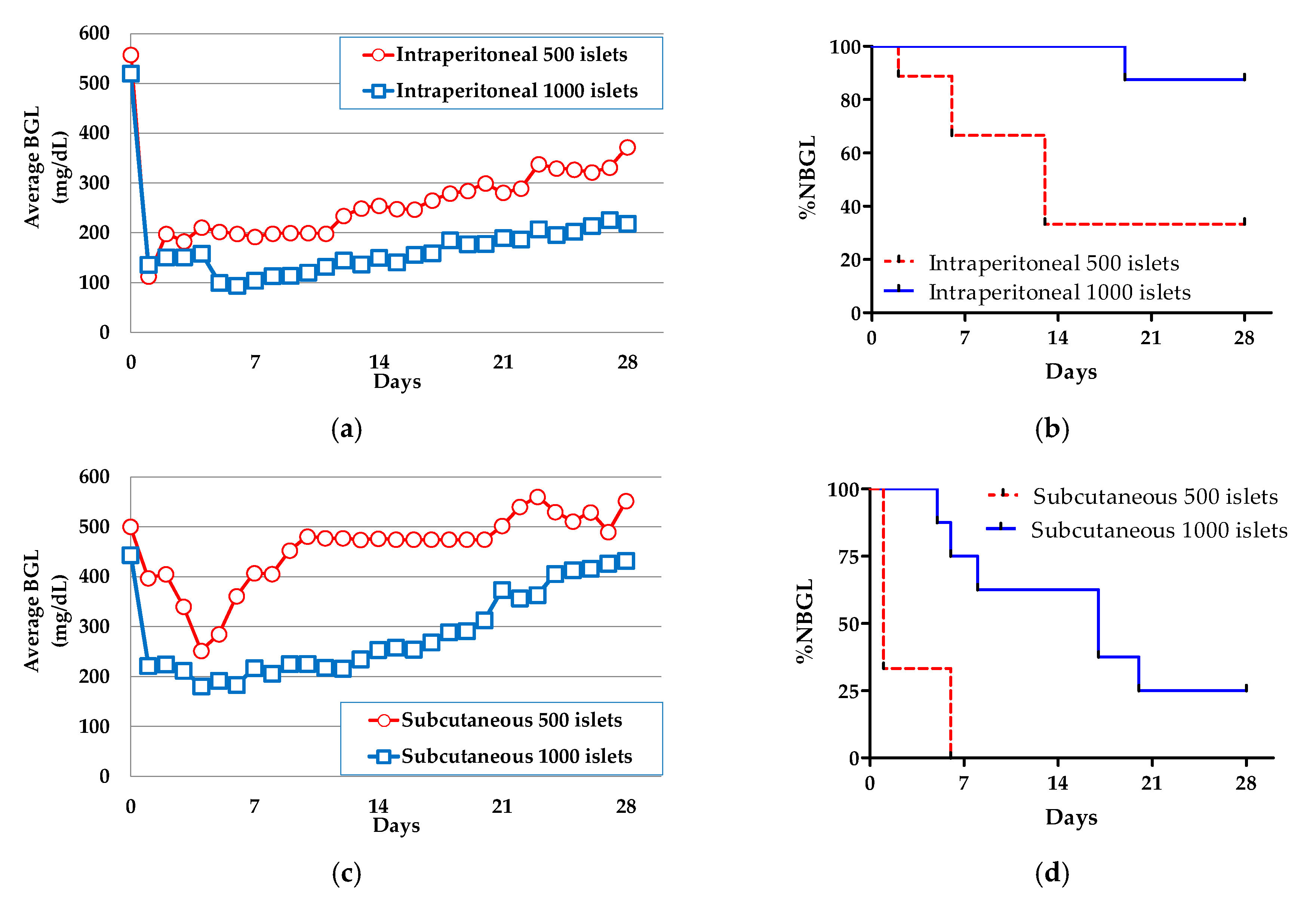
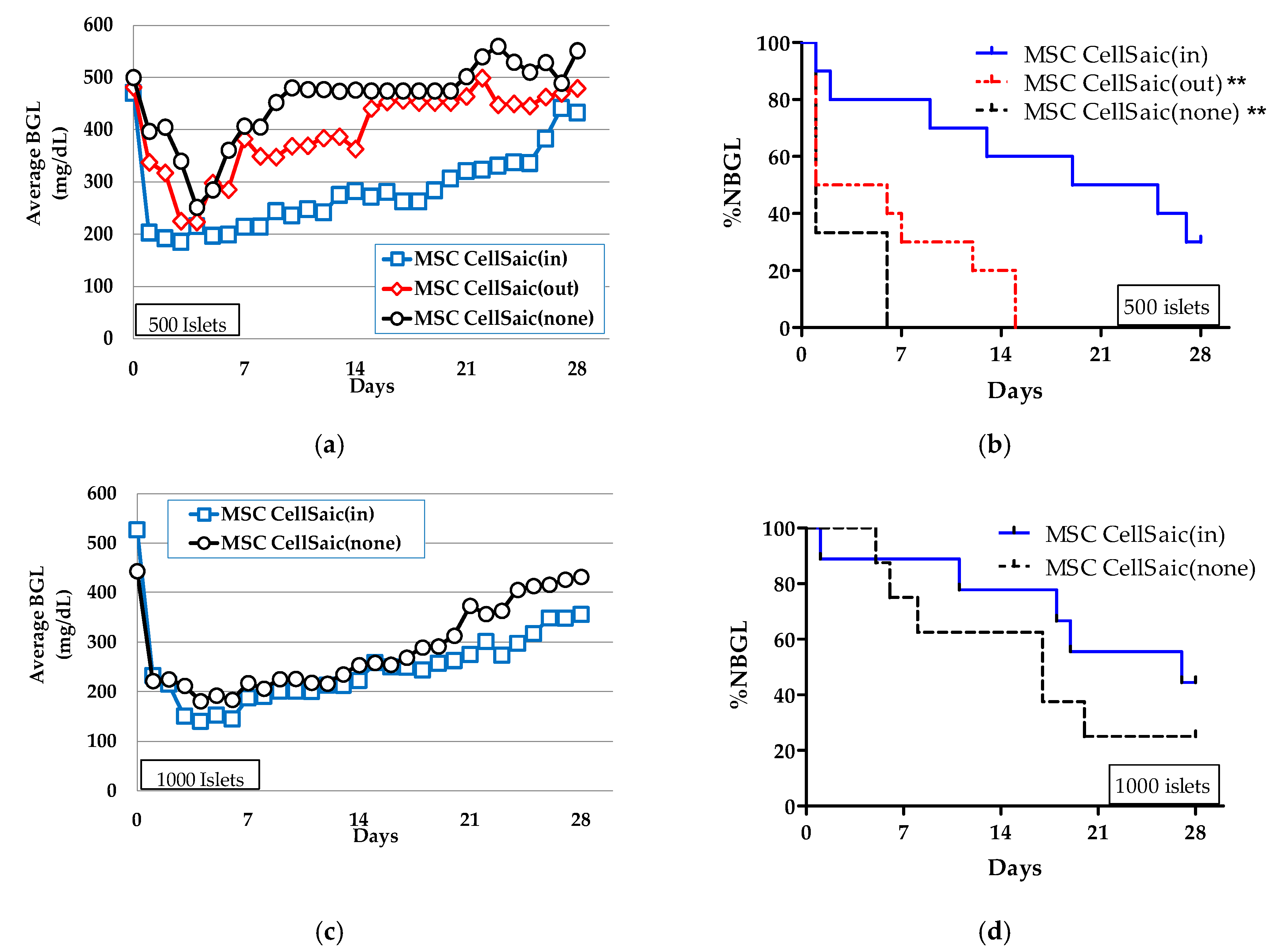
3.2. The Effect of Co-Microencapusulation of the MSC CellSaics and Islets Inside on %NBGL
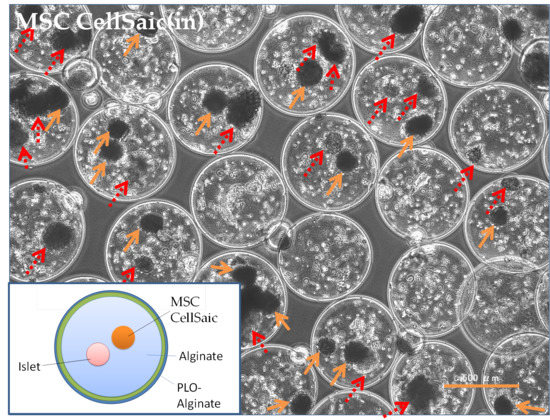
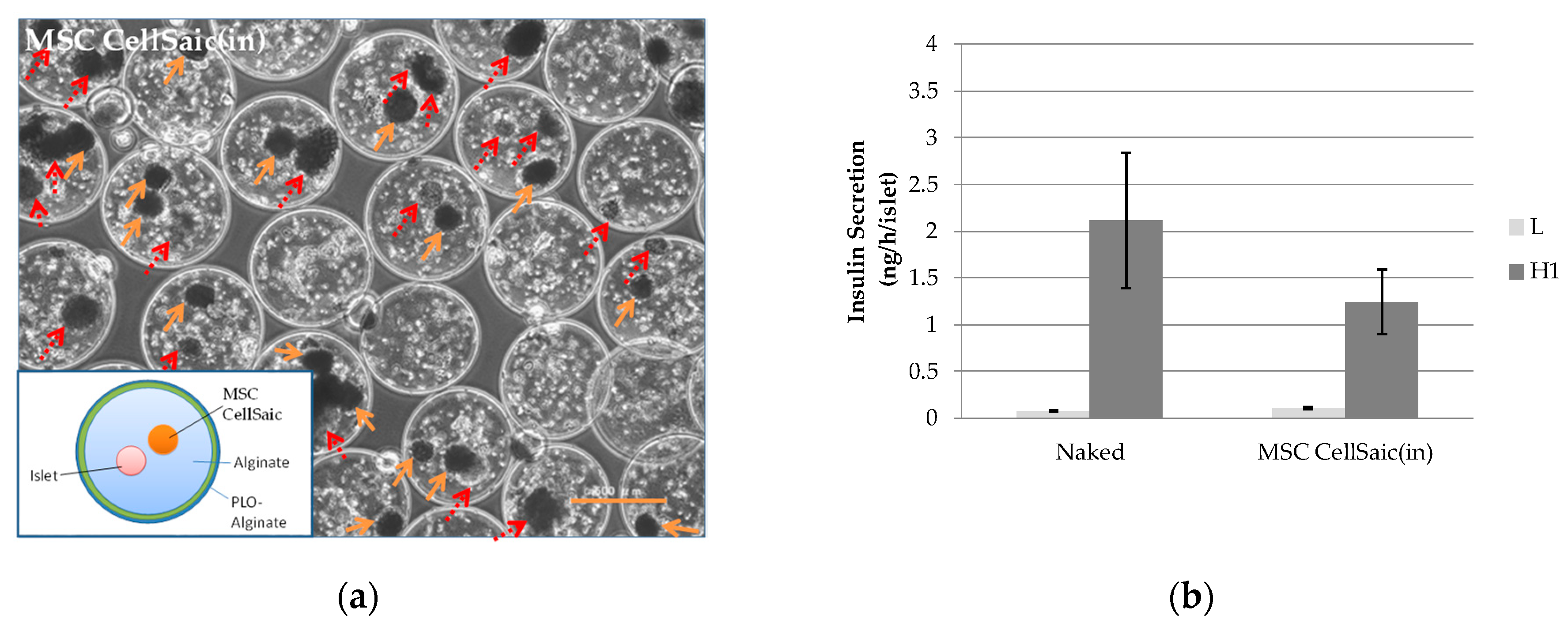
3.2.1. Preparation of MSC CellSaic(in)
3.2.2. In Vivo Evaluations of MSC CellSaic(in) and MSC CellSaic(out)
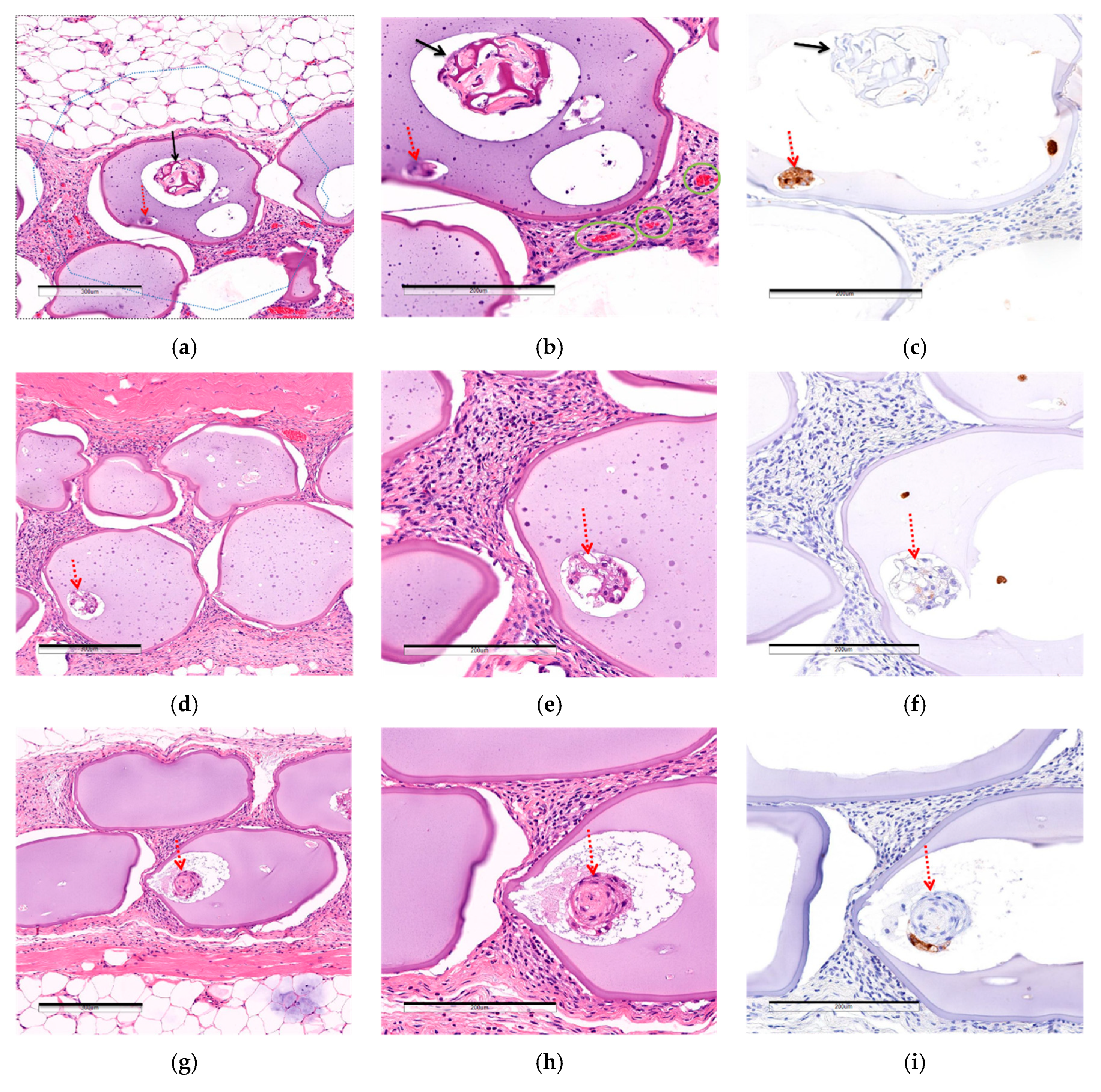
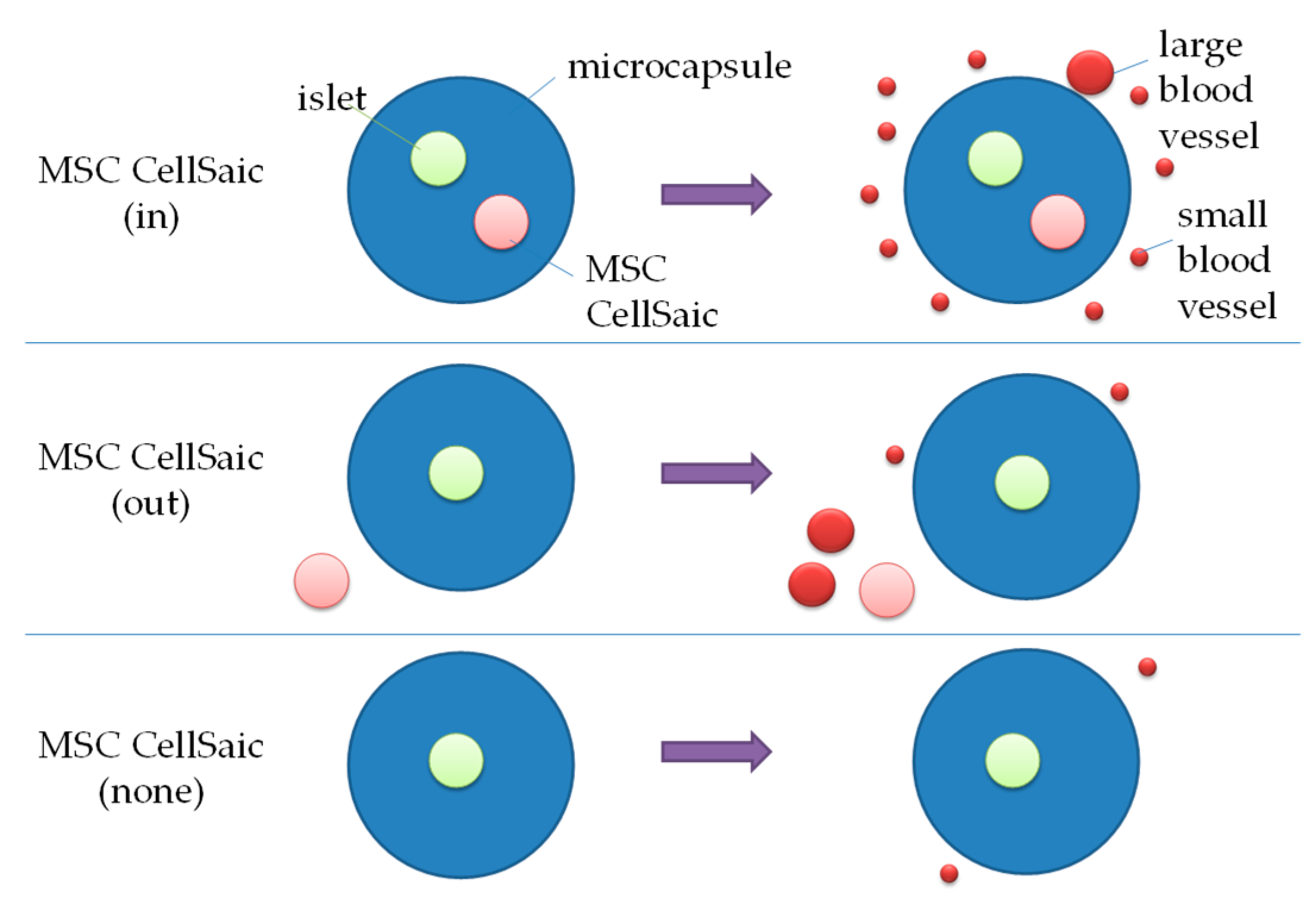
3.2.3. Comparison of Post-Implantation Section Images
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shapiro, A.M.; Ricordi, C.; Hering, B.J.; Auchincloss, H.; Lindblad, R.; Robertson, R.P.; Secchi, A.; Brendel, M.D.; Berney, T.; Brennan, D.C.; et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006, 355, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, E.S.; Vegas, A.; Anderson, D.G.; Weir, G.C. Islets transplanted in immunoisolation devices: A review of the progress and the challenges that remain. Endocr. Rev. 2011, 32, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, R.; Barton, F.B.; Hering, B.J.; Wease, S.; Investigators, C.I.T.R. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation 2008, 86, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Sumi, S.; Yoshimatsu, G.; Goto, M.; Egawa, S.; Unno, M. Encapsulated islets transplantation: Past, present and future. World J. Gastrointest. Pathophysiol. 2012, 3, 19–26. [Google Scholar] [CrossRef]
- Korsgren, O. Islet Encapsulation: Physiological Possibilities and Limitations. Diabetes 2017, 66, 1748–1754. [Google Scholar] [CrossRef]
- Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Orive, G.; Hernández, R.M.M.; Saenz Del Burgo, L.; Pedraz, J.L. Review of Advanced Hydrogel-Based Cell Encapsulation Systems for Insulin Delivery in Type 1 Diabetes Mellitus. Pharmaceutics 2019, 11, 597. [Google Scholar] [CrossRef]
- Matsumoto, S.; Abalovich, A.; Wechsler, C.; Wynyard, S.; Elliott, R.B. Clinical Benefit of Islet Xenotransplantation for the Treatment of Type 1 Diabetes. EBioMedicine 2016, 12, 255–262. [Google Scholar] [CrossRef]
- Jo, E.H.; Hwang, Y.H.; Lee, D.Y. Encapsulation of pancreatic islet with HMGB1 fragment for attenuating inflammation. Biomater. Res. 2015, 19, 21. [Google Scholar] [CrossRef]
- Barkai, U.; Rotem, A.; de Vos, P. Survival of encapsulated islets: More than a membrane story. World J. Transplant. 2016, 6, 69–90. [Google Scholar] [CrossRef]
- Pepper, A.R.; Gala-Lopez, B.; Pawlick, R.; Merani, S.; Kin, T.; Shapiro, A.M. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat. Biotechnol. 2015, 33, 518–523. [Google Scholar] [CrossRef]
- Farah, S.; Doloff, J.C.; Muller, P.; Sadraei, A.; Han, H.J.; Olafson, K.; Vyas, K.; Tam, H.H.; Hollister-Lock, J.; Kowalski, P.S.; et al. Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations. Nat. Mater. 2019, 18, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Tabata, Y. A new fluorescent imaging of renal inflammation with RCP. J. Control. Release 2010, 148, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Iwazawa, R.; Yoshioka, Y. Introduction to a new cell transplantation platform via recombinant peptide petaloid pieces and its application to islet transplantation with mesenchymal stem cells. Transpl. Int. 2016, 29, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Figliuzzi, M.; Bonandrini, B.; Silvani, S.; Remuzzi, A. Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes. World J. Stem Cells 2014, 6, 163–172. [Google Scholar] [CrossRef]
- Iyer, S.S.; Rojas, M. Anti-inflammatory effects of mesenchymal stem cells: Novel concept for future therapies. Expert Opin. Biol. Ther. 2008, 8, 569–581. [Google Scholar] [CrossRef]
- Ueyama, H.; Okano, T.; Orita, K.; Mamoto, K.; Sobajima, S.; Iwaguro, H.; Nakamura, H. Local transplantation of adipose-derived stem cells has a significant therapeutic effect in a mouse model of rheumatoid arthritis. Sci. Rep. 2020, 10, 3076. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Pontecorvi, P.; Anastasiadou, E.; Napoli, C.; Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020, 8, 236. [Google Scholar] [CrossRef]
- Berman, D.M.; Willman, M.A.; Han, D.; Kleiner, G.; Kenyon, N.M.; Cabrera, O.; Karl, J.A.; Wiseman, R.W.; O’Connor, D.H.; Bartholomew, A.M.; et al. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 2010, 59, 2558–2568. [Google Scholar] [CrossRef]
- Figliuzzi, M.; Cornolti, R.; Perico, N.; Rota, C.; Morigi, M.; Remuzzi, G.; Remuzzi, A.; Benigni, A. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant. Proc. 2009, 41, 1797–1800. [Google Scholar] [CrossRef]
- Iwazawa, R.; Kozakai, S.; Kitahashi, T.; Nakamura, K.; Hata, K.I. The Therapeutic Effects of Adipose-Derived Stem Cells and Recombinant Peptide Pieces on Mouse Model of DSS Colitis. Cell Transplant. 2018, 27, 1390–1400. [Google Scholar] [CrossRef]
- de Vos, P.; Faas, M.M.; Strand, B.; Calafiore, R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006, 27, 5603–5617. [Google Scholar] [CrossRef] [PubMed]
- Alagpulinsa, D.A.; Cao, J.J.L.; Driscoll, R.K.; Sirbulescu, R.F.; Penson, M.F.E.; Sremac, M.; Engquist, E.N.; Brauns, T.A.; Markmann, J.F.; Melton, D.A.; et al. Alginate-microencapsulation of human stem cell-derived beta cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am. J. Transplant. 2019, 19, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Feilen, P.J.; Brunnenmeier, F.; Minnemann, T.; Zimmermann, H.; Zimmermann, U.; Weber, M.M. Long-term graft function of adult rat and human islets encapsulated in novel alginate-based microcapsules after transplantation in immunocompetent diabetic mice. Diabetes 2005, 54, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Iizuka, N.; Fujita, Y.; Sawamoto, O.; Matsumoto, S. Effects of encapsulated porcine islets on glucose and C-peptide concentrations in diabetic nude mice 6 months after intraperitoneal transplantation. Xenotransplantation 2017, 24. [Google Scholar] [CrossRef]
- Morch, Y.A.; Donati, I.; Strand, B.L.; Skjak-Braek, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Hillberg, A.L.; Kathirgamanathan, K.; Lam, J.B.; Law, L.Y.; Garkavenko, O.; Elliott, R.B. Improving alginate-poly-L-ornithine-alginate capsule biocompatibility through genipin crosslinking. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 258–268. [Google Scholar] [CrossRef]
- Liu, Q.; Chiu, A.; Wang, L.H.; An, D.; Zhong, M.; Smink, A.M.; de Haan, B.J.; de Vos, P.; Keane, K.; Vegge, A.; et al. Zwitterionically modified alginates mitigate cellular overgrowth for cell encapsulation. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Stock, A.A.; Manzoli, V.; De Toni, T.; Abreu, M.M.; Poh, Y.C.; Ye, L.; Roose, A.; Pagliuca, F.W.; Thanos, C.; Ricordi, C.; et al. Conformal Coating of Stem Cell-Derived Islets for beta Cell Replacement in Type 1 Diabetes. Stem Cell Rep. 2020, 14, 91–104. [Google Scholar] [CrossRef]
- Nakanishi, W.; Imura, T.; Inagaki, A.; Nakamura, Y.; Sekiguchi, S.; Fujimori, K.; Satomi, S.; Goto, M. Ductal Injection Does Not Increase the Islet Yield or Function after Cold Storage in a Vascular Perfusion Model. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Zha, M.; Li, F.; Xu, W.; Chen, B.; Sun, Z. Isolation and characterization of islet stellate cells in rat. Islets 2014, 6, e28701. [Google Scholar] [CrossRef][Green Version]
- Yañez, R.; Lamana, M.L.; García-Castro, J.; Colmenero, I.; Ramírez, M.; Bueren, J.A. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 2006, 24, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, J.M.; Chang, P.L. Osmotic pressure test: A simple, quantitative method to assess the mechanical stability of alginate microcapsules. J. Biomed. Mater. Res. 2001, 54, 264–271. [Google Scholar] [CrossRef]
- Sakata, N.; Egawa, S.; Sumi, S.; Unno, M. Optimization of glucose level to determine the stimulation index of isolated rat islets. Pancreas 2008, 36, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Hata, Y.; Nishinakamura, H.; Kumano, K.; Takahashi, H.; Kodama, S. Islet-derived damage-associated molecular pattern molecule contributes to immune responses following microencapsulated neonatal porcine islet xenotransplantation in mice. Xenotransplantation 2016, 23, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, V.; Evans, M.D.M.; Lewy, D.M.; Bean, P.A.; Bal, S.; Tuch, B.E. Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted. Sci. Rep. 2017, 7, 10059. [Google Scholar] [CrossRef]
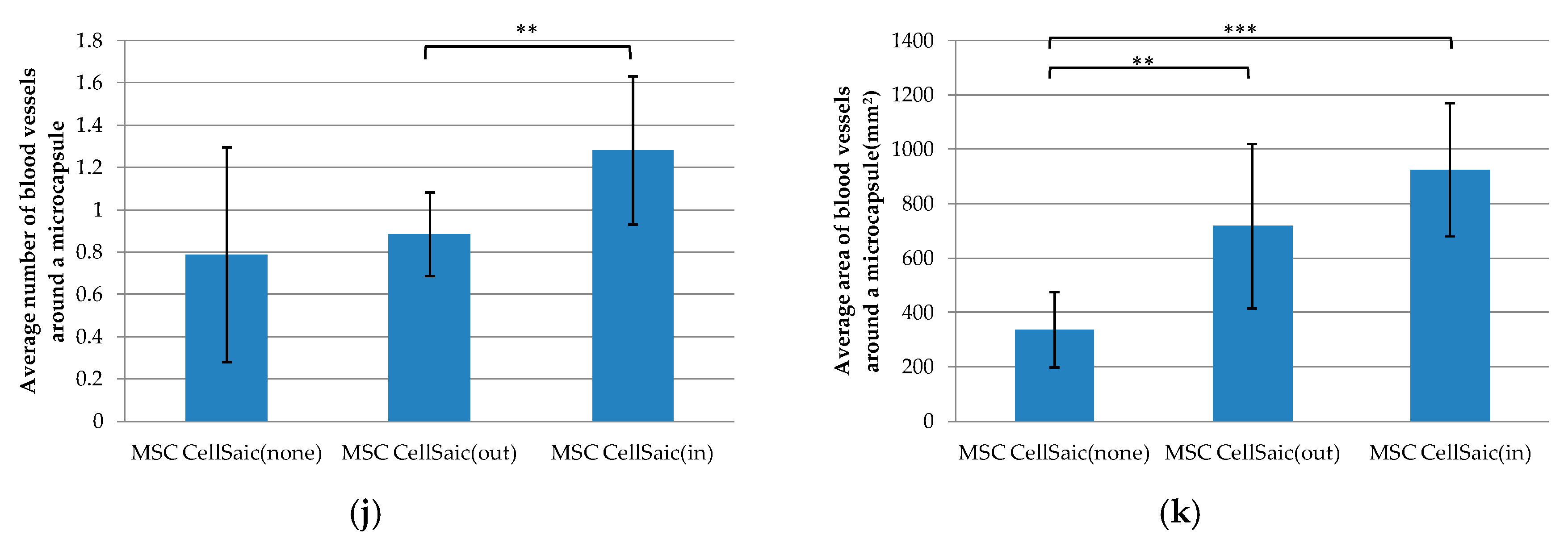
| Microcapsule Constitution | Resistance against Osmotic Pressure (%) | Duration Time for IgG Blocking (min) |
|---|---|---|
| Alginate/Ca2+ | 0 | 10 |
| Alginate/Ba2+ | 100 | 30 |
| Alginate/PLO | 100 | ≥120 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochizuki, Y.; Kogawa, R.; Takegami, R.; Nakamura, K.; Wakabayashi, A.; Ito, T.; Yoshioka, Y. Co-Microencapsulation of Islets and MSC CellSaics, Mosaic-Like Aggregates of MSCs and Recombinant Peptide Pieces, and Therapeutic Effects of Their Subcutaneous Transplantation on Diabetes. Biomedicines 2020, 8, 318. https://doi.org/10.3390/biomedicines8090318
Mochizuki Y, Kogawa R, Takegami R, Nakamura K, Wakabayashi A, Ito T, Yoshioka Y. Co-Microencapsulation of Islets and MSC CellSaics, Mosaic-Like Aggregates of MSCs and Recombinant Peptide Pieces, and Therapeutic Effects of Their Subcutaneous Transplantation on Diabetes. Biomedicines. 2020; 8(9):318. https://doi.org/10.3390/biomedicines8090318
Chicago/Turabian StyleMochizuki, Yusuke, Ryo Kogawa, Ryuta Takegami, Kentaro Nakamura, Akira Wakabayashi, Tadashi Ito, and Yasuhiro Yoshioka. 2020. "Co-Microencapsulation of Islets and MSC CellSaics, Mosaic-Like Aggregates of MSCs and Recombinant Peptide Pieces, and Therapeutic Effects of Their Subcutaneous Transplantation on Diabetes" Biomedicines 8, no. 9: 318. https://doi.org/10.3390/biomedicines8090318
APA StyleMochizuki, Y., Kogawa, R., Takegami, R., Nakamura, K., Wakabayashi, A., Ito, T., & Yoshioka, Y. (2020). Co-Microencapsulation of Islets and MSC CellSaics, Mosaic-Like Aggregates of MSCs and Recombinant Peptide Pieces, and Therapeutic Effects of Their Subcutaneous Transplantation on Diabetes. Biomedicines, 8(9), 318. https://doi.org/10.3390/biomedicines8090318