Application of 3D Bioprinting Technologies to the Management and Treatment of Diabetic Foot Ulcers
Abstract
1. Introduction
2. The Human Skin Structure
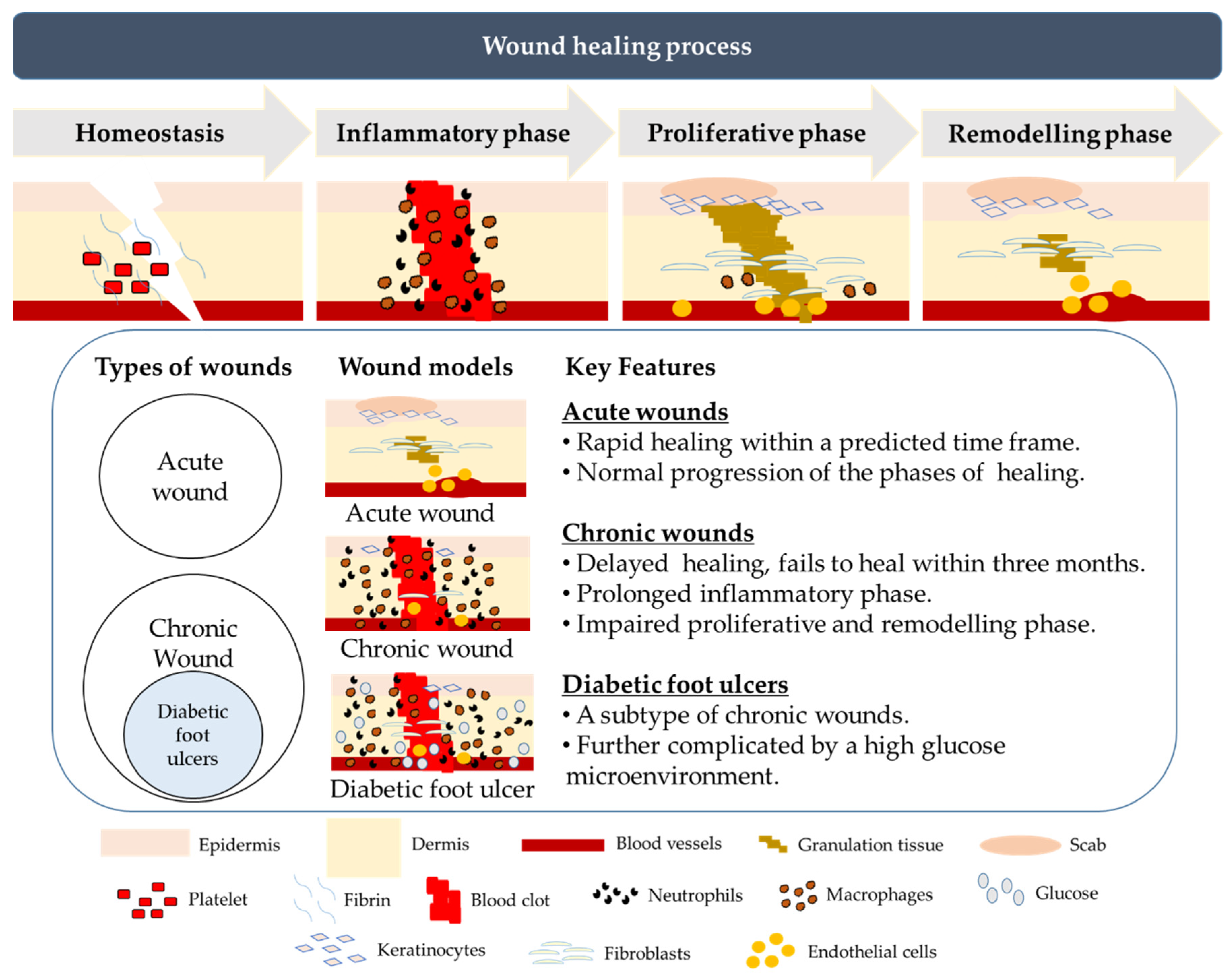
3. Normal and Chronic Wound Healing
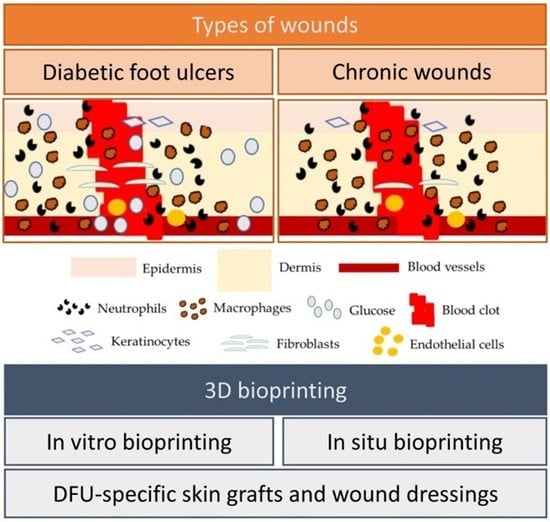
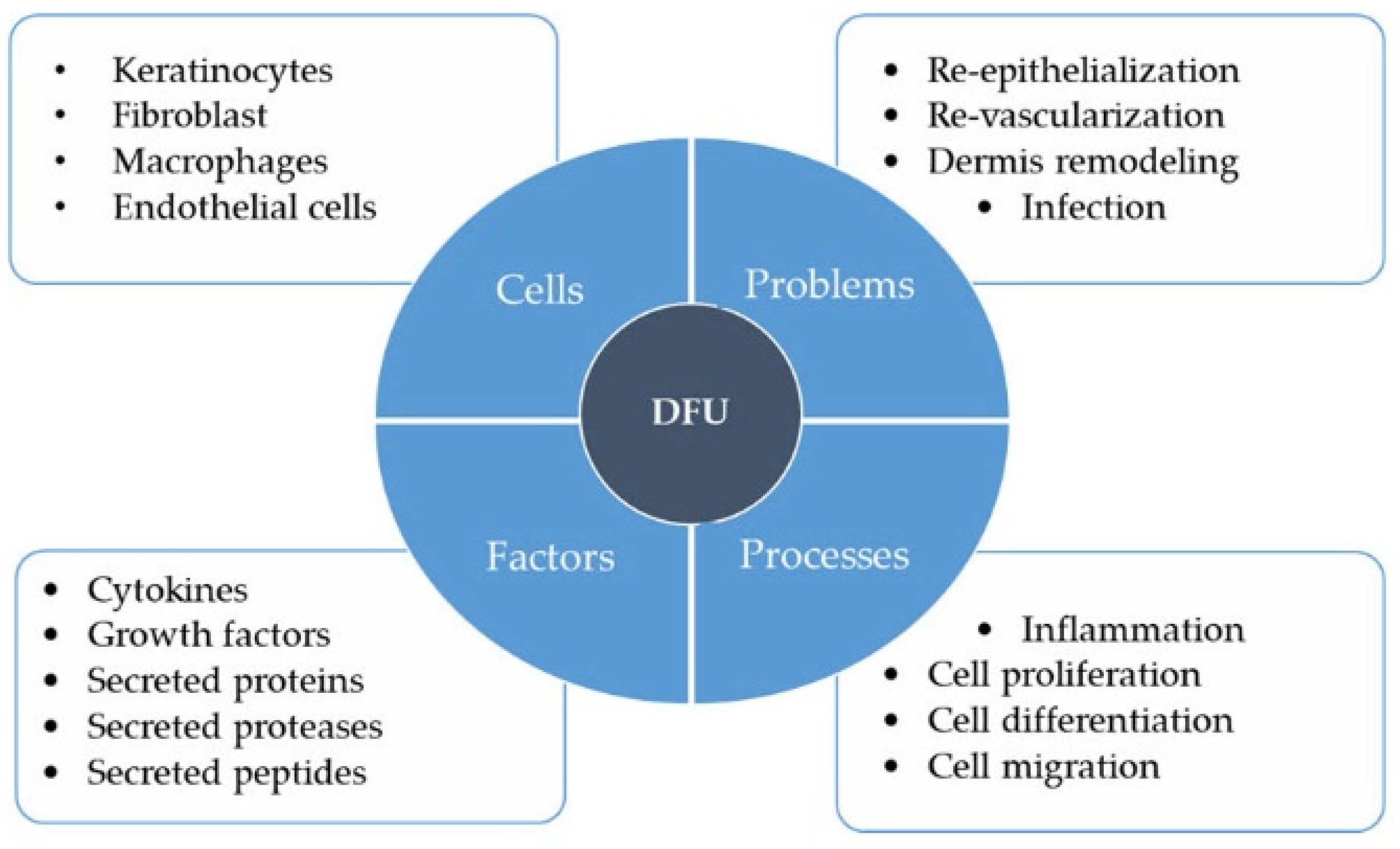
4. Diabetic Foot Ulcers
4.1. Keratinocytes
4.2. Fibroblasts
4.3. Macrophages
4.4. Endothelial Cells
4.5. Overall Impact of High Glucose on DFU Wound Healing
5. Treatment of Diabetic Ulcer/Foot Ulcer
5.1. Wound Dressings
5.2. Skin Grafts
5.3. Cell-Based Therapy
6. Three-Dimensional Bioprinting Approaches to Aid Wound Repair
6.1. Three-Dimensional Bioprinting Techniques
6.2. Application of 3D Bioprinting Approaches to Wound Treatment
6.2.1. In Vitro Bioprinting
6.2.2. In Situ Bioprinting
6.3. Future Directions for 3D Bioprinting Strategies in Diabetic Wound Repair
6.3.1. Development of Novel Biocompatible ECM-Based Hydrogels as Bioinks
6.3.2. Personalized Treatment
6.3.3. Infusion of Bioactives in Acellular 3D-Printed Wound Dressings
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raghav, A.; Khan, Z.A.; Labala, R.K.; Ahmad, J.; Noor, S.; Mishra, B.K. Financial Burden of Diabetic Foot Ulcers to World: A Progressive Topic to Discuss Always. Ther. Adv. Endocrinol. Metab. 2018, 9, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global Epidemiology of Diabetic Foot Ulceration: A Systematic Review and Meta-Analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Selvarajah, S.; Mathioudakis, N.; Sherman, R.E.; Hines, K.F.; Black, J.H.; Abularrage, C.J. Burden of Infected Diabetic Foot Ulcers on Hospital Admissions and Costs. Ann. Vasc. Surg. 2016, 33, 149–158. [Google Scholar] [CrossRef]
- Jiang, Y.; Ran, X.; Jia, L.; Yang, C.; Wang, P.; Ma, J.; Chen, B.; Yu, Y.; Feng, B.; Chen, L.; et al. Epidemiology of Type 2 Diabetic Foot Problems and Predictive Factors for Amputation in China. Int. J. Low. Extrem. Wounds 2015, 14, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V. Wound Healing and Its Impairment in the Diabetic Foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Lavery, L.A.; Armstrong, D.G.; Wunderlich, R.P.; Tredwell, J.; Boulton, A.J.M. Diabetic Foot Syndrome: Evaluating the Prevalence and Incidence of Foot Pathology in Mexican Americans and Non-Hispanic Whites from a Diabetes Disease Management Cohort. Diabetes Care 2003, 26, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, A. Type 1 Diabetes Mellitus. Nat. Rev. Dis. Prim. 2017, 3, 1–17. [Google Scholar] [CrossRef]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 Diabetes Mellitus: A Review of Current Trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Hoepelman, A.I. Immune Dysfunction in Patients with Diabetes Mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef]
- Ferlita, S.; Yegiazaryan, A.; Noori, N.; Lal, G.; Nguyen, T.; To, K.; Venketaraman, V. Type 2 Diabetes Mellitus and Altered Immune System Leading to Susceptibility to Pathogens, Especially Mycobacterium Tuberculosis. J. Clin. Med. 2019, 8, 2219. [Google Scholar] [CrossRef]
- Duby, J.J.; Campbell, R.K.; Setter, S.M.; White, J.R.; Rasmussen, K.A. Diabetic Neuropathy: An Intensive Review. Am. J. Health-Syst. Pharm. 2004, 61, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Greenman, R.L.; Panasyuk, S.; Wang, X.; Lyons, T.E.; Dinh, T.; Longoria, L.; Giurini, J.M.; Freeman, J.; Khaodhiar, L.; Veves, A. Early Changes in the Skin Microcirculation and Muscle Metabolism of the Diabetic Foot. Lancet 2005, 366, 1711–1717. [Google Scholar] [CrossRef]
- Alexiadou, K.; Doupis, J. Management of Diabetic Foot Ulcers. Diabetes Ther. 2012, 3, 1:1–1:15. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula, W.V.; Bus, S.A. Five Year Mortality and Direct Costs of Care for People with Diabetic Foot Complications Are Comparable to Cancer. J. Foot Ankle Res. 2020, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kruse, I.; Edelman, S. Evaluation and Treatment of Diabetic Foot Ulcers. Clin. Diabetes 2006, 24, 91–93. [Google Scholar] [CrossRef]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global Estimates of Diabetes Prevalence for 2013 and Projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D Bioprinting System to Produce Human-Scale Tissue Constructs with Structural Integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, F.; Shi, J.; Shen, S.; Teng, H.; Yang, J.; Wang, X.; Jiang, Q. In Situ Repair of Bone and Cartilage Defects Using 3D Scanning and 3D Printing. Sci. Rep. 2017, 7, 9416. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344:1–1900344:10. [Google Scholar] [CrossRef]
- Ackland, D.C.; Robinson, D.; Redhead, M.; Lee, P.V.S.; Moskaljuk, A.; Dimitroulis, G. A Personalized 3D-Printed Prosthetic Joint Replacement for the Human Temporomandibular Joint: From Implant Design to Implantation. J. Mech. Behav. Biomed. Mater. 2017, 69, 404–411. [Google Scholar] [CrossRef]
- Powell, J. Skin Physiology. Women’s Health Med. 2006, 3, 130–133. [Google Scholar] [CrossRef]
- Anderson, B.E. The Netter Collection of Medical Illustrations. Integumentary System, 2nd ed.; Elsevier Saunders: Philadelphia, PA, USA, 2012; Volume 4, ISBN 978-1437756548. [Google Scholar]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and Physiology of the Skin. J. Dermatol. Nurses’ Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The Structure and Function of the Stratum Corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Fenner, J.; Clark, R.A. Anatomy, Physiology, Histology, and Immunohistochemistry of Human Skin. In Skin Tissue Engineering and Regenerative Medicine; Albanna, M.Z., Holmes, J.H., IV, Eds.; Academic Press: Boston, MA, USA, 2016; ISBN 9780128017975. [Google Scholar]
- Mann, E.R.; Smith, K.M.; Bernardo, D.; Al-Hassi, H.O.; Knight, S.C.; Hart, A.L. Review: Skin and the Immune System. J. Clin. Exp. Dermatol. Res. 2012, S2, 003. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound Healing—A Literature Review. Anais Brasileiros de Dermatologia 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Gale, A.J. Continuing Education Course #2: Current Understanding of Hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Palta, S.; Saroa, R.; Palta, A. Overview of the Coagulation System. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef]
- Oettgen, H.; Broide, D.H. Allergy. In Introduction to Mechanisms of Allergic Diesase, 4th ed.; Holgate, S.T., Church, M.K., Broide, D.H., Martinez, F.D., Eds.; WB Saunders: Edinburgh, UK, 2011; pp. 1–32. ISBN 978-0723436584. [Google Scholar]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Schmidtke, D.W.; Diamond, S.L. Direct Observation of Membrane Tethers Formed during Neutrophil Attachment to Platelets or P-Selectin under Physiological Flow. J. Cell Biol. 2000, 149, 719–729. [Google Scholar] [CrossRef]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages That Have Ingested Apoptotic Cells in Vitro Inhibit Proinflammatory Cytokine Production through Autocrine/Paracrine Mechanisms Involving TGF-β, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gómez, A.; Perretti, M.; Soehnlein, O. Resolution of Inflammation: An Integrated View. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-Smooth Muscle Actin Expression Upregulates Fibroblast Contractile Activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M.W. Tissue-Specific Contribution of Macrophages to Wound Healing. Semin. Cell Dev. Biol. 2017, 61, 3–11. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Hart, C.E.; Loewen-Rodriguez, A.; Lessem, J. Dermagraft: Use in the Treatment of Chronic Wounds. Adv. Wound Care 2012, 1, 138–141. [Google Scholar] [CrossRef]
- Dash, N.R.; Dash, S.N.; Routray, P.; Mohapatra, S.; Mohapatra, P.C. Targeting Nonhealing Ulcers of Lower Extremity in Human through Autologous Bone Marrow-Derived Mesenchymal Stem Cells. Rejuvenation Res. 2009, 12, 359–366. [Google Scholar] [CrossRef]
- Suga, H.; Sugaya, M.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. TLR4, Rather than TLR2, Regulates Wound Healing through TGF-β and CCL5 Expression. J. Dermatol. Sci. 2014, 73, 117–124. [Google Scholar] [CrossRef]
- Kasuya, A.; Tokura, Y. Attempts to Accelerate Wound Healing. J. Dermatol. Sci. 2014, 76, 169–172. [Google Scholar] [CrossRef]
- Carey, I.M.; Critchley, J.A.; Dewilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of Infection in Type 1 and Type 2 Diabetes Compared with the General Population: A Matched Cohort Study. Diabetes Care 2018, 41, 513–521. [Google Scholar] [CrossRef]
- Stegenga, M.E.; Van Der Crabben, S.N.; Blümer, R.M.E.; Levi, M.; Meijers, J.C.M.; Serlie, M.J.; Tanck, M.W.T.; Sauerwein, H.P.; Van Der Poll, T. Hyperglycemia Enhances Coagulation and Reduces Neutrophil Degranulation, Whereas Hyperinsulinemia Inhibits Fibrinolysis during Human Endotoxemia. Blood 2008, 112, 82–89. [Google Scholar] [CrossRef]
- Kumar, M.; Roe, K.; Nerurkar, P.V.; Orillo, B.; Thompson, K.S.; Verma, S.; Nerurkar, V.R. Reduced Immune Cell Infiltration and Increased Pro-Inflammatory Mediators in the Brain of Type 2 Diabetic Mouse Model Infected with West Nile Virus. J. Neuroinflamm. 2014, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.E.; Wu, C.S.; Huang, S.M.; Kuo, H.Y.; Wu, I.H.; Wen, C.H.; Chai, C.Y.; Fang, A.H.; Chen, G.S. High-Glucose Environment Inhibits P38MAPK Signaling and Reduces Human β-3 Expression in Keratinocytes. Mol. Med. 2011, 17, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Trimble, H.C.; Carey, B.W. On the True Sugar Content of Skin and of Muscle in Diabetic and Non-Diabetic Persons. J. Biol. Chem. 1931, 3, 655–663. [Google Scholar]
- Jensen, B.M.; Bjerring, P.; Christiansen, J.S.; ørskov, H. Glucose Content in Human Skin: Relationship with Blood Glucose Levels. Scand. J. Clin. Lab. Investig. 1995, 55, 427–432. [Google Scholar] [CrossRef]
- Miller, D.I.; Ridolfo, A.S. The Skin-Surface-Glucose Test: An Aid in the Diagnosis of Diabetes Mellitus. Diabetes 1960, 9, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.E.; Wu, C.S.; Huang, S.M.; Wu, I.H.; Chen, G.S. High-Glucose Environment Enhanced Oxidative Stress and Increased Interleukin-8 Secretion from Keratinocytes. Diabetes 2013, 62, 2530–2538. [Google Scholar] [CrossRef]
- Loots, M.A.M.; Lamme, E.N.; Mekkes, J.R.; Bos, J.D.; Middelkoop, E. Cultured Fibroblasts from Chronic Diabetic Wounds on the Lower Extremity (Non-Insulin-Dependent Diabetes Mellitus) Show Disturbed Proliferation. Arch. Dermatol. Res. 1999, 291, 93–99. [Google Scholar] [CrossRef]
- Pang, L.; Wang, Y.; Zheng, M.; Wang, Q.; Lin, H.; Zhang, L.; Wu, L. Transcriptomic Study of High-Glucose Effects on Human Skin Fibroblast Cells. Mol. Med. Rep. 2016, 13, 2627–2634. [Google Scholar] [CrossRef]
- Madhyastha, R.; Madhyastha, H.; Nakajima, Y.; Omura, S.; Maruyama, M. MicroRNA Signature in Diabetic Wound Healing: Promotive Role of MiR-21 in Fibroblast Migration. Int. Wound J. 2012, 9, 355–361. [Google Scholar] [CrossRef]
- Hehenberger, K.; Kratz, G.; Hansson, A.; Brismar, K. Fibroblasts Derived from Human Chronic Diabetic Wounds Have a Decreased Proliferation Rate, Which Is Recovered by the Addition of Heparin. J. Dermatol. Sci. 1998, 16, 144–151. [Google Scholar] [CrossRef]
- Andreea, S.I.; Marieta, C.; Anca, D. AGEs and Glucose Levels Modulate Type I and III Procollagen MRNA Synthesis in Dermal Fibroblasts Cells Culture. Exp. Diabetes Res. 2008, 2008, 473603–473603. [Google Scholar] [CrossRef]
- Torres-Castro, I.; Arroyo-Camarena, Ú.D.; Martínez-Reyes, C.P.; Gómez-Arauz, A.Y.; Dueñas-Andrade, Y.; Hernández-Ruiz, J.; Béjar, Y.L.; Zaga-Clavellina, V.; Morales-Montor, J.; Terrazas, L.I.; et al. Human Monocytes and Macrophages Undergo M1-Type Inflammatory Polarization in Response to High Levels of Glucose. Immunol. Lett. 2016, 176, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Grosick, R.; Alvarado-Vazquez, P.A.; Messersmith, A.R.; Romero-Sandoval, E.A. High Glucose Induces a Priming Effect in Macrophages and Exacerbates the Production of Pro-Inflammatory Cytokines after a Challenge. J. Pain Res. 2018, 11, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dai, L.; Li, L.; Chen, H.; Chai, Y. NLRP3 Inflammasome Expression and Signaling in Human Diabetic Wounds and in High Glucose Induced Macrophages. J. Diabetes Res. 2017, 2017, 5281358. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, J.J.; Kim, H.J.; Shong, M.; Ku, B.J.; Jo, E.K. Upregulated NLRP3 Inflammasome Activation in Patients with Type 2 Diabetes. Diabetes 2013, 62, 194–204. [Google Scholar] [CrossRef]
- Bitto, A.; Altavilla, D.; Pizzino, G.; Irrera, N.; Pallio, G.; Colonna, M.R.; Squadrito, F. Inhibition of Inflammasome Activation Improves the Impaired Pattern of Healing in Genetically Diabetic Mice. Br. J. Pharmacol. 2014, 171, 2300–2307. [Google Scholar] [CrossRef]
- Hu, S.C.S.; Lan, C.C.E. High-Glucose Environment Disturbs the Physiologic Functions of Keratinocytes: Focusing on Diabetic Wound Healing. J. Dermatol. Sci. 2016, 84, 121–127. [Google Scholar] [CrossRef]
- Weinheimer-Haus, E.M.; Mirza, R.E.; Koh, T.J. Nod-like Receptor Protein-3 Inflammasome Plays an Important Role during Early Stages of Wound Healing. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Huang, S.-M.; Wu, C.-S.; Chao, D.; Wu, C.-H.; Li, C.-C.; Chen, G.-S.; Lan, C.-C.E. High-Glucose-Cultivated Peripheral Blood Mononuclear Cells Impaired Keratinocyte Function via Reduced IL-22 Expression: Implications on Impaired Diabetic Wound Healing. Exp. Dermatol. 2015, 24, 639–641. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.T.; Zhang, B.; Zhou, Q.; Shen, C.X.; Wang, C.Q. Downregulation of MicroRNA-126 in Endothelial Progenitor Cells from Diabetes Patients, Impairs Their Functional Properties, via Target Gene Spred-1. J. Mol. Cell. Cardiol. 2012, 53, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The Endothelial-Specific MicroRNA MiR-126 Governs Vascular Integrity and Angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Walz, A.; Kunkel, S.L. Perspectives Neutrophil-Activating Peptide-1/Lnterleukin 8, a Novel Cytokine That Activates Neutrophils. J. Clin. Investig. 1989, 84, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Wetzler, C.; Kampfer, H.; Stallmeyer, B.; Pfeilschifter, J.; Frank, S. Large and Sustained Induction of Chemokines during Impaired Wound Healing in the Genetically Diabetic Mouse: Prolonged Persistence of Neutrophils and Macrophages during the Late Phase of Repair. J. Investig. Dermatol. 2000, 115, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.-C.E.; Wu, C.-S.; Huang, S.-M.; Kuo, H.-Y.; Wu, I.-H.; Liang, C.W.; Chen, G.-S. High-Glucose Environment Reduces Human β-Defensin-2 Expression in Human Keratinocytes: Implications for Poor Diabetic Wound Healing. Br. J. Dermatol. 2012, 166, 1221–1229. [Google Scholar] [CrossRef]
- Park, H.-Y.; Kim, J.-H.; Jung, M.; Chung, C.H.; Hasham, R.; Park, C.S.; Choi, E.H. A Long-Standing Hyperglycaemic Condition Impairs Skin Barrier by Accelerating Skin Ageing Process. Exp. Dermatol. 2011, 20, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; Zhang, Q.; Zhang, D.; Xiang, F.; Jia, J.; Wei, P.; Zhang, J.; Hu, J.; Huang, Y. High Glucose Suppresses Keratinocyte Migration Through the Inhibition of P38 MAPK/Autophagy Pathway. Front. Physiol. 2019, 10, 24. [Google Scholar] [CrossRef]
- Lan, C.-C.E.; Wu, C.-S.; Kuo, H.-Y.; Huang, S.-M.; Chen, G.-S. Hyperglycaemic Conditions Hamper Keratinocyte Locomotion via Sequential Inhibition of Distinct Pathways: New Insights on Poor Wound Closure in Patients with Diabetes. Br. J. Dermatol. 2009, 160, 1206–1214. [Google Scholar] [CrossRef]
- Lan, C.-C.E.; Liu, I.-H.; Fang, A.-H.; Wen, C.-H.; Wu, C.-S. Hyperglycaemic Conditions Decrease Cultured Keratinocyte Mobility: Implications for Impaired Wound Healing in Patients with Diabetes. Br. J. Dermatol. 2008, 159, 1103–1115. [Google Scholar] [CrossRef]
- Spravchikov, N.; Sizyakov, G.; Gartsbein, M.; Accili, D.; Tennenbaum, T.; Wertheimer, E. Glucose Effects on Skin Keratinocytes Implications for Diabetes Skin Complications. Diabetes 2001, 50, 1627–1635. [Google Scholar] [CrossRef]
- Terashi, H.; Izumi, K.; Deveci, M.; Rhodes, L.M.; Marcelo, C.L. High Glucose Inhibits Human Epidermal Keratinocyte Proliferation for Cellular Studies on Diabetes Mellitus. Int. Wound J. 2005, 2, 298–304. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-Based Biomaterials for Accelerated Diabetic Wound Healing: A Critical Review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.J.; Padmapriya, B. A Pragmatic Review on the Property, Role and Significance of Polymers in Treating Diabetic Foot Ulcer. Mater. Today Proc. 2019, 23, 91–99. [Google Scholar] [CrossRef]
- Gonzalez, S.R.; Wolter, K.G.; Yuen, J.C. Infectious Complications Associated with the Use of Integra: A Systematic Review of the Literature. Plast. Reconstr. Surg. Glob. Open 2020, 8. [Google Scholar] [CrossRef]
- Dalla Paola, L.; Cimaglia, P.; Carone, A.; Boscarino, G.; Scavone, G. Use of Integra Dermal Regeneration Template for Limb Salvage in Diabetic Patients With No-Option Critical Limb Ischemia. Int. J. Low. Extrem. Wounds 2020, 1534734620905741. [Google Scholar] [CrossRef]
- Duplantier, A.J.; van Hoek, M.L. The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds. Front. Immunol. 2013, 4, 143. [Google Scholar] [CrossRef]
- McCrudden, M.T.C.; McLean, D.T.F.; Zhou, M.; Shaw, J.; Linden, G.J.; Irwin, C.R.; Lundy, F.T. The Host Defence Peptide LL-37 Is Susceptible to Proteolytic Degradation by Wound Fluid Isolated from Foot Ulcers of Diabetic Patients. Int. J. Pept. Res. Ther. 2014, 20, 457–464. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Holroyd, K.J.; Zasloff, M. Topical versus Systemic Antimicrobial Therapy for Treating Mildly Infected Diabetic Foot Ulcers: A Randomized, Controlled, Double-Blinded, Multicenter Trial of Pexiganan Cream. Clin. Infect. Dis. 2008, 47, 1537–1545. [Google Scholar] [CrossRef]
- McCartan, B.; Dinh, T. The Use of Split-Thickness Skin Grafts on Diabetic Foot Ulcerations: A Literature Review. Plast. Surg. Int. 2012, 2012, 715273. [Google Scholar] [CrossRef]
- Brem, H.; Balledux, J.; Bloom, T.; Kerstein, M.D.; Hollier, L. Healing of Diabetic Foot Ulcers and Pressure Ulcers with Human Skin Equivalent: A New Paradigm in Wound Healing. Arch. Surg. 2000, 135, 627–634. [Google Scholar] [CrossRef]
- Streit, M.; Braathen, L.R. Apligraf—A Living Human Skin Equivalent for the Treatment of Chronic Wounds. Int. J. Artif. Organs 2000, 23, 831–833. [Google Scholar] [CrossRef]
- Dinh, T.L.; Veves, A. The Efficacy of Apligraf in the Treatment of Diabetic Foot Ulcers. Plast. Reconstr. Surg. 2006, 117, 152S–157S. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Midha, S.; Giri, S.; Mohanty, S. Functional Skin Grafts: Where Biomaterials Meet Stem Cells. Stem Cells Int. 2019, 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Isaacs, C.; Paquette, D.; Downing, G.; Kouttab, N.; Butmarc, J.; Badiavas, E.; Hardin-Young, J. Wounding of Bioengineered Skin: Cellular and Molecular Aspects after Injury. J. Investig. Dermatol. 2002, 119, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kirsner, R.S.; Falanga, V.; Phillips, T.; Eaglstein, W.H. Evaluation of ApligrafR Persistence and Basement Membrane Restoration in Donor Site Wounds: A Pilot Study. Wound Repair Regen. 2006, 14, 427–433. [Google Scholar] [CrossRef]
- Griffiths, M.; Ojeh, N.; Livingstone, R.; Price, R.; Navsaria, H. Survival of Apligraf in Acute Human Wounds. Tissue Eng. 2004, 10, 1180–1195. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gang, X.; Sun, C.; Wang, G. Mesenchymal Stem Cells Improve Healing of Diabetic Foot Ulcer. J. Diabetes Res. 2017, 2017, 9328347. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, R.; Nakao, S.; Shibusawa, Y.; Ishikawa, O.; Moro, T.; Mikami, K.; Fukumitsu, H.; Ueda, Y.; Minakawa, K.; Tabata, Y.; et al. Differential Contribution of Dermal Resident and Bone Marrow-Derived Cells to Collagen Production during Wound Healing and Fibrogenesis in Mice. J. Investig. Dermatol. 2011, 131, 529–536. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Scott, P.G.; Tredget, E.E. Bone Marrow-Derived Stem Cells in Wound Healing: A Review. Wound Repair Regen. 2007, 15, S18–S26. [Google Scholar] [CrossRef]
- Deng, W.; Han, Q.; Liao, L.; Li, C.; Ge, W.; Zhao, Z.; You, S.; Deng, H.; Murad, F.; Zhao, R.C.H. Engrafted Bone Marrow-Derived Flk-1+ Mesenchymal Stem Cells Regenerate Skin Tissue. Tissue Eng. 2005, 11, 110–119. [Google Scholar] [CrossRef]
- Fathke, C. Contribution of Bone Marrow-Derived Cells to Skin: Collagen Deposition and Wound Repair. Stem Cells 2004, 22, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Badiavas, E.V.; Abedi, M.; Butmarc, J.; Falanga, V.; Quesenberry, P. Participation of Bone Marrow Derived Cells in Cutaneous Wound Healing. J. Cell. Physiol. 2003, 196, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Procházka, V.; Gumulec, J.; Jalůvka, F.; Salounová, D.; Jonszta, T.; Czerný, D.; Krajča, J.; Urbanec, R.; Klement, P.; Martinek, J.; et al. Cell Therapy, a New Standard in Management of Chronic Critical Limb Ischemia and Foot Ulcer. Cell Transplant. 2010, 19, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.H.; Abd Elmageed, Z.Y.; Nair, D.; Partyka, M.I.; Kadowitz, P.J.; Belmadani, S.; Matrougui, K. Modified Multipotent Stromal Cells with Epidermal Growth Factor Restore Vasculogenesis and Blood Flow in Ischemic Hind-Limb of Type II Diabetic Mice. Lab. Investig. 2010, 90, 985–996. [Google Scholar] [CrossRef]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; et al. Comparison of Bone Marrow Mesenchymal Stem Cells with Bone Marrow-Derived Mononuclear Cells for Treatment of Diabetic Critical Limb Ischemia and Foot Ulcer: A Double-Blind, Randomized, Controlled Trial. Diabetes Res. Clin. Pract. 2011, 92, 26–36. [Google Scholar] [CrossRef]
- Amann, B.; Luedemann, C.; Ratei, R.; Schmidt-Lucke, J.A. Autologous Bone Marrow Cell Transplantation Increases Leg Perfusion and Reduces Amputations in Patients with Advanced Critical Limb Ischemia Due to Peripheral Artery Disease. Cell Transplant. 2009, 18, 371–380. [Google Scholar] [CrossRef]
- Vojtaššák, J.; Danišovič, Ľ.; Kubeš, M.; Bakoš, D.; Jarábek, Ľ.; Uličná, M.; Blaško, M. Autologous Biograft and Mesenchymal Stem Cells in Treatment of the Diabetic Foot. Neuro Endocrinol. Lett. 2006, 27, 134–137. [Google Scholar]
- Lopes, L.; Setia, O.; Aurshina, A.; Liu, S.; Hu, H.; Isaji, T.; Liu, H.; Wang, T.; Ono, S.; Guo, X.; et al. Stem Cell Therapy for Diabetic Foot Ulcers: A Review of Preclinical and Clinical Research. Stem Cell Res. Ther. 2018, 91, 188. [Google Scholar] [CrossRef]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef]
- Otero-Viñas, M.; Falanga, V. Mesenchymal Stem Cells in Chronic Wounds: The Spectrum from Basic to Advanced Therapy. Adv. Wound Care 2016, 5, 149–163. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Gou, M.; Da, L.-C.; Zhang, W.-Q.; Xie, H.-Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part B Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Veves, A.; Sheehan, P.; Pham, H.T. A Randomized, Controlled Trial of Promogran (a Collagen/Oxidized Regenerated Cellulose Dressing) vs Standard Treatment in the Management of Diabetic Foot Ulcers. Arch. Surg. 2002, 137, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of Bioactive Glasses on Angiogenesis: A Review of in Vitro and in Vivo Evidences. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.S.; Schmid, P. Epidermal-Dermal Interactions Regulate Gelatinase Activity in Apligraf®, a Tissue-Engineered Human Skin Equivalent. Br. J. Dermatol. 2002, 146, 26–31. [Google Scholar] [CrossRef] [PubMed]
- McColgan, M.; Foster, A.; Edmonds, M. Dermagraft in the Treatment of Diabetic Foot Ulcers. Diabet. Foots 1998, 1, 75–78. [Google Scholar]
- Kim, S.-W.; Zhang, H.-Z.; Guo, L.; Kim, J.-M.; Kim, H. Amniotic Mesenchymal Stem Cells Enhance Wound Healing in Diabetic NOD/SCID Mice through High Angiogenic and Engraftment Capabilities. PLoS ONE 2012, 7, e41105. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zheng, Z.-H.; Li, X.-Y.; Guo, J.; Zhang, Y.; Li, H.; Wang, Y.-W.; Ren, J.; Wu, Z.-B. Treatment of Foot Disease in Patients with Type 2 Diabetes Mellitus Using Human Umbilical Cord Blood Mesenchymal Stem Cells: Response and Correction of Immunological Anomalies. Curr. Pharm. Des. 2013, 19, 4893–4899. [Google Scholar] [CrossRef]
- van Kogelenberg, S.; Yue, Z.; Dinoro, J.N.; Baker, C.S.; Wallace, G.G. Three-Dimensional Printing and Cell Therapy for Wound Repair. Adv. Wound Care 2018, 7, 145–156. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Douroumis, D.; Boateng, J. 3D Printed Scaffolds for Wound Healing and Tissue Regeneration. Ther. Dress. Wound Healing Appl. 2020, 2020, 385–398. [Google Scholar] [CrossRef]
- Saunders, R.E.; Derby, B. Inkjet Printing Biomaterials for Tissue Engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Angelopoulos, I.; Allenby, M.C.; Lim, M.; Zamorano, M. Engineering Inkjet Bioprinting Processes toward Translational Therapies. Biotechnol. Bioeng. 2020, 117, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel Crosslinking Methods to Design Hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet Printing of Viable Mammalian Cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Murphy, S.V.; Crowell, K.; Mack, D.; Atala, A.; Soker, S. A Tunable Hydrogel System for Long-Term Release of Cell-Secreted Cytokines and Bioprinted in Situ Wound Cell Delivery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1986–2000. [Google Scholar] [CrossRef]
- Velasco, D.; Quílez, C.; Garcia, M.; del Cañizo, J.F.; Jorcano, J.L. 3D Human Skin Bioprinting: A View from the Bio Side. J. 3D Print. Med. 2018, 2, 141–162. [Google Scholar] [CrossRef]
- Chang, R.; Nam, J.; Sun, W. Effects of Dispensing Pressure and Nozzle Diameter on Cell Survival from Solid Freeform Fabrication-Based Direct Cell Writing. Tissue Eng. Part A. 2008, 14, 41–48. [Google Scholar] [CrossRef]
- Ghibaudo, C. Design of a 3D Printed Nanocellulose Based Moisturizer for Wound Dressing Applications. Ph.D. Dissertation, Politecnico di Torino, Turin, Italy, 2018. [Google Scholar]
- Bohandy, J.; Kim, B.F.; Adrian, F.J. Metal Deposition from a Supported Metal Film Using an Excimer Laser. J. Appl. Phys. 1986, 60, 1538–1539. [Google Scholar] [CrossRef]
- Morales, M.; Munoz-Martin, D.; Marquez, A.; Lauzurica, S.; Molpeceres, C. Laser-Induced Forward Transfer Techniques and Applications. In Advances in Laser Materials Processing; Woodhead Publishing: Cambridge, UK, 2018; pp. 339–379. [Google Scholar] [CrossRef]
- Yanez, M.; Rincon, J.; Dones, A.; De Maria, C.; Gonzales, R.; Boland, T. In Vivo Assessment of Printed Microvasculature in a Bilayer Skin Graft to Treat Full-Thickness Wounds. Tissue Eng. Part A 2015, 21, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng. Part A 2020, 26, 227–238. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D Cell Printing of in Vitro Stabilized Skin Model and in Vivo Pre-Vascularized Skin Patch Using Tissue-Specific Extracellular Matrix Bioink: A Step towards Advanced Skin Tissue Engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef]
- Hiller, T.; Berg, J.; Elomaa, L.; Röhrs, V.; Ullah, I.; Schaar, K.; Dietrich, A.-C.; Al-Zeer, M.; Kurtz, A.; Hocke, A.; et al. Generation of a 3D Liver Model Comprising Human Extracellular Matrix in an Alginate/Gelatin-Based Bioink by Extrusion Bioprinting for Infection and Transduction Studies. Int. J. Mol. Sci. 2018, 19, 3129. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Ha, D.H.; Jang, J.; Han, H.H.; Rhie, J.W.; Cho, D.W. Biomimetic 3D Tissue Printing for Soft Tissue Regeneration. Biomaterials 2015, 62, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Varkey, M.; Wang, Z.; Xie, B.; Hou, R.; Atala, A. ECM Concentration and Cell-mediated Traction Forces Play a Role in Vascular Network Assembly in 3D Bioprinted Tissue. Biotechnol. Bioeng. 2020, 117, 1148–1158. [Google Scholar] [CrossRef]
- Brugués, A.; Anon, E.; Conte, V.; Veldhuis, J.H.; Gupta, M.; Colombelli, J.; Muñoz, J.J.; Brodland, G.W.; Ladoux, B.; Trepat, X. Forces Driving Epithelial Wound Healing. Nat. Phys. 2014, 10, 683–690. [Google Scholar] [CrossRef]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of PH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef]
- Garbern, J.C.; Hoffman, A.S.; Stayton, P.S. Injectable PH- and Temperature-Responsive Poly(N-Isopropylacrylamide-Co-Propylacrylic Acid) Copolymers for Delivery of Angiogenic Growth Factors. Biomacromolecules 2010, 11, 1833–1839. [Google Scholar] [CrossRef]
- Varkey, M.; Visscher, D.O.; van Zuijlen, P.P.M.; Atala, A.; Yoo, J.J. Skin Bioprinting: The Future of Burn Wound Reconstruction? Burns Trauma 2019, 7, s41038–019. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and Innovative Approaches for Wound Healing and Skin Regeneration: Current Status and Advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.C. 3D Printing of a Wearable Personalized Oral Delivery Device: A First-in-Human Study. Sci. Adv. 2018, 4, eaat2544. [Google Scholar] [CrossRef] [PubMed]
- Poldervaart, M.T.; Gremmels, H.; Van Deventer, K.; Fledderus, J.O.; Öner, F.C.; Verhaar, M.C.; Dhert, W.J.A.; Alblas, J. Prolonged Presence of VEGF Promotes Vascularization in 3D Bioprinted Scaffolds with Defined Architecture. J. Control. Release 2014, 184, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.H.; Lee, M.K.; Baek, K.; Jeong, J.H.; Kim, D.H.; Millet, L.J.; Bashir, R.; Kong, H. Material-Mediated Proangiogenic Factor Release Pattern Modulates Quality of Regenerated Blood Vessels. J. Control. Release 2014, 196, 363–369. [Google Scholar] [CrossRef]
- Park, J.Y.; Shim, J.H.; Choi, S.A.; Jang, J.; Kim, M.; Lee, S.H.; Cho, D.W. 3D Printing Technology to Control BMP-2 and VEGF Delivery Spatially and Temporally to Promote Large-Volume Bone Regeneration. J. Mater. Chem. B 2015, 3, 5415–5425. [Google Scholar] [CrossRef]
- Wong, H.K.; Lam, C.R.I.; Wen, F.; Chong, S.K.M.; Tan, N.S.; Chan, J.; Pal, M.; Tan, L.P. Novel Method to Improve Vascularization of Tissue Engineered Constructs with Biodegradable Fibers—IOPscience. Biofabrication 2016, 8, 015004:1–015004:12. [Google Scholar] [CrossRef]
| Treatments/Key Examples | Mechanisms | Effects |
|---|---|---|
Wound dressings
|
|
|
Skin grafting
|
| |
Stem-cell therapy
|
|
| Bioprinting Devices | Biomaterials for Printing and Construct Applications | Outcomes |
|---|---|---|
In vitro
| Bioink: Fibrin and collagen Cells: Neonatal human dermal fibroblast, human dermal microvascular endothelial cells and neonatal human epidermal keratinocytes Application: Full-thickness skin excision (1.7 cm × 1.7 cm) on athymic nude mice | Bioprinted skin graft showed accelerated wound healing with 17% improvement in wound contraction and formation of microvasculature [126]. |
| Bioink: Decellularized extracellular matrix of porcine skin tissue Cells: Adipose-derived stem cells (ASCs) + endothelial progenitor cells (EPCs) Application: 10 mm biopsy punch full-thickness excisional wound on BALB/c A-nu/nu | Bioprinted skin patch remarkably enhanced neovascularization as well as wound closure and re-epithelization. Cell-printed dECM patch also exhibited better-wound healing activity compared to only ASC/EPCs mixture [128]. |
| Bioink: Rat tail type I collagen Cells (dermis): human foreskin dermal fibroblasts, human endothelial cells derived from cord blood endothelial colony-forming cells and placental pericytes (PCs) Cells (epidermis): Human foreskin keratinocytes Application: Excised dorsal mouse skin | Skin substitutes formulated with human ECs and PCs contained vascular structures 2-weeks post-engraftment and a higher degree of epidermal organization. Grafts containing PCs in addition to ECs appeared to evoke a more extensive angiogenic host response [127]. |
In situ
| Bioink: Fibrin and collagen Cells: Human fibroblasts and keratinocytes Application: Full-thickness excisional skin defect (3 × 2.5 cm) on female outbred nu/nu mice | Bioprinted constructs promoted accelerated wound closure with fully formed and organized dermis and epidermis. Fibroblasts and keratinocytes remained 6 weeks post engraftment [119]. |
| Bioink: Fibrin and collagen Cells: Amniotic fluid-derived stem cells (AFS) and Bone marrow-derived mesenchymal stem cells (MSC) Application: Full-thickness skin wound (2.0 × 2.0 cm) on nu/nu mice. | Wounds treated with AFS and MSC cells had faster-wound closure, re-epithelialization and better neovascularization than cell-free controls [129]. |
| Bioink: Heparin-conjugated hyaluronic acid (HA) and thiolated HA Cells: Amniotic fluid-derived stem (AFS) cells Application: Full-thickness skin wound (2.0 × 2.0 cm) on nu/nu mice. | HA-HP hydrogel supports the extended release of AFS-secreted cytokines by heparin-associated sequestration that improved wound closure, re-epithelialization, and vascularization [120]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.T.; Liang, K.; Ngo, Z.H.; Dube, C.T.; Lim, C.Y. Application of 3D Bioprinting Technologies to the Management and Treatment of Diabetic Foot Ulcers. Biomedicines 2020, 8, 441. https://doi.org/10.3390/biomedicines8100441
Tan CT, Liang K, Ngo ZH, Dube CT, Lim CY. Application of 3D Bioprinting Technologies to the Management and Treatment of Diabetic Foot Ulcers. Biomedicines. 2020; 8(10):441. https://doi.org/10.3390/biomedicines8100441
Chicago/Turabian StyleTan, Chew Teng, Kun Liang, Zong Heng Ngo, Christabel Thembela Dube, and Chin Yan Lim. 2020. "Application of 3D Bioprinting Technologies to the Management and Treatment of Diabetic Foot Ulcers" Biomedicines 8, no. 10: 441. https://doi.org/10.3390/biomedicines8100441
APA StyleTan, C. T., Liang, K., Ngo, Z. H., Dube, C. T., & Lim, C. Y. (2020). Application of 3D Bioprinting Technologies to the Management and Treatment of Diabetic Foot Ulcers. Biomedicines, 8(10), 441. https://doi.org/10.3390/biomedicines8100441