The Role of Ras-Associated Protein 1 (Rap1) in Cancer: Bad Actor or Good Player?
Abstract
1. Introduction
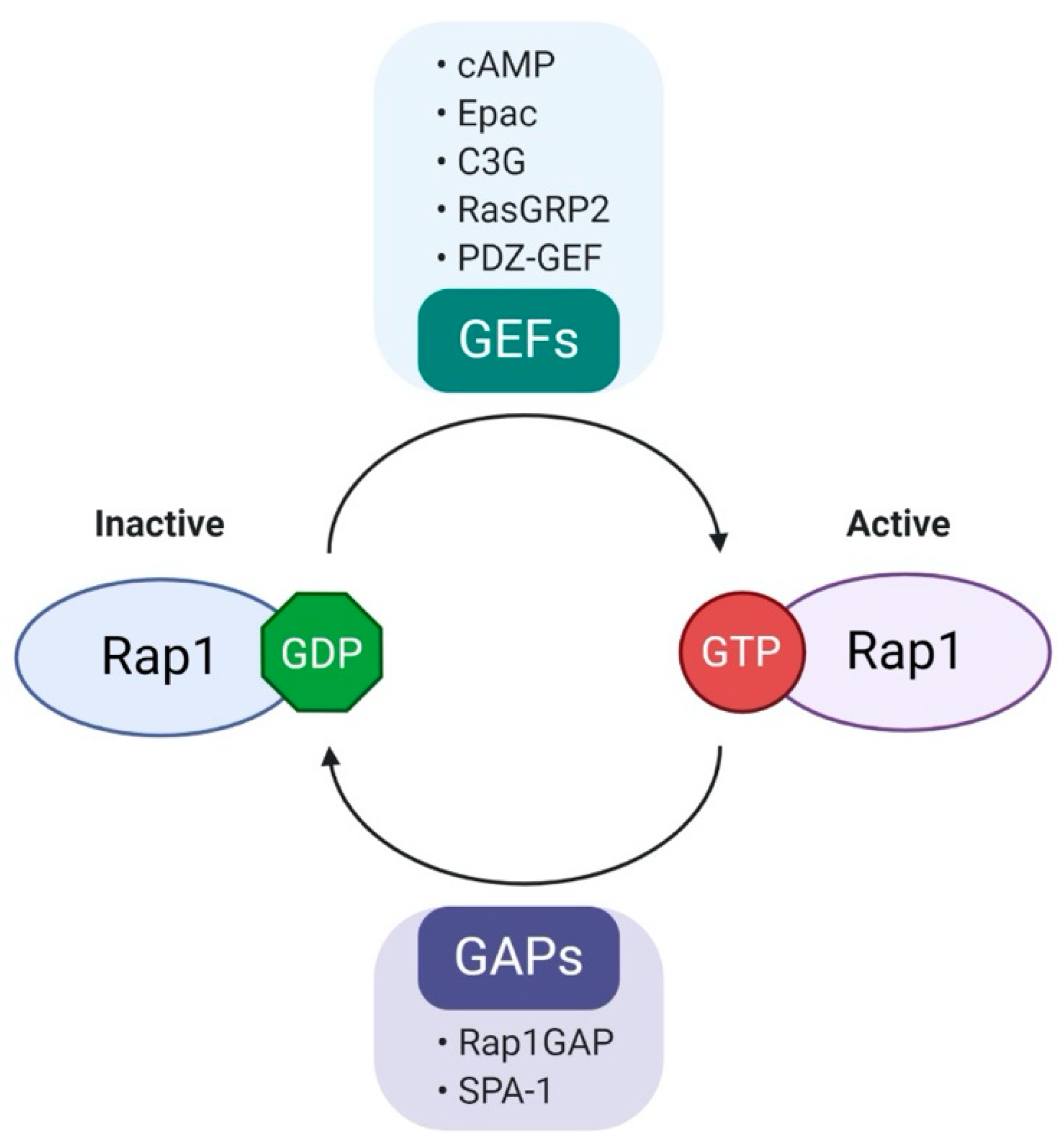
2. Biology and Regulation of Rap1
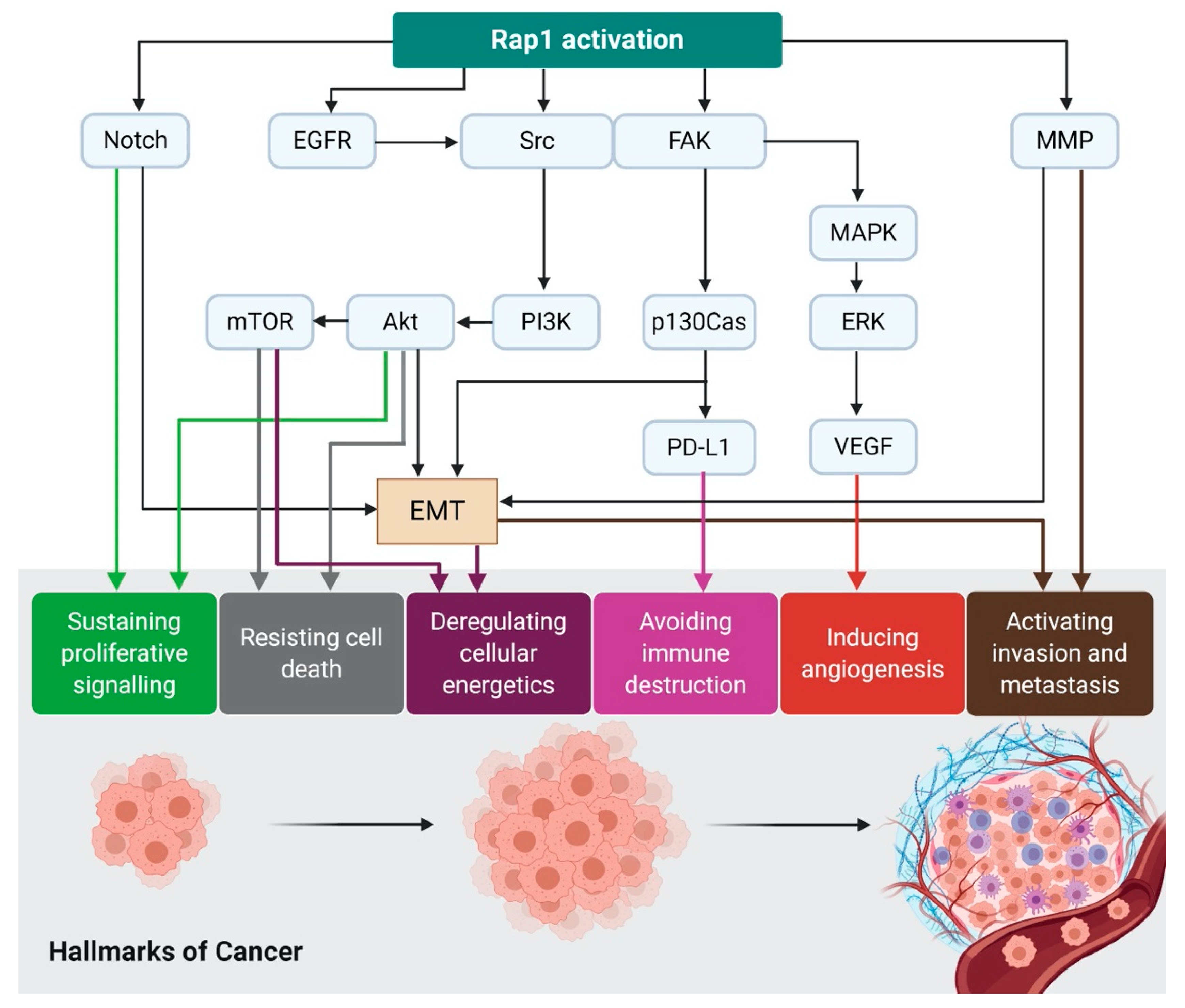
3. Rap1 and Rap1GAP in Cancer
3.1. Rap1 Regulates Autophagy and Cancer Metabolism via mTORC1
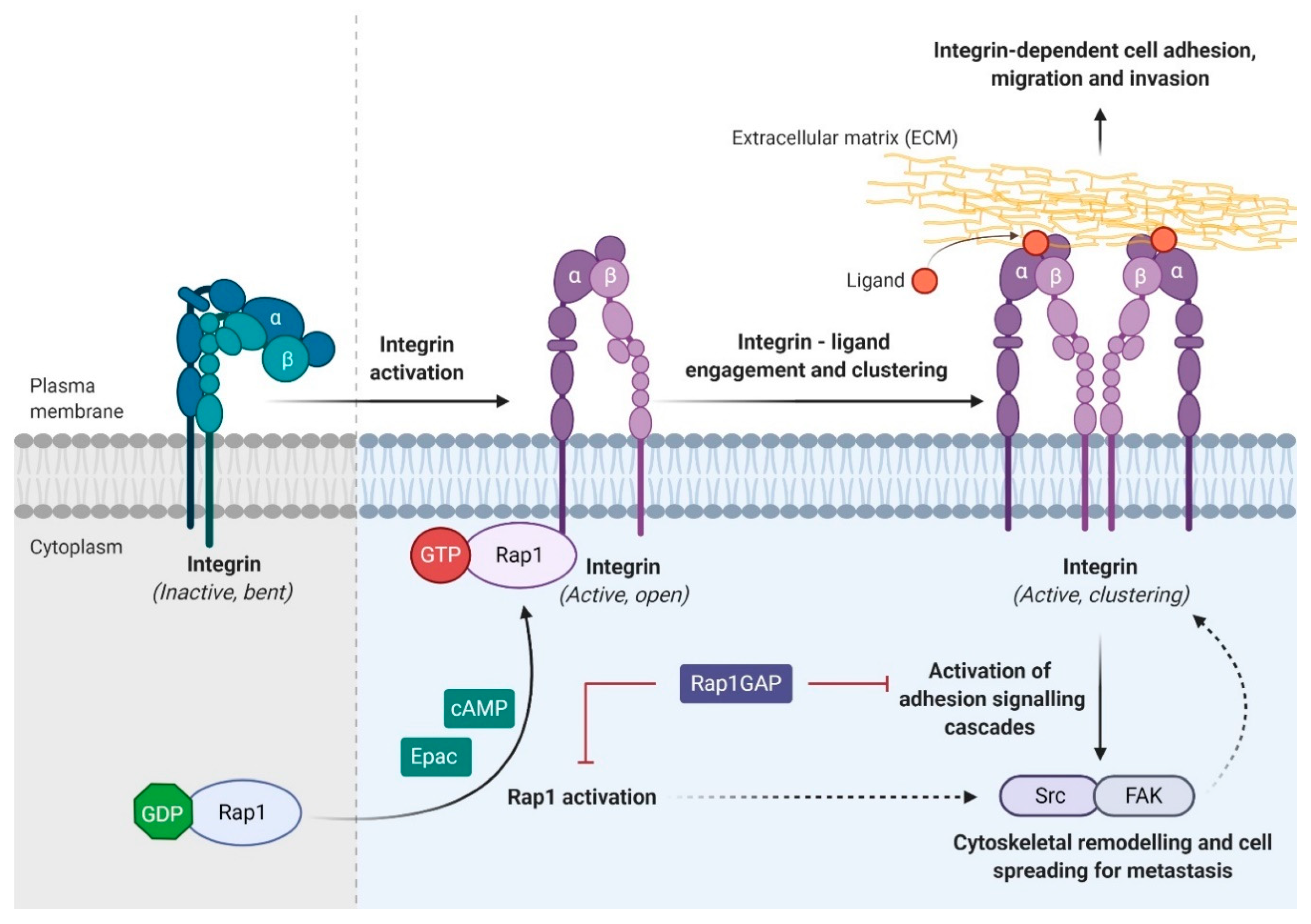
3.2. Rap1 Signalling Promotes Integrin-Mediated Cell Adhesion
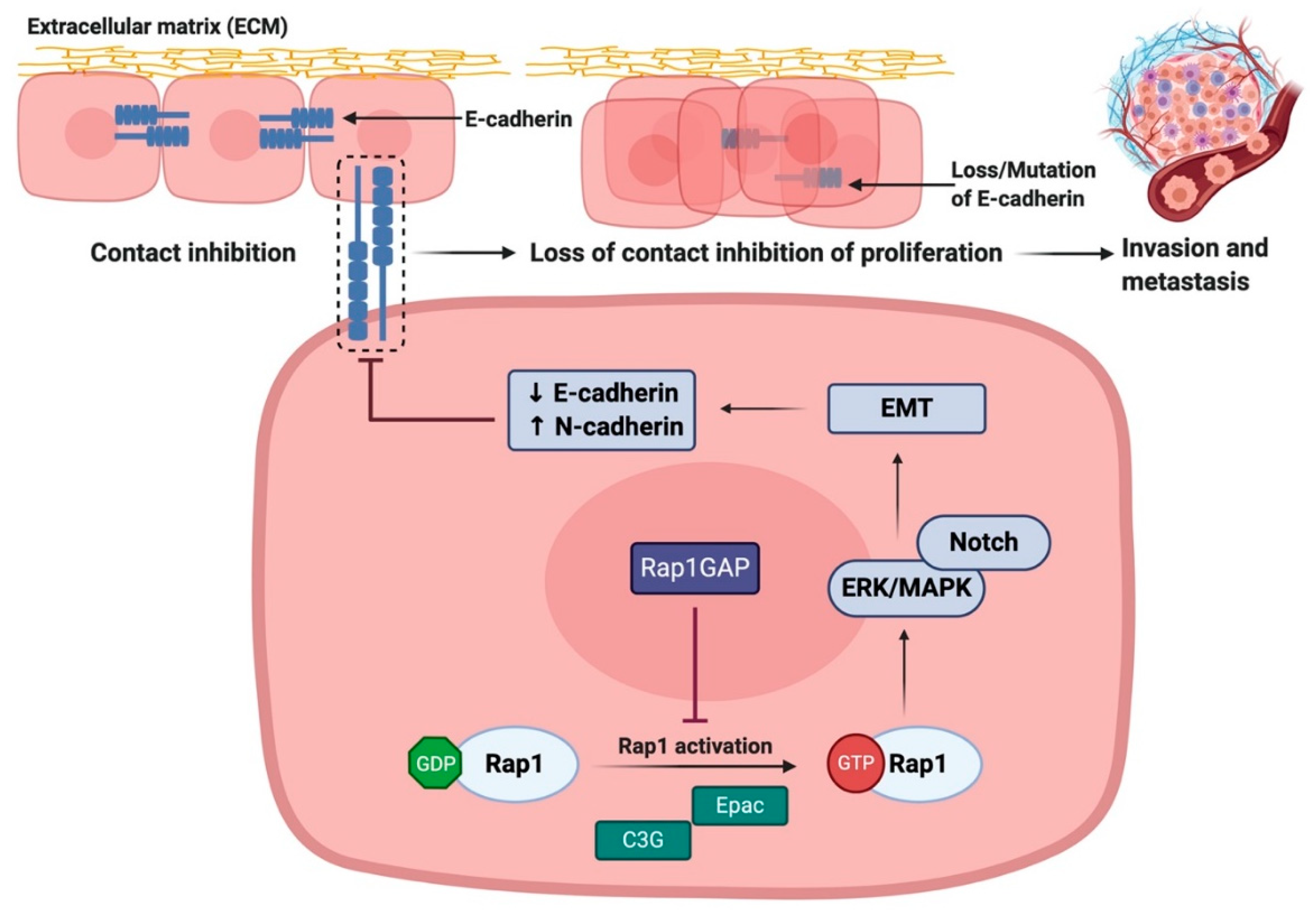
3.3. Rap1 Promotes EMT via Downregulation of E-cadherin Expression
3.4. Rap1 Promotes Angiogenesis in Cancer
3.5. Rap1 Signaling Promotes Cancer Cell Proliferation and Survival
3.6. Rap1 Signaling Regulates Matrix Metalloproteinases (MMPs)-Dependent Tumor Invasion and Metastasis
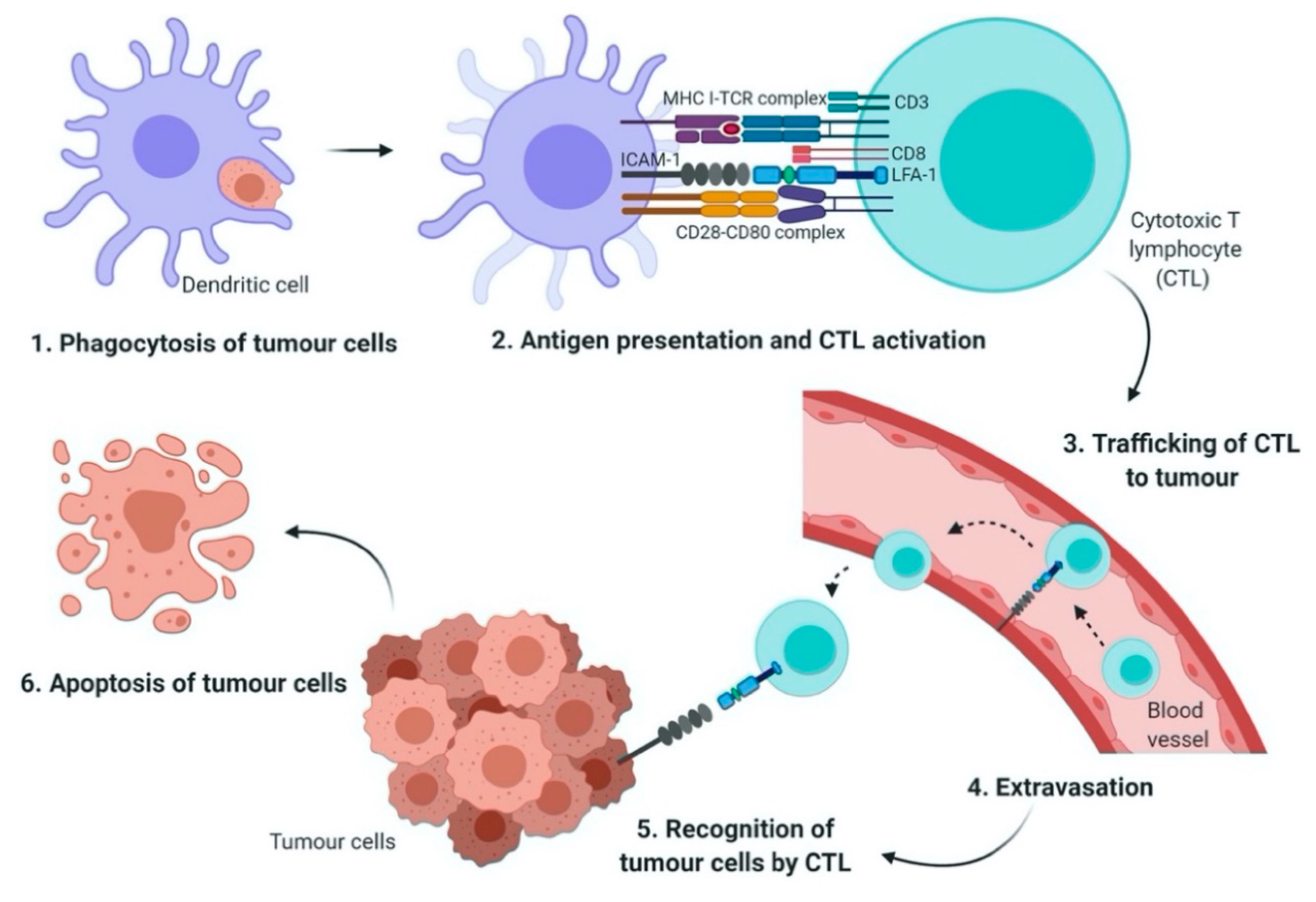
3.7. Rap1 Signaling Mediates Chemoresistance and Immune Evasion in Cancer
4. Therapeutic Potential of Rap1 Signaling in Cancers
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Smith, B.D.; Smith, G.L.; Hurria, A.; Hortobagyi, G.N.; Buchholz, T.A. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J. Clin. Oncol. 2009, 27, 2758–2765. [Google Scholar] [CrossRef]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef]
- Wong, S.H.M.; Fang, C.M.; Chuah, L.H.; Leong, C.O.; Ngai, S.C. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit. Rev. Oncol. Hematol. 2018, 121, 11–22. [Google Scholar] [CrossRef]
- Wu, Y.S.; Lee, Z.Y.; Chuah, L.-H.; Mai, C.W.; Ngai, S.C. Epigenetics in metastatic breast cancer: Its regulation and implications in diagnosis, prognosis and therapeutics. Curr. Cancer Drug Targets 2018, 19, 82–100. [Google Scholar] [CrossRef]
- Shinde, A.; Paez, J.S.; Libring, S.; Hopkins, K.; Solorio, L.; Wendt, M.K. Transglutaminase-2 facilitates extracellular vesicle-mediated establishment of the metastatic niche. Oncogenesis 2020, 9, 16. [Google Scholar] [CrossRef]
- Shinde, A.; Libring, S.; Alpsoy, A.; Abdullah, A.; Schaber, J.A.; Solorio, L.; Wendt, M.K. Autocrine fibronectin inhibits breast cancer metastasis. Mol. Cancer Res. 2018, 16, 1579–1589. [Google Scholar] [CrossRef]
- Shinde, A.; Hardy, S.D.; Kim, D.; Akhand, S.S.; Jolly, M.K.; Wang, W.H.; Anderson, J.C.; Khodadadi, R.B.; Brown, W.S.; George, J.T.; et al. Spleen tyrosine kinase–mediated autophagy is required for epithelial–mesenchymal plasticity and metastasis in breast cancer. Cancer Res. 2019, 79, 1831–1843. [Google Scholar] [CrossRef]
- Gan, L.L.; Hii, L.W.; Wong, S.F.; Leong, C.O.; Mai, C.W. Molecular mechanisms and potential therapeutic reversal of pancreatic cancer-induced immune evasion. Cancers 2020, 12, 1872. [Google Scholar] [CrossRef]
- Looi, C.-K.; Chung, F.F.-L.; Leong, C.-O.; Wong, S.-F.; Rosli, R.; Mai, C.-W. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, 162. [Google Scholar] [CrossRef]
- Mai, C.-W.; Chung, F.F.-L.; Leong, C.-O. Targeting legumain as a novel therapeutic strategy in cancers. Curr. Drug Targets. 2017, 18, 1259–1268. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Spurrell, E.L.; Lockley, M. Adaptive immunity in cancer immunology and therapeutics. Ecancermedicalscience 2014, 8, 441. [Google Scholar] [CrossRef]
- Harjunpää, H.; Asens, M.L.; Guenther, C.; Fagerholm, S.C. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Verma, N.K.; Kelleher, D. Not Just an Adhesion Molecule: LFA-1 contact tunes the T lymphocyte program. J. Immunol. 2017, 199, 1213–1221. [Google Scholar] [CrossRef]
- Fiore, E.; Fusco, C.; Romero, P.; Stamenkovic, I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene 2002, 21, 5213–5223. [Google Scholar] [CrossRef]
- Minato, N.; Kometani, K.; Hattori, M. Regulation of immune responses and hematopoiesis by the Rap1 signal. Adv. Immunol. 2007, 93, 229–264. [Google Scholar] [CrossRef]
- Katagiri, K.; Hattori, M.; Minato, N.; Kinashi, T. Rap1 Functions as a key regulator of T-cell and antigen-presenting cell interactions and modulates T-cell responses. Mol. Cell. Biol. 2002, 22, 1001–1015. [Google Scholar] [CrossRef]
- Katagiri, K.; Hattori, M.; Minato, N.; Irie, S.; Takatsu, K.; Kinashi, T. Rap1 Is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol. Cell. Biol. 2000, 20, 1956–1969. [Google Scholar] [CrossRef]
- Sebzda, E.; Bracke, M.; Tugal, T.; Hogg, N.; Cantrell, D.A. Rap 1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat. Immunol. 2002, 3, 251–258. [Google Scholar] [CrossRef]
- Boussiotis, V.A.; Freeman, G.J.; Berezovskaya, A.; Barber, D.L.; Nadler, L.M. Maintenance of human T cell anergy: Blocking of IL-2 gene transcription by activated Rap1. Science 1997, 278, 124–128. [Google Scholar] [CrossRef]
- Ishida, D.; Yang, H.; Masuda, K.; Uesugi, K.; Kawamoto, H.; Hattori, M.; Minato, N. Antigen-driven T cell anergy and defective memory T cell response via deregulated Rap1 activation in SPA-1-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10919–10924. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, R.C.; Cheng, K.; Ring, B.Z.; Su, L. Roles of Rap1 signaling in tumor cell migration and invasion. Cancer Biol. Med. 2017, 14, 90–99. [Google Scholar] [CrossRef]
- Jeyaraj, S.C.; Unger, N.T.; Chotani, M.A. Rap1 GTPases: An emerging role in the cardiovasculature. Life Sci. 2011, 88, 645–652. [Google Scholar] [CrossRef]
- Wittchen, E.S.; Aghajanian, A.; Burridge, K. Isoform-specific differences between Rap1A and Rap1B GTPases in the formation of endothelial cell junctions. Small GTPases 2011, 2, 65–76. [Google Scholar] [CrossRef]
- Bailey, C.L.; Kelly, P.; Casey, P.J. Activation of Rap1 promotes prostate cancer metastasis. Cancer Res. 2009, 69, 4962–4968. [Google Scholar] [CrossRef]
- Goto, M.; Mitra, R.S.; Liu, M.; Lee, J.; Henson, B.S.; Carey, T.; Bradford, C.; Prince, M.; Wang, C.Y.; Fearon, E.R.; et al. Rap1 stabilizes β-catenin and enhances β-catenin-dependent transcription and invasion in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2010, 16, 65–76. [Google Scholar] [CrossRef]
- Lin, K.T.; Yeh, Y.M.; Chuang, C.M.; Yang, S.Y.; Chang, J.W.; Sun, S.P.; Wang, Y.S.; Chao, K.C.; Wang, L.H. Glucocorticoids mediate induction of microRNA-708 to suppress ovarian cancer metastasis through targeting Rap1B. Nat. Commun. 2015, 6, 5917. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, S.; Zhang, L.; Zhang, J.; Cai, H.; Zhu, J.; Huang, C.; Wang, J. MiR-518b is down-regulated, and involved in cell proliferation and invasion by targeting Rap1b in esophageal squamous cell carcinoma. FEBS Lett. 2012, 586, 3508–3521. [Google Scholar] [CrossRef]
- Alemayehu, M.; Dragan, M.; Pape, C.; Siddiqui, I.; Sacks, D.B.; Di Guglielmo, G.M.; Babwah, A.V.; Bhattacharya, M. β-Arrestin2 regulates lysophosphatidic acid-induced human breast tumor cell migration and invasion via Rap1 and IQGAP1. PLoS ONE 2013, 8, e56174. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Yan, Y.; Zhang, J.; Sun, K.; Qu, J.K.; Wang, J.S.; Duan, X.Y. Expression of RAP1B is associated with poor prognosis and promotes an aggressive phenotype in gastric cancer. Oncol. Rep. 2015, 34, 2385–2394. [Google Scholar] [CrossRef]
- Kitayama, H.; Sugimoto, Y.; Matsuzaki, T.; Ikawa, Y.; Noda, M. A ras-related gene with transformation suppressor activity. Cell 1989, 56, 77–84. [Google Scholar] [CrossRef]
- Bos, J.L. All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. EMBO J. 1998, 17, 6776–6782. [Google Scholar] [CrossRef] [PubMed]
- Soo, H.-C.; Chung, F.F.-L.; Lim, K.-H.; Yap, V.A.; Bradshaw, T.D.; Hii, L.-W.; Tan, S.-H.; See, S.-J.; Tan, Y.-F.; Leong, C.-O.; et al. Cudraflavone C induces tumor-specific apoptosis in colorectal cancer cells through inhibition of the phosphoinositide 3-kinase (PI3K)-AKT pathway. PLoS ONE 2017, 12, e0170551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Xu, K.; Xiao, Z.; Sun, J.; Xu, J.; Wang, J.; Tang, Q. PTEN/PI3K/mTOR/B7-H1 signaling pathway regulates cell progression and immunoresistance in pancreatic cancer. Hepatogastroenterology 2013, 60, 1766–1772. [Google Scholar] [PubMed]
- Ohtsuka, T.; Shimizu, K.; Yamamori, B.; Kuroda, S.; Takai, Y. Activation of brain B-Raf protein kinase by Rap1B small GTP-binding protein. J. Biol. Chem. 1996, 271, 1258–1261. [Google Scholar] [CrossRef]
- Vetter, I.R.; Linnemann, T.; Wohlgemuth, S.; Geyer, M.; Kalbitzer, H.R.; Herrmann, C.; Wittinghofer, A. structural and biochemical analysis of Ras-effector signaling via RalGDS. FEBS Lett. 1999, 451, 175–180. [Google Scholar] [CrossRef]
- Mochizuki, N.; Yamashita, S.; Kurokawa, K.; Ohba, Y.; Nagai, T.; Miyawaki, A.; Matsuda, M. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 2001, 411, 1065–1068. [Google Scholar] [CrossRef]
- Wang, H.; Singh, S.R.; Zheng, Z.; Oh, S.W.; Chen, X.; Edwards, K.; Hou, S.X. Rap-GEF signaling controls stem cell anchoring to their niche through regulating DE-cadherin-mediated cell adhesion in the Drosophila testis. Dev. Cell 2006, 10, 117–126. [Google Scholar] [CrossRef]
- Knox, A.L.; Brown, N.H. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science 2002, 295, 1285–1288. [Google Scholar] [CrossRef]
- Lee, M.R.; Jeon, T.J. Cell Migration: Regulation of cytoskeleton by Rap1 in Dictyostelium discoideum. J. Microbiol. 2012, 50, 555–561. [Google Scholar] [CrossRef]
- Shah, B.; Lutter, D.; Tsytsyura, Y.; Glyvuk, N.; Sakakibara, A.; Klingauf, J.; Püschel, A.W. Rap1 GTPases are master regulators of neural cell polarity in the developing neocortex. Cereb. Cortex. 2017, 27, 1253–1269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Itoh, M.; Nelson, C.M.; Myers, C.A.; Bissell, M.J. Rap1 integrates tissue polarity, lumen formation, and tumorigenic potential in human breast epithelial cells. Cancer Res. 2007, 67, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Brock, E.J.; Jackson, R.M.; Ji, K.; Boerner, J.L.; Sloane, B.F.; Mattingly, R.R. Downregulation of Rap1Gap: A switch from DCIS to invasive breast carcinoma via ERK/MAPK activation. Neoplasia (United States) 2018, 20, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Onodera, Y.; Nam, J.M.; Bissell, M.J. Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J. Clin. Investig. 2014, 124, 367–384. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Yan, Y.; Cai, H.; Li, M.; Sun, K.; Wang, J.; Liu, X.; Wang, J.; Duan, X. Low expression of Rap1GAP is associated with epithelialmesenchymal transition (EMT) and poor prognosis in gastric cancer. Oncotarget 2017, 8, 8057–8068. [Google Scholar] [CrossRef]
- Zhang, Z.; Mitra, R.S.; Henson, B.S.; Datta, N.S.; McCauley, L.K.; Kumar, P.; Lee, J.S.J.; Carey, T.E.; D’Silva, N.J. Rap1GAP inhibits tumor growth in oropharyngeal squamous cell carcinoma. Am. J. Pathol. 2006, 168, 585–596. [Google Scholar] [CrossRef]
- Sayyah, J.; Bartakova, A.; Nogal, N.; Quilliam, L.A.; Stupack, D.G.; Brown, J.H. The Ras-related protein, Rap1A, mediates thrombin-stimulated, integrin-dependent glioblastoma cell proliferation and tumor growth. J. Biol. Chem. 2014, 289, 17689–17698. [Google Scholar] [CrossRef]
- Xiao, L.; Lan, X.; Shi, X.; Zhao, K.; Wang, D.; Wang, X.; Li, F.; Huang, H.; Liu, J. Cytoplasmic RAP1 mediates cisplatin resistance of non-small cell lung cancer. Cell Death Dis. 2017, 8, e2803. [Google Scholar] [CrossRef]
- Zhou, S.; Liang, Y.; Zhang, X.; Liao, L.; Yang, Y.; Ouyang, W.; Xu, H. SHARPIN promotes melanoma progression via Rap1 signaling pathway. J. Investig. Dermatol. 2020, 140, 395–403. [Google Scholar] [CrossRef]
- McSherry, E.A.; Brennan, K.; Hudson, L.; Hill, A.D.K.; Hopkins, A.M. Breast cancer cell migration is regulated through junctional adhesion molecule-A-mediated activation of Rap1 GTPase. Breast Cancer Res. 2011, 13, R31. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Anand, S.; Murphy, E.A.; Desgrosellier, J.S.; Stupack, D.G.; Shattil, S.J.; Schlaepfer, D.D.; Cheresh, D.A. EGFR-dependent pancreatic carcinoma cell metastasis through Rap1 activation. Oncogene 2012, 31, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Vallés, A.M.; Beuvin, M.; Boyer, B. Activation of Rac1 by paxillin-Crk-DOCK180 signaling complex is antagonized by Rap1 in migrating NBT-II cells. J. Biol. Chem. 2004, 279, 44490–44496. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.S.; Goto, M.; Lee, J.S.; Maldonado, D.; Taylor, J.M.G.; Pan, Q.; Carey, T.E.; Bradford, C.R.; Prince, M.E.; Cordell, K.G.; et al. Rap1GAP promotes invasion via induction of matrix metalloproteinase 9 secretion, which is associated with poor survival in low N-stage squamous cell carcinoma. Cancer Res. 2008, 68, 3959–3969. [Google Scholar] [CrossRef][Green Version]
- Qiu, T.; Qi, X.; Cen, J.; Chen, Z. Rap1GAP alters leukemia cell differentiation, apoptosis and invasion in vitro. Oncol. Rep. 2012, 28, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.L.; Ye, G.C.; Liu, L.W.; Wei, L. The downregulation of Rap1 GTPase-activating protein is associated with a poor prognosis in colorectal cancer and may impact on tumor progression. Oncol. Lett. 2018, 15, 7661–7668. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, L.; Feng, Y.; Yuan, L.; Zhao, H.; Cornelius, L.A. Down-regulation of Rap1GAP via promoter hypermethylation promotes melanoma cell proliferation, survival, and migration. Cancer Res. 2009, 69, 449–457. [Google Scholar] [CrossRef]
- Tamate, M.; Tanaka, R.; Osogami, H.; Matsuura, M.; Satohisa, S.; Iwasaki, M.; Saito, T. Rap1GAP inhibits tumor progression in endometrial cancer. Biochem. Biophys. Res. Commun. 2017, 485, 476–483. [Google Scholar] [CrossRef]
- Zhang, L.; Chenwei, L.; Mahmood, R.; Van Golen, K.; Greenson, J.; Li, G.; D’Silva, N.J.; Li, X.; Burant, C.F.; Logsdon, C.D.; et al. Identification of a putative tumor suppressor gene Rap1GAP in pancreatic cancer. Cancer Res. 2006, 66, 898–906. [Google Scholar] [CrossRef]
- Zuo, H.; Gandhi, M.; Edreira, M.M.; Hochbaum, D.; Nimgaonkar, V.L.; Zhang, P.; DiPaola, J.; Evdokimova, V.; Altschuler, D.L.; Nikiforov, Y.E. Downregulation of Rap1GAP through epigenetic silencing and loss of heterozygosity promotes invasion and progression of thyroid tumors. Cancer Res. 2010, 70, 1389–1397. [Google Scholar] [CrossRef]
- Kim, W.J.; Gersey, Z.; Daaka, Y. Rap1GAP regulates renal cell carcinoma invasion. Cancer Lett. 2012, 320, 65–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banerjee, R.; Mani, R.S.; Russo, N.; Scanlon, C.S.; Tsodikov, A.; Jing, X.; Cao, Q.; Palanisamy, N.; Metwally, T.; Inglehart, R.C.; et al. The tumor suppressor gene rap1GAP is silenced by miR-101-mediated EZH2 overexpression in invasive squamous cell carcinoma. Oncogene 2011, 30, 4339–4349. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H.; et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. 2018, 37, 87. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gélinas, C.; Fan, Y.; et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006, 10, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Ugland, H.; Naderi, S.; Brech, A.; Collas, P.; Blomhoff, H.K. cAMP induces autophagy via a novel pathway involving ERK, cyclin E and Beclin 1. Autophagy 2011, 7, 1199–1211. [Google Scholar] [CrossRef]
- Goldsmith, J.; Levine, B.; Debnath, J. Autophagy and cancer metabolism. Methods Enzymol. 2014, 542, 25–57. [Google Scholar] [CrossRef]
- Karantza-Wadsworth, V.; Patel, S.; Kravchuk, O.; Chen, G.; Mathew, R.; Jin, S.; White, E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007, 21, 1621–1635. [Google Scholar] [CrossRef]
- Paquette, M.; El-Houjeiri, L.; Pause, A. mTOR pathways in cancer and autophagy. Cancers 2018, 10, 18. [Google Scholar] [CrossRef]
- Lu, Z.; Luo, R.Z.; Lu, Y.; Zhang, X.; Yu, Q.; Khare, S.; Kondo, S.; Kondo, Y.; Yu, Y.; Mills, G.B.; et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J. Clin. Investig. 2008, 118, 3917–3929. [Google Scholar] [CrossRef]
- Efeyan, A.; Sabatini, D.M. mTOR and cancer: Many loops in one pathway. Curr. Opin. Cell Biol. 2010, 22, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Mutvei, A.P.; Nagiec, M.J.; Hamann, J.C.; Kim, S.G.; Vincent, C.T.; Blenis, J. Rap1-GTPases control mTORC1 activity by coordinating lysosome organization with amino acid availability. Nat. Commun. 2020, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Gao, L.; Feng, Y.; Bowers, R.; Becker-Hapak, M.; Gardner, J.; Council, L.; Linette, G.; Zhao, H.; Cornelius, L.A. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: Two processes important to melanoma tumorigenesis and metastasis. Cancer Res. 2006, 66, 7880–7888. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Palmieri, M.; Chaudhury, A.; Klisch, T.J.; di Ronza, A.; Neilson, J.R.; Rodney, G.G.; Sardiello, M. Src regulates amino acid-mediated mTORC1 activation by disrupting GATOR1-Rag GTPase interaction. Nat. Commun. 2018, 9, 4351. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Wankell, M.; Ho, V.; White, R.; Deo, N.; Devine, C.; Dewdney, B.; Bhathal, P.; Govaere, O.; Roskams, T.; et al. Targeting mTOR and Src restricts hepatocellular carcinoma growth in a novel murine liver cancer model. PLoS ONE 2019, 14, e0212860. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Weber, G.F.; Bjerke, M.A.; DeSimone, D.W. Integrins and cadherins join forces to form adhesive networks. J. Cell Sci. 2011, 124, 1183–1193. [Google Scholar] [CrossRef]
- Symowicz, J.; Adley, B.P.; Gleason, K.J.; Johnson, J.J.; Ghosh, S.; Fishman, D.A.; Hudson, L.G.; Stack, M.S. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007, 67, 2030–2039. [Google Scholar] [CrossRef]
- Retta, S.F.; Balzac, F.; Avolio, M. Rap1: A turnabout for the crosstalk between cadherins and integrins. Eur. J. Cell Biol. 2006, 85, 283–293. [Google Scholar] [CrossRef]
- Wittchen, E.S.; Worthylake, R.A.; Kelly, P.; Casey, P.J.; Quilliam, L.A.; Burridge, K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J. Biol. Chem. 2005, 280, 11675–11682. [Google Scholar] [CrossRef]
- Stefanini, L.; Bergmeier, W. RAP1-GTPase signaling and platelet function. J. Mol. Med. 2016, 94, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, N.; Hattori, M.; Yang, H.; Bos, J.L.; Minato, N. Rap1 GTPase-activating protein SPA-1 negatively regulates cell adhesion. J. Biol. Chem. 1999, 274, 18463–18469. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, S.; Enserink, J.M.; Kuiperij, H.B.; De Rooij, J.; Price, L.S.; Schwede, F.; Bos, J.L. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the β2-adrenergic receptor. J. Cell Biol. 2003, 160, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Enserink, J.M.; Price, L.S.; Methi, T.; Mahic, M.; Sonnenberg, A.; Bos, J.L.; Taskén, K. The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through, the α3β1 integrin but not the α6β4 integrin. J. Biol. Chem. 2004, 279, 44889–44896. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Okura, M.; Imamoto, A. Focal adhesions require catalytic activity of Src family kinases to mediate integrin-matrix adhesion. Mol. Cell. Biol. 2002, 22, 1203–1217. [Google Scholar] [CrossRef]
- Nagano, M.; Hoshino, D.; Koshikawa, N.; Akizawa, T.; Seiki, M. Turnover of focal adhesions and cancer cell migration. Int. J. Cell Biol. 2012, 2012, 310616. [Google Scholar] [CrossRef]
- Webb, D.J.; Donais, K.; Whitmore, L.A.; Thomas, S.M.; Turner, C.E.; Parsons, J.T.; Horwitz, A.F. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004, 6, 154–161. [Google Scholar] [CrossRef]
- Dong, X.; Tang, W.; Stopenski, S.; Brose, M.S.; Korch, C.; Meinkoth, J.L. RAP1GAP inhibits cytoskeletal remodeling and motility in thyroid cancer cells. Endocr. Relat. Cancer 2012, 19, 575–588. [Google Scholar] [CrossRef][Green Version]
- Mendonsa, A.M.; Na, T.Y.; Gumbiner, B.M. E-cadherin in contact inhibition and cancer. Oncogene 2018, 37, 4769–4780. [Google Scholar] [CrossRef]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef]
- Price, L.S.; Hajdo-Milasinovic, A.; Zhao, J.; Zwartkruis, F.J.T.; Collard, J.G.; Bos, J.L. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J. Biol. Chem. 2004, 279, 35127–35132. [Google Scholar] [CrossRef]
- Lu, L.; Wang, J.; Wu, Y.; Wan, P.; Yang, G. Rap1A promotes ovarian cancer metastasis via activation of ERK/p38 and notch signaling. Cancer Med. 2016, 5, 3544–3554. [Google Scholar] [CrossRef] [PubMed]
- Tsygankova, O.M.; Prendergast, G.V.; Puttaswamy, K.; Wang, Y.; Feldman, M.D.; Wang, H.; Brose, M.S.; Meinkoth, J.L. Downregulation of Rap1GAP Contributes to Ras Transformation. Mol. Cell. Biol. 2007, 27, 6647–6658. [Google Scholar] [CrossRef] [PubMed]
- Javan, M.R.; Khosrojerdi, A.; Moazzeni, S.M. New insights into implementation of mesenchymal stem cells in cancer therapy: Prospects for anti-angiogenesis treatment. Front. Oncol. 2019, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Byzova, T.V.; Goldman, C.K.; Pampori, N.; Thomas, K.A.; Bett, A.; Shattil, S.J.; Plow, E.F. A mechanism for modulation of cellular responses to VEGF: Activation of the integrins. Mol. Cell. 2000, 6, 851–860. [Google Scholar] [CrossRef]
- Carmona, G.; Göttig, S.; Orlandi, A.; Scheele, J.; Bäuerle, T.; Jugold, M.; Kiessling, F.; Henschler, R.; Zeiher, A.M.; Dimmeler, S.; et al. Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood 2009, 113, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Lakshmikanthan, S.; Sobczak, M.; Chun, C.; Henschel, A.; Dargatz, J.; Ramchandran, R.; Chrzanowska-Wodnicka, M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin αvβ3. Blood 2011, 118, 2015–2026. [Google Scholar] [CrossRef]
- Li, W.; Jin, B.; Cornelius, L.A.; Zhou, B.; Fu, X.; Shang, D.; Zheng, H. Inhibitory effects of Rap1GAP overexpression on proliferation and migration of endothelial cells via ERK and Akt pathways. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 721–727. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Che, Y.L.; Luo, S.J.; Li, G.; Cheng, M.; Gao, Y.M.; Li, X.M.; Dai, J.M.; He, H.; Wang, J.; Peng, H.J.; et al. The C3G/Rap1 pathway promotes secretion of MMP-2 and MMP-9 and is involved in serous ovarian cancer metastasis. Cancer Lett. 2015, 359, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Yuan, P.; Lin, S.S.; Song, C.F.; Guan, W.Y.; Yuan, L.; Lai, R.B.; Gao, Y.; Wang, Y. Matrix metalloproteinase 9 expression and prognosis in colorectal cancer: A meta-analysis. Tumor Biol. 2013, 34, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ye, G.; Liu, L.; Gu, D. Down-regulated expression of Rap1GAP predicts unfavorable prognosis in diffuse type gastric cancer and may be correlated with EMT. Int. J. Clin. Exp. Med. 2017, 10, 12101–12111. [Google Scholar]
- Cowden Dahl, K.D.; Symowicz, J.; Ning, Y.; Gutierrez, E.; Fishman, D.A.; Adley, B.P.; Stack, M.S.; Hudson, L.G. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent E-cadherin loss in ovarian carcinoma cells. Cancer Res. 2008, 68, 4606–4613. [Google Scholar] [CrossRef]
- Zheng, G.; Lyons, J.G.; Thian, K.T.; Wang, Y.; Hsu, T.T.; Min, D.; Succar, L.; Rangan, G.K.; Hu, M.; Henderson, B.R.; et al. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-β1 in renal tubular epithelial cells. Am. J. Pathol. 2009, 175, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhan, H. Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion. Cancer Lett. 2020, 468, 72–81. [Google Scholar] [CrossRef]
- Chen, L.; Gibbons, D.L.; Goswami, S.; Cortez, M.A.; Ahn, Y.H.; Byers, L.A.; Zhang, X.; Yi, X.; Dwyer, D.; Lin, W.; et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014, 5, 5241. [Google Scholar] [CrossRef]
- Arumugam, T.; Ramachandran, V.; Fournier, K.F.; Wang, H.; Marquis, L.; Abbruzzese, J.L.; Gallick, G.E.; Logsdon, C.D.; McConkey, D.J.; Choi, W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009, 69, 5820–5828. [Google Scholar] [CrossRef]
- Kumar, S.; Davra, V.; Obr, A.E.; Geng, K.; Wood, T.L.; Birge, R.B. Crk adaptor protein promotes PD-L1 expression, EMT and immune evasion in a murine model of triple-negative breast cancer. Oncoimmunology 2017, 7, e1413845. [Google Scholar] [CrossRef]
- Er, J.L.; Goh, P.N.; Lee, C.Y.; Tan, Y.J.; Hii, L.-W.; Mai, C.W.; Chung, F.F.-L.; Leong, C.-O. Identification of inhibitors synergizing gemcitabine sensitivity in the squamous subtype of pancreatic ductal adenocarcinoma (PDAC). Apoptosis 2018, 23, 343–355. [Google Scholar] [CrossRef]
- Hii, L.W.; Chung, F.F.L.; Soo, J.S.S.; Tan, B.S.; Mai, C.W.; Leong, C.O. Histone deacetylase (HDAC) inhibitors and doxorubicin combinations target both breast cancer stem cells and non-stem breast cancer cells simultaneously. Breast Cancer Res. Treat. 2020, 179, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Hii, L.W.; Lim, S.H.E.; Leong, C.O.; Chin, S.Y.; Tan, N.P.; Lai, K.S.; Mai, C.W. The synergism of Clinacanthus nutans Lindau extracts with gemcitabine: Downregulation of anti-apoptotic markers in squamous pancreatic ductal adenocarcinoma. BMC Complement. Altern. Med. 2019, 19, 257. [Google Scholar] [CrossRef] [PubMed]
- Nellore, A.; Paziana, K.; Ma, C.; Tsygankova, O.M.; Wang, Y.; Puttaswamy, K.; Iqbal, A.U.; Franks, S.R.; Lv, Y.; Troxel, A.B.; et al. Loss of rap1GAP in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2009, 94, 1026–1032. [Google Scholar] [CrossRef]
- Almahariq, M.; Tsalkova, T.; Mei, F.C.; Chen, H.; Zhou, J.; Sastry, S.K.; Schwede, F.; Cheng, X. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol. Pharmacol. 2013, 83, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Rehmann, H. Epac-inhibitors: Facts and artefacts. Sci. Rep. 2013, 3, 3032. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, H.; Boulton, S.; Mei, F.; Ye, N.; Melacini, G.; Zhou, J.; Cheng, X. Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: Defining the ESI-09 therapeutic window. Sci. Rep. 2015, 5, 9344. [Google Scholar] [CrossRef]
- Mor, A.; Haklai, R.; Ben-Moshe, O.; Mekori, Y.A.; Kloog, Y. Inhibition of contact sensitivity by farnesylthiosalicylic acid-amide, a potential rap1 inhibitor. J. Invest. Dermatol. 2011, 131, 2040–2048. [Google Scholar] [CrossRef]
- Lobell, R.B.; Omer, C.A.; Abrams, M.T.; Bhimnathwala, H.G.; Brucker, M.J.; Buser, C.A.; Davide, J.P.; DeSolms, S.J.; Dinsmore, C.J.; Ellis-Hutchings, M.S.; et al. Evaluation of farnesyl:protein transferase and geranylgeranyl:protein transferase inhibitor combinations in preclinical models. Cancer Res. 2001, 61, 8758–8768. [Google Scholar]
- Sequera, C.; Manzano, S.; Guerrero, C.; Porras, A. How Rap and its GEFs control liver physiology and cancer development. C3G alterations in human hepatocarcinoma. Hepatic Oncol. 2018, 5, HEP05. [Google Scholar] [CrossRef]
- Evelyn, C.R.; Biesiada, J.; Duan, X.; Tang, H.; Shang, X.; Papoian, R.; Seibel, W.L.; Nelson, S.; Meller, J.; Zheng, Y. Combined rational design and a high throughput screening platform for identifying chemical inhibitors of a ras-activating enzyme. J. Biol. Chem. 2015, 290, 12879–12898. [Google Scholar] [CrossRef]
| Roles of Rap1 | Tumor Types | Signaling Molecules | Results | Reference |
|---|---|---|---|---|
| Tumor promoter | Glioblastoma | Rap1A, thrombin, β1-integrin | Knockdown of Rap1A reduced more than 70% of tumor growth compared to control | [48] |
| NSCLC | Rap1-GTP | Rap1-GTP depletion reduced growth of NSCLC cells and increased cisplatin sensitivity | [49] | |
| Melanoma | Rap1-GTP, p38 | Rap1-GTP expression promoted melanoma migration and invasion | [50] | |
| Breast cancer | Rap1-GTP, β1-integrin | Pharmacological inhibition of Rap1-GTP and β1-integrin reduced cell migration in breast cancer | [51] | |
| Rap1 | Expression of dominant-active Rap1 in breast epithelial cells increased invasiveness and tumorigenicity | [43] | ||
| Prostate cancer | Rap1A, α4, β3 -integrins | Rap1A activation increased expression of α4- and β3-integrins, leading to increased tumor cell invasion | [26] | |
| Pancreatic cancer | Rap1-GTP, EGFR | Rap1-GTP activation promoted migration and EGFR-mediated metastasis of pancreatic cancer cells | [52] | |
| Tumor suppressor | Bladder cancer | Rap1-GTP | Rap1-GTP activation suppressed migration of NBT-II bladder carcinoma cells | [53] |
| Roles of Rap1 | Tumor Types | Signaling Molecules | Results | Reference |
|---|---|---|---|---|
| Tumor promotor | HNSCC | Rap1, MMP9 | Rap1GAP promoted SCC cell invasion via MMP9 production | [54] |
| Acute myeloid leukemia | Rap1, MMP9 | Overexpression of Rap1GAP promoted MMP9-mediated cell invasion | [55] | |
| Tumor suppressor | Colorectal cancer | Rap1, MMP9, E-cadherin | Loss of Rap1GAP leading to tumor metastasis and poor prognosis | [56] |
| Prostate cancer Melanoma | Rap1, integrin | Overexpression of Rap1GAP impaired tumor cell progression | [26] | |
| Melanoma | Rap1, ERK | Rap1GAP blocked Rap1 activation and inhibiting tumor proliferation and survival | [57] | |
| Endometrial cancer | Rap1, E-cadherin | Patients with higher expression of both Rap1GAP and E-cadherin had better survival | [58] | |
| Gastric cancer | Rap1, MMP2, E-cadherin | Rap1GAP increased E-cadherin and reduced cell migration and invasion | [46] | |
| Pancreatic cancer | Rap1, integrin | Overexpression of Rap1GAP blocked Rap1 activation and tumor invasion | [59] | |
| Oropharyngeal SCC | Rap1, ERK | Loss of Rap1GAP promoted Rap1 and ERK activation and tumor proliferation. | [47] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Looi, C.-K.; Hii, L.-W.; Ngai, S.C.; Leong, C.-O.; Mai, C.-W. The Role of Ras-Associated Protein 1 (Rap1) in Cancer: Bad Actor or Good Player? Biomedicines 2020, 8, 334. https://doi.org/10.3390/biomedicines8090334
Looi C-K, Hii L-W, Ngai SC, Leong C-O, Mai C-W. The Role of Ras-Associated Protein 1 (Rap1) in Cancer: Bad Actor or Good Player? Biomedicines. 2020; 8(9):334. https://doi.org/10.3390/biomedicines8090334
Chicago/Turabian StyleLooi, Chin-King, Ling-Wei Hii, Siew Ching Ngai, Chee-Onn Leong, and Chun-Wai Mai. 2020. "The Role of Ras-Associated Protein 1 (Rap1) in Cancer: Bad Actor or Good Player?" Biomedicines 8, no. 9: 334. https://doi.org/10.3390/biomedicines8090334
APA StyleLooi, C.-K., Hii, L.-W., Ngai, S. C., Leong, C.-O., & Mai, C.-W. (2020). The Role of Ras-Associated Protein 1 (Rap1) in Cancer: Bad Actor or Good Player? Biomedicines, 8(9), 334. https://doi.org/10.3390/biomedicines8090334





