The Heat Shock Protein 27 Immune Complex Enhances Exosomal Cholesterol Efflux
Abstract
:1. Introduction
2. Experimental Section
2.1. Patient Information
2.2. Cell Culture and Treatment
2.3. Recombinant Protein Preparation
2.4. Preparation of HSP27 Polyclonal Antibody
2.5. Exosome Purification
2.6. Flow Cytometry Analysis
2.7. Exosomal HSP27 ELISA Detection
2.8. Fast Protein Liquid Chromatography (FPLC)
2.9. Immunoblotting Analysis
2.10. Statistical Analysis
3. Results
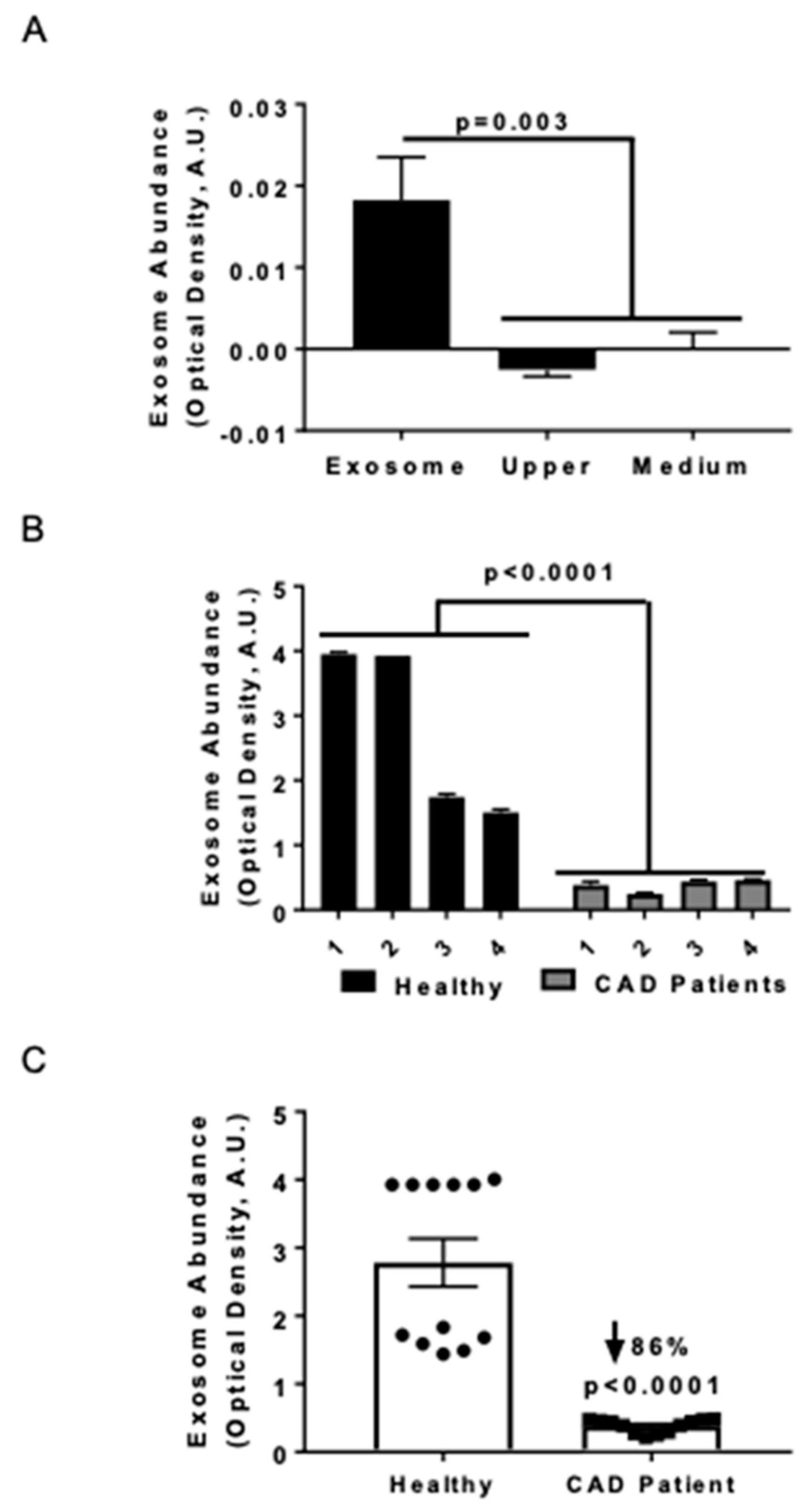
3.1. Serum Exosome Levels were Lower in CAD Patients Compared to Healthy Control Subjects
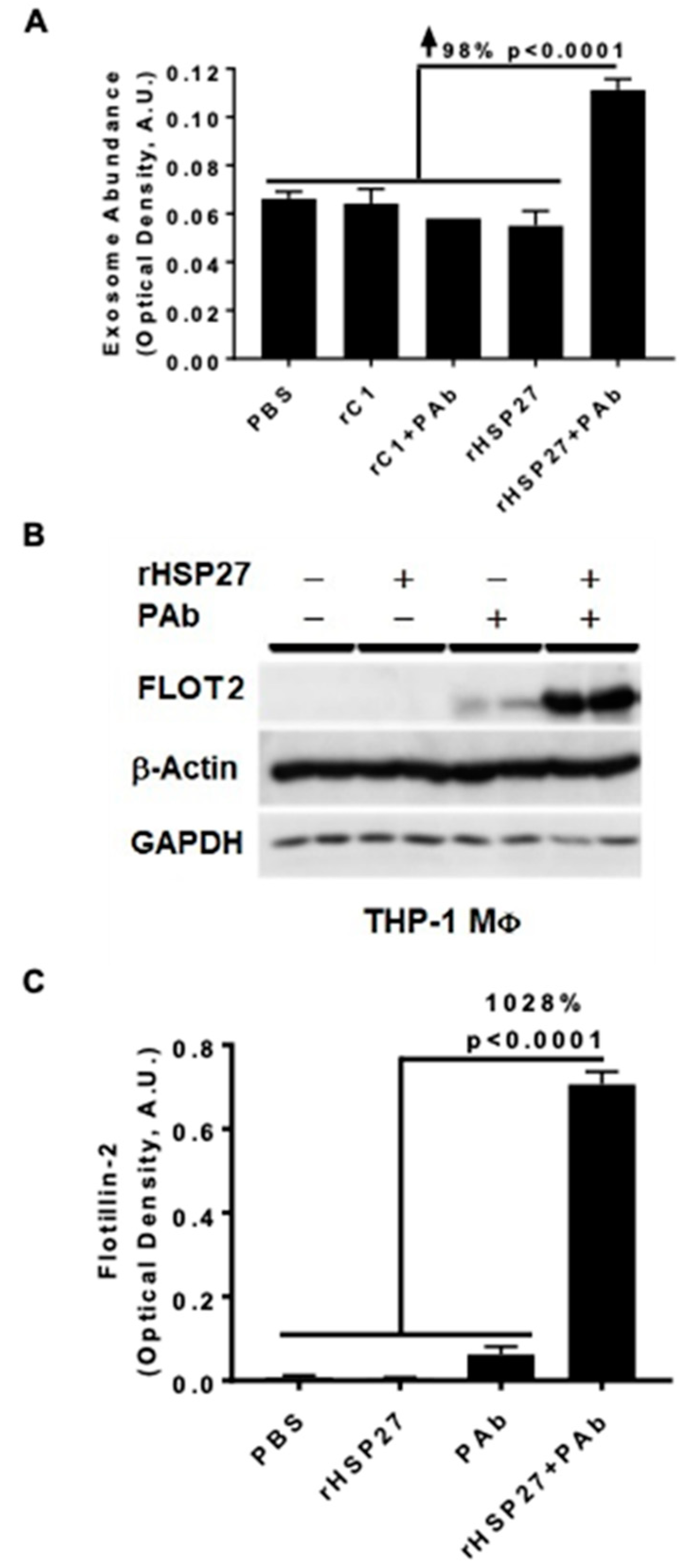
3.2. HSP27 IC Increases Exosomal Release and Flotillin-2
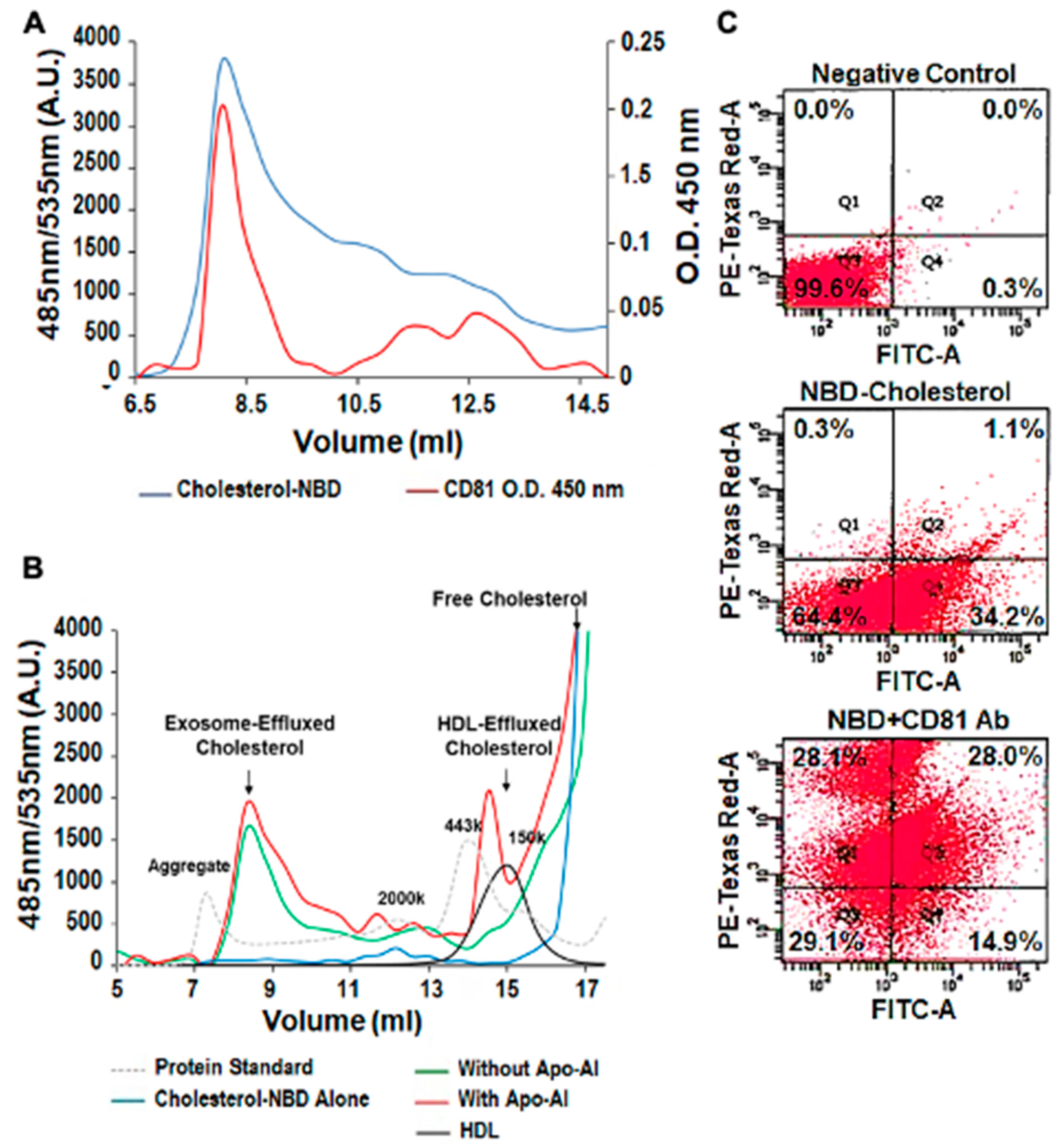
3.3. Exosomal Cholesterol Export is Independent of Apo AI or HDL
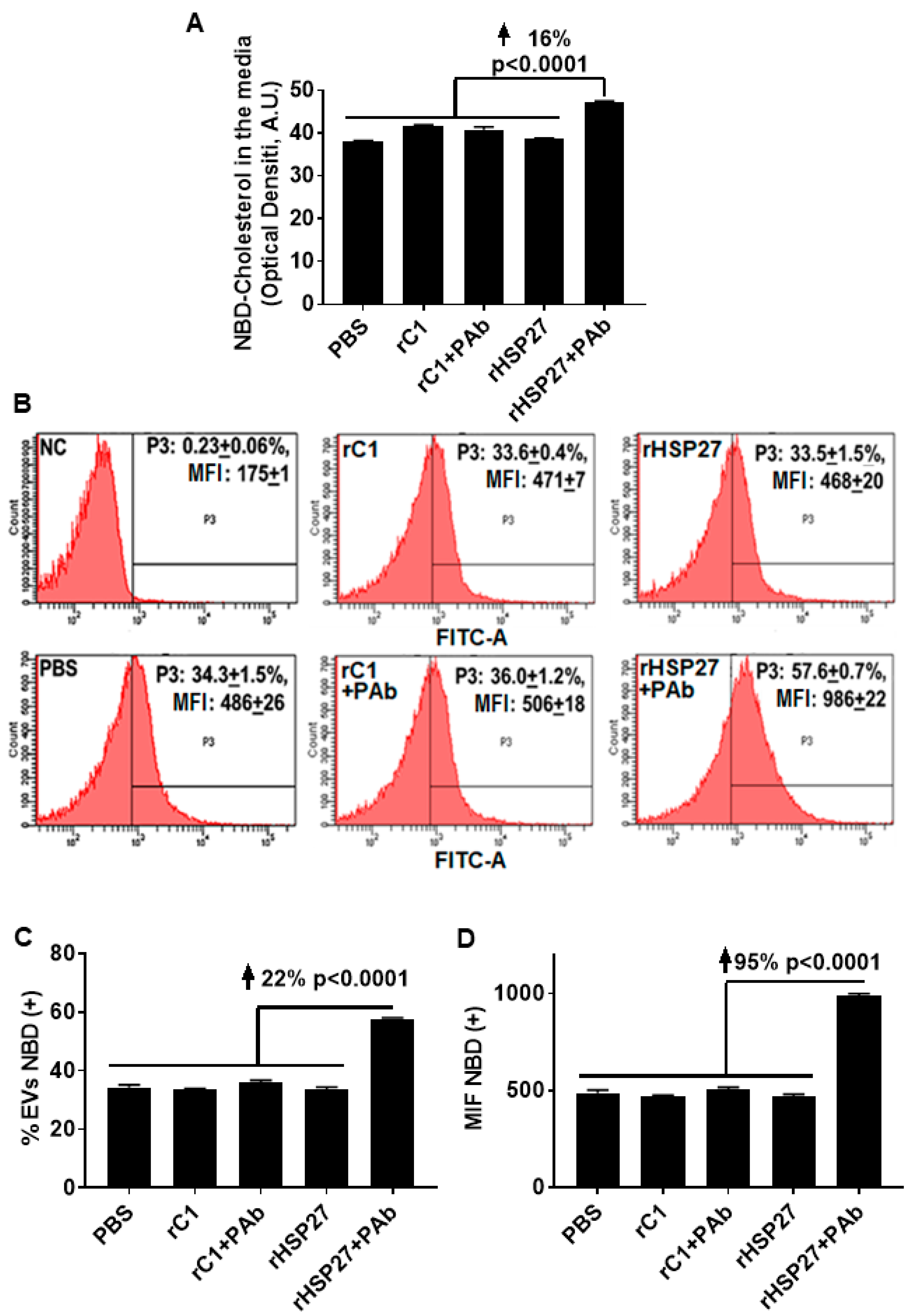
3.4. HSP27 Immune Complex Increases Exosomal Cholesterol Efflux in THP-1 MΦ
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Merchant, M.L.; Rood, I.M.; Deegens, J.K.J.; Klein, J.B. Isolation and characterization of urinary extracellular vesicles: Implications for biomarker discovery. Nat. Rev. Nephrol. 2017, 13, 731–749. [Google Scholar] [CrossRef] [Green Version]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Raposo, G. Exosomes–vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, L.; Wei, Y.; Ea, V.L.; Nian, H.; Wei, R. Recent advances of exosomes in immune-mediated eye diseases. Stem. Cell Res. Ther. 2019, 10, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, I.M.; Rajakumar, G.; Venkidasamy, B.; Subramanian, U.; Thiruvengadam, M. Exosomes: Current use and future applications. Clin. Chim. Acta 2020, 500, 226–232. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef]
- Zakharova, L.; Svetlova, M.; Fomina, A.F. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J. Cell Physiol. 2007, 212, 174–181. [Google Scholar] [CrossRef]
- Blanchard, N.; Lankar, D.; Faure, F.; Regnault, A.; Dumont, C.; Raposo, G.; Hivroz, C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/ζ complex. J. Immunol. 2002, 168, 3235–3241. [Google Scholar] [CrossRef] [Green Version]
- Baixauli, F.; López-Otín, C.; Mittelbrunn, M. Exosomes and autophagy: Coordinated mechanisms for the maintenance of cellular fitness. Front. Immunol. 2014, 5, 403. [Google Scholar] [CrossRef] [Green Version]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. BBA-Mol. Cell. Biol. Lipids 2014, 1841, 108–120. [Google Scholar] [CrossRef]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of cytosolic proteins by late endosomes. Dev. cell 2011, 20, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurley, J.H. ESCRT complexes and the biogenesis of multivesicular bodies. Curr. Opin. Cell Biol. 2008, 20, 4–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Baixauli, F.; Gutiérrez-Vázquez, C.; Sánchez-Madrid, F.; Mittelbrunn, M. Sorting it out: Regulation of exosome loading. In Proceedings of Seminars in Cancer Biology; Academic Press: London, UK, 2014; Volume 28, pp. 3–13. [Google Scholar]
- Meister, M.; Tikkanen, R. Endocytic trafficking of membrane-bound cargo: A flotillin point of view. Membranes 2014, 4, 356–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, W.; Hao, Y.; He, C.; Li, L.; Zhu, G. Exosome-orchestrated hypoxic tumor microenvironment. Mol. Cancer 2019, 18, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibbert, B.; Lavoie, J.R.; Ma, X.L.; Seibert, T.; Raizman, J.E.; Simard, T.; Chen, Y.X.; Stewart, D.; O’Brien, E.R. Glycogen Synthase Kinase-3 beta Inhibition Augments Diabetic Endothelial Progenitor Cell Abundance and Functionality via Cathepsin B: A Novel Therapeutic Opportunity for Arterial Repair. Diabetes 2014, 63, 1410–1421. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-X.; Shi, C.; Deng, J.; Diao, C.; Maarouf, N.; Rosin, M.; Shrivastava, V.; Hu, A.; Bharadwa, S.; Adijiang, A.; et al. Heat Shock Protein 27 Immune Complex Upregulates LDLR Expression Thereby Reducing Plasma Cholesterol and Atherogenesis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Martin-Ventura, J.L.; Duran, M.C.; Blanco-Colio, L.M.; Meilhac, O.; Leclercq, A.; Michel, J.B.; Jensen, O.N.; Hernandez-Merida, S.; Tunon, J.; Vivanco, F.; et al. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation 2004, 110, 2216–2219. [Google Scholar] [CrossRef] [Green Version]
- Park, H.K.; Park, E.C.; Bae, S.W.; Park, M.Y.; Kim, S.W.; Yoo, H.S.; Tudev, M.; Ko, Y.H.; Choi, Y.H.; Kim, S.; et al. Expression of heat shock protein 27 in human atherosclerotic plaques and increased plasma level of heat shock protein 27 in patients with acute coronary syndrome. Circulation 2006, 114, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Lepedda, A.J.; Cigliano, A.; Cherchi, G.M.; Spirito, R.; Maggioni, M.; Carta, F.; Turrini, F.; Edelstein, C.; Scanu, A.M.; Formato, M. A proteomic approach to differentiate histologically classified stable and unstable plaques from human carotid arteries. Atherosclerosis 2009, 203, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Ward, L.J.; Karlsson, H.; Ljunggren, S.A.; Li, W.; Lindahl, M.; Yuan, X.M. Distinctive proteomic profiles among different regions of human carotid plaques in men and women. Sci. Rep. 2016, 6, 26231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibert, T.A.; Hibbert, B.; Chen, Y.X.; Rayner, K.; Simard, T.; Hu, T.; Cuerrier, C.M.; Zhao, X.; de Belleroche, J.; Chow, B.J.; et al. Serum heat shock protein 27 levels represent a potential therapeutic target for atherosclerosis: Observations from a human cohort and treatment of female mice. J. Am. Coll. Cardiol. 2013, 62, 1446–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuerrier, C.M.; Chen, Y.X.; Tremblay, D.; Rayner, K.; McNulty, M.; Zhao, X.; Kennedy, C.R.; de BelleRoche, J.; Pelling, A.E.; O’Brien, E.R. Chronic over-expression of heat shock protein 27 attenuates atherogenesis and enhances plaque remodeling: A combined histological and mechanical assessment of aortic lesions. PLoS ONE 2013, 8, e55867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.; Ulke-Lemee, A.; Deng, J.; Batulan, Z.; O’Brien, E.R. Characterization of heat shock protein 27 in extracellular vesicles: A potential anti-inflammatory therapy. FASEB J. 2019, 33, 1617–1630. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Deng, J.; Chiu, M.; Chen, Y.-X.; O’Brien, E.R. Heat Shock Protein 27 Immune Complex Altered Signaling and Transport (ICAST): Novel Mechanisms of Attenuating Inflammation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Raizman, J.E.; Chen, Y.X.; Seibert, T.; Hibbert, B.; Cuerrier, C.M.; Salari, S.; Zhao, X.; Hu, T.; Shi, C.; Ma, X.; et al. Heat shock protein-27 attenuates foam cell formation and atherogenesis by down-regulating scavenger receptor-A expression via NF-kappaB signaling. Biochim. Biophys. Acta 2013, 1831, 1721–1728. [Google Scholar] [CrossRef]
- Salari, S.; Seibert, T.; Chen, Y.X.; Hu, T.; Shi, C.; Zhao, X.; Cuerrier, C.M.; Raizman, J.E.; O’Brien, E.R. Extracellular HSP27 acts as a signaling molecule to activate NF-kappaB in macrophages. Cell Stress Chaperon 2013, 18, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Pulakazhi Venu, V.K.; Adijiang, A.; Seibert, T.; Chen, Y.X.; Shi, C.; Batulan, Z.; O’Brien, E.R. Heat shock protein 27-derived atheroprotection involves reverse cholesterol transport that is dependent on GM-CSF to maintain ABCA1 and ABCG1 expression in ApoE(-/-) mice. FASEB J. 2017, 31, 2364–2379. [Google Scholar] [CrossRef] [Green Version]
- Boulanger, C.M.; Loyer, X.; Rautou, P.-E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Rader, D.J.; Tall, A.R. The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat. Med. 2012, 18, 1344–1346. [Google Scholar] [CrossRef]
- Strauss, K.; Goebel, C.; Runz, H.; Möbius, W.; Weiss, S.; Feussner, I.; Simons, M.; Schneider, A. Exosome Secretion Ameliorates Lysosomal Storage of Cholesterol in Niemann-Pick Type C Disease. J. Biol. Chem. 2010, 285, 26279–26288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijnen, H.; Schiel, E.A.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Souza, L.; Asselbergh, B.; De Winter, V.; Goethals, S.; Timmerman, V.; Janssens, S. HSPB1 facilitates the formation of non-centrosomal microtubules. PLoS ONE 2013, 8, e66541. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Alvarez-Olmedo, D.; Zhang, Y.; Pattar, B.S.B.; O’Brien, E.R. The Heat Shock Protein 27 Immune Complex Enhances Exosomal Cholesterol Efflux. Biomedicines 2020, 8, 290. https://doi.org/10.3390/biomedicines8080290
Shi C, Alvarez-Olmedo D, Zhang Y, Pattar BSB, O’Brien ER. The Heat Shock Protein 27 Immune Complex Enhances Exosomal Cholesterol Efflux. Biomedicines. 2020; 8(8):290. https://doi.org/10.3390/biomedicines8080290
Chicago/Turabian StyleShi, Chunhua, Daiana Alvarez-Olmedo, Yuan Zhang, Badal S. B. Pattar, and Edward R. O’Brien. 2020. "The Heat Shock Protein 27 Immune Complex Enhances Exosomal Cholesterol Efflux" Biomedicines 8, no. 8: 290. https://doi.org/10.3390/biomedicines8080290
APA StyleShi, C., Alvarez-Olmedo, D., Zhang, Y., Pattar, B. S. B., & O’Brien, E. R. (2020). The Heat Shock Protein 27 Immune Complex Enhances Exosomal Cholesterol Efflux. Biomedicines, 8(8), 290. https://doi.org/10.3390/biomedicines8080290





