Vivostat Platelet-Rich Fibrin® for Complicated or Chronic Wounds—A Pilot Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Definition of A Chronic Wound
2.2. Definition of A Complicated Wound
2.3. Clinical Study Population
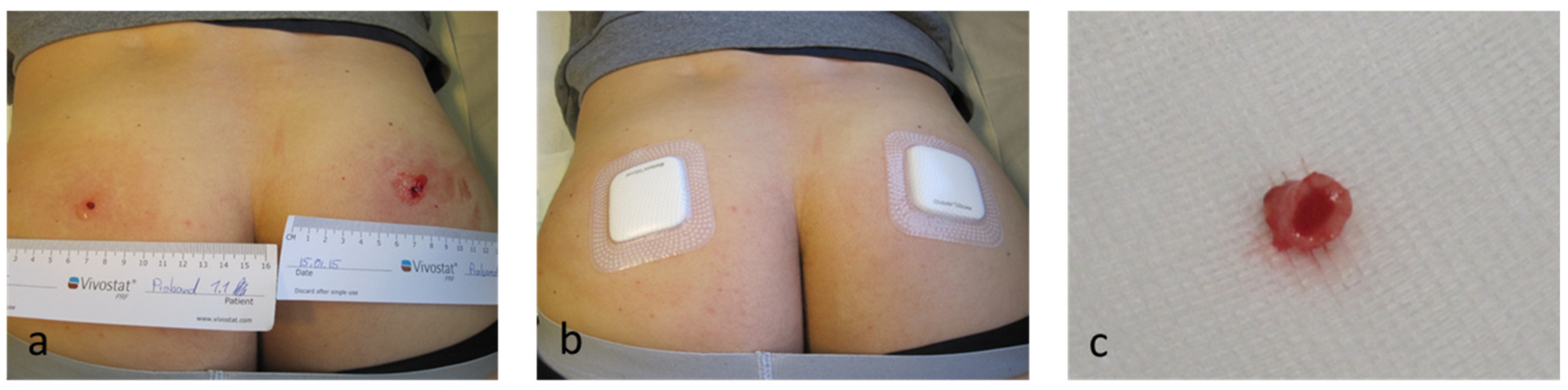
2.4. Vivostat PRF® Treatment
2.5. Analyses of the Clinical Efficacy of the Vivostat PRF® Treatment
- (a)
- wounds with a size <10 cm2
- (b)
- wounds with a size >10 cm2 and <30 cm2
- (c)
- wounds with a size >30 cm2.
- (a)
- “no improvement of the wound” in patients where the wound size was reduced <10% under Vivostat PRF® treatment
- (b)
- “improvement of the wound” in patients with a reduced size >10% under Vivostat PRF® treatment
- (c)
- “complete epithelialization” in patients with completely re-epithelialized wounds after Vivostat PRF® treatment.
2.6. Statistics
2.7. Experimental Study Population
3. Results in the Clinical Study Population
3.1. Entire Study Population
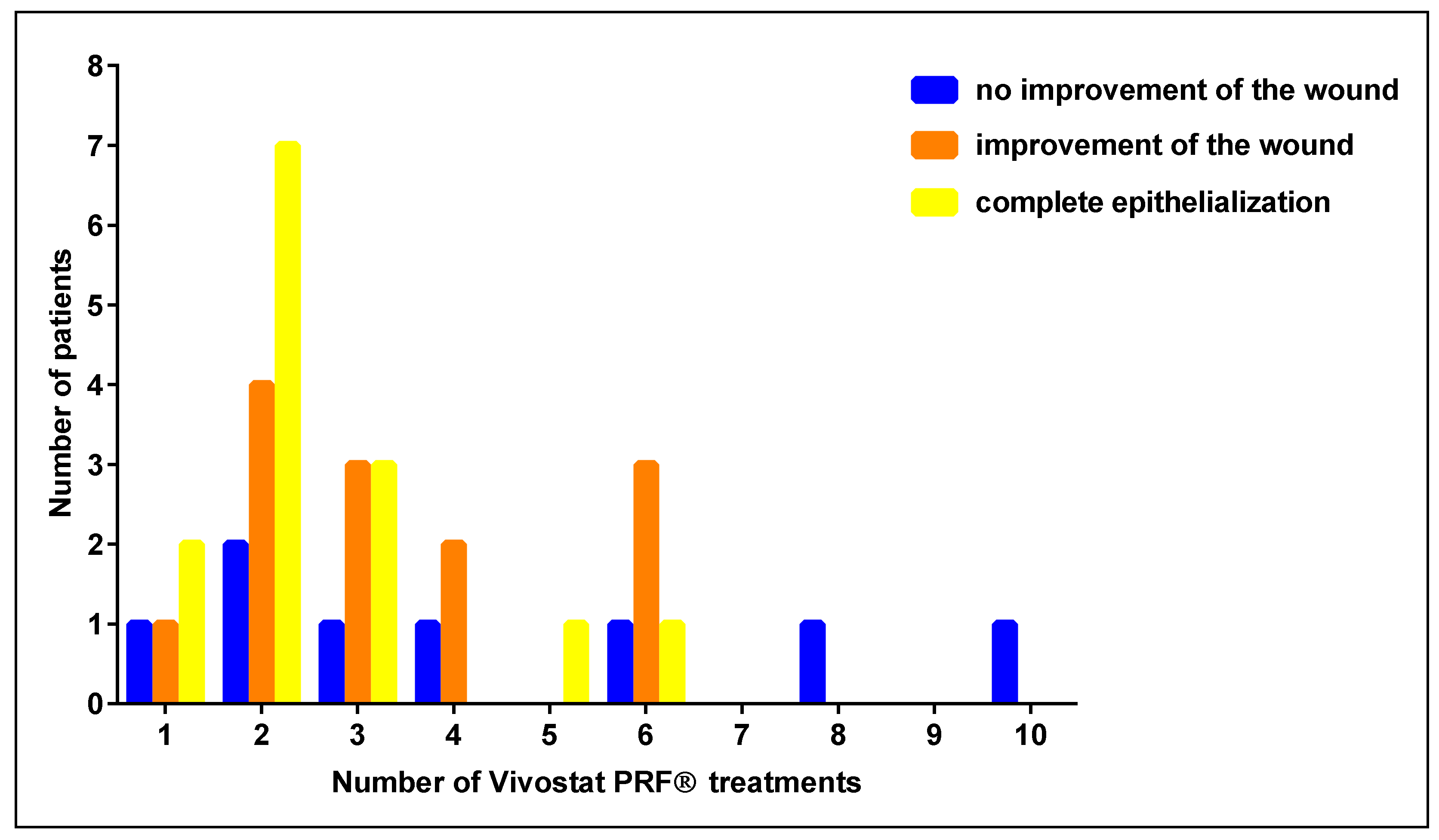
3.2. Number of Vivostat PRF® Treatments
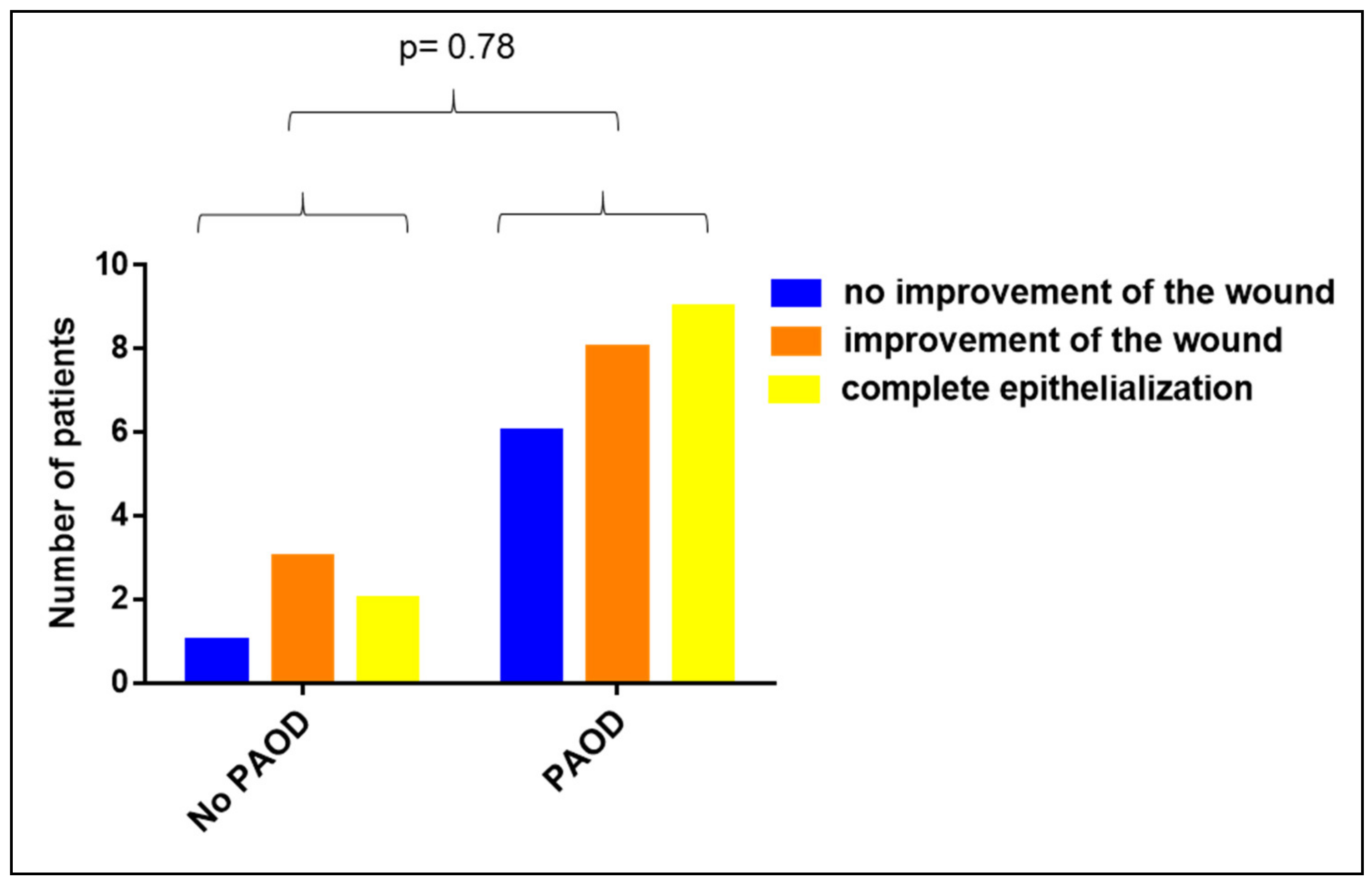
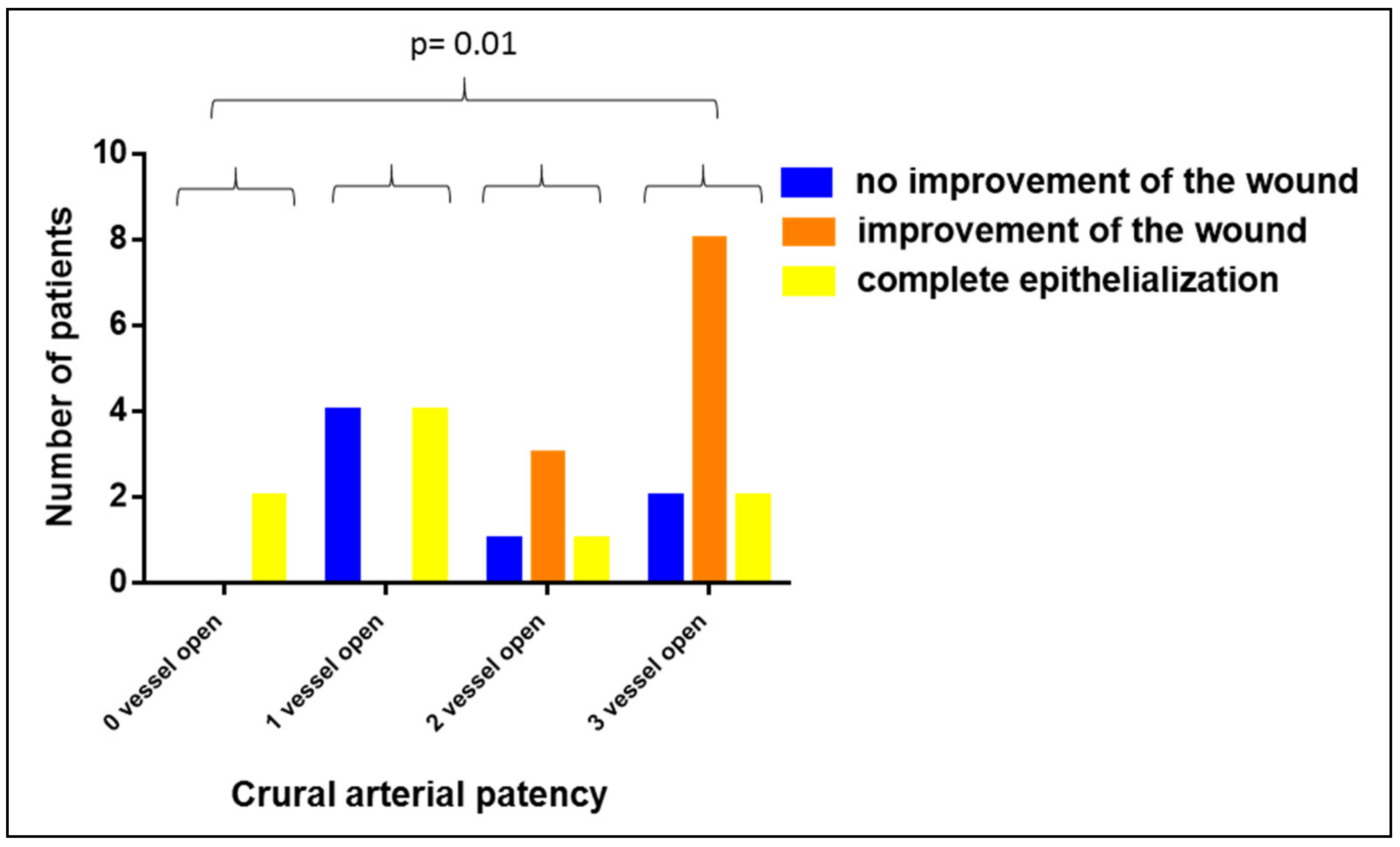
3.3. Impact of PAOD on the Clinical Efficacy of Vivostat PRF®
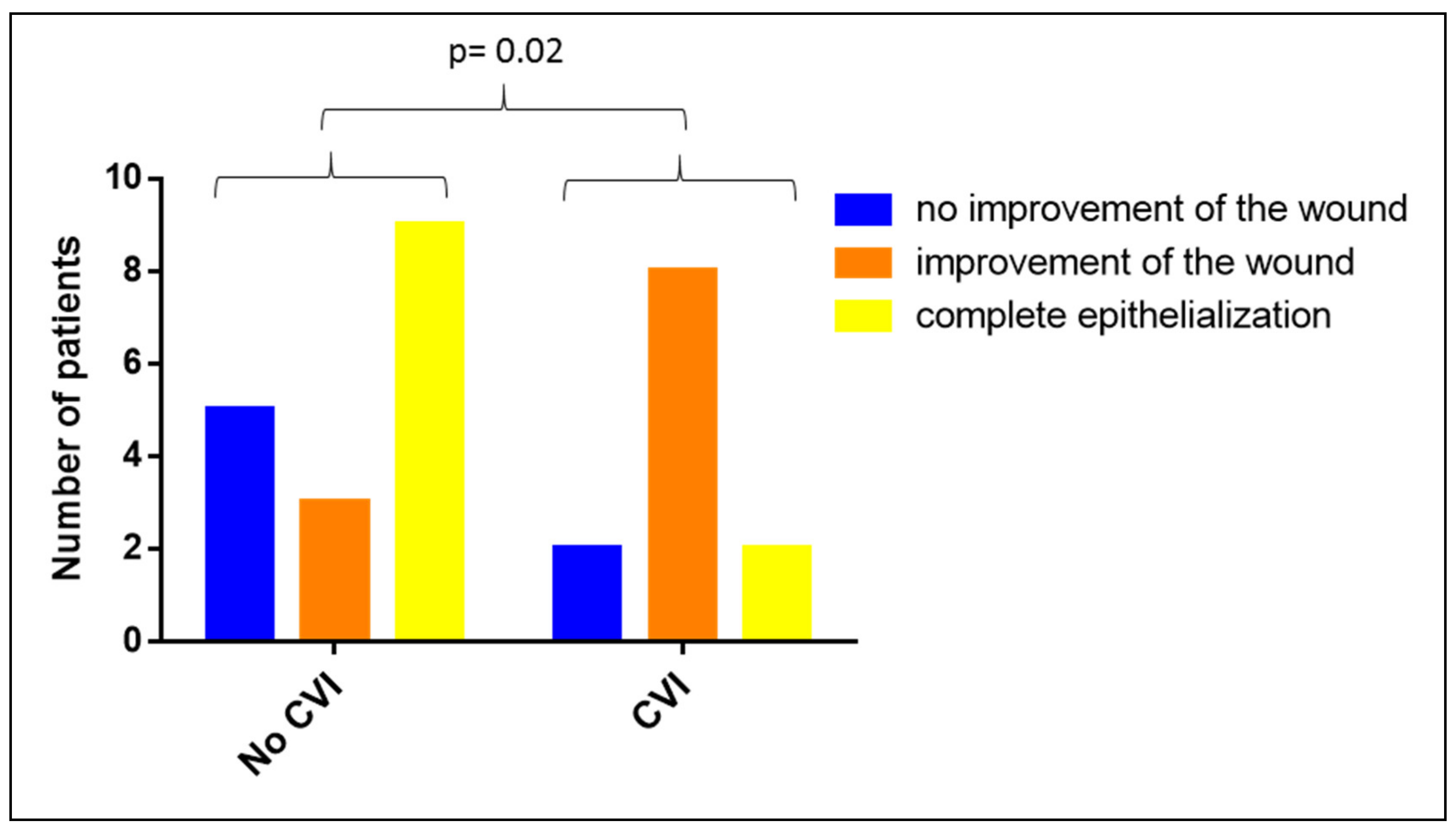
3.4. Impact of CVI on the Clinical Efficacy of Vivostat PRF®
3.5. Impact of Diabetic Foot Syndrome (DFS) on the Clinical Efficacy of Vivostat PRF®
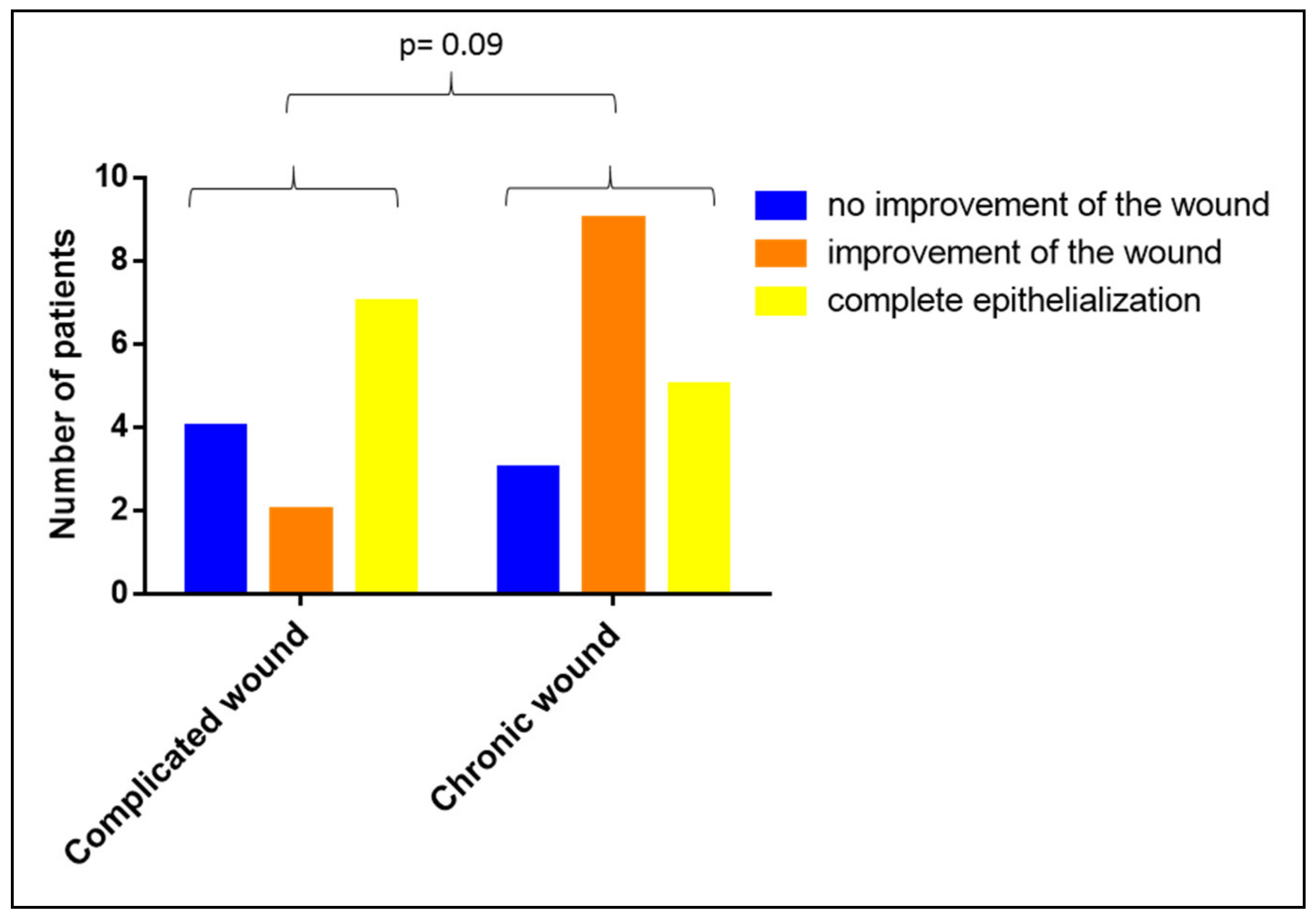
3.6. Impact of the Duration of the Wound-Existence on the Clinical Efficacy of Vivostat PRF®
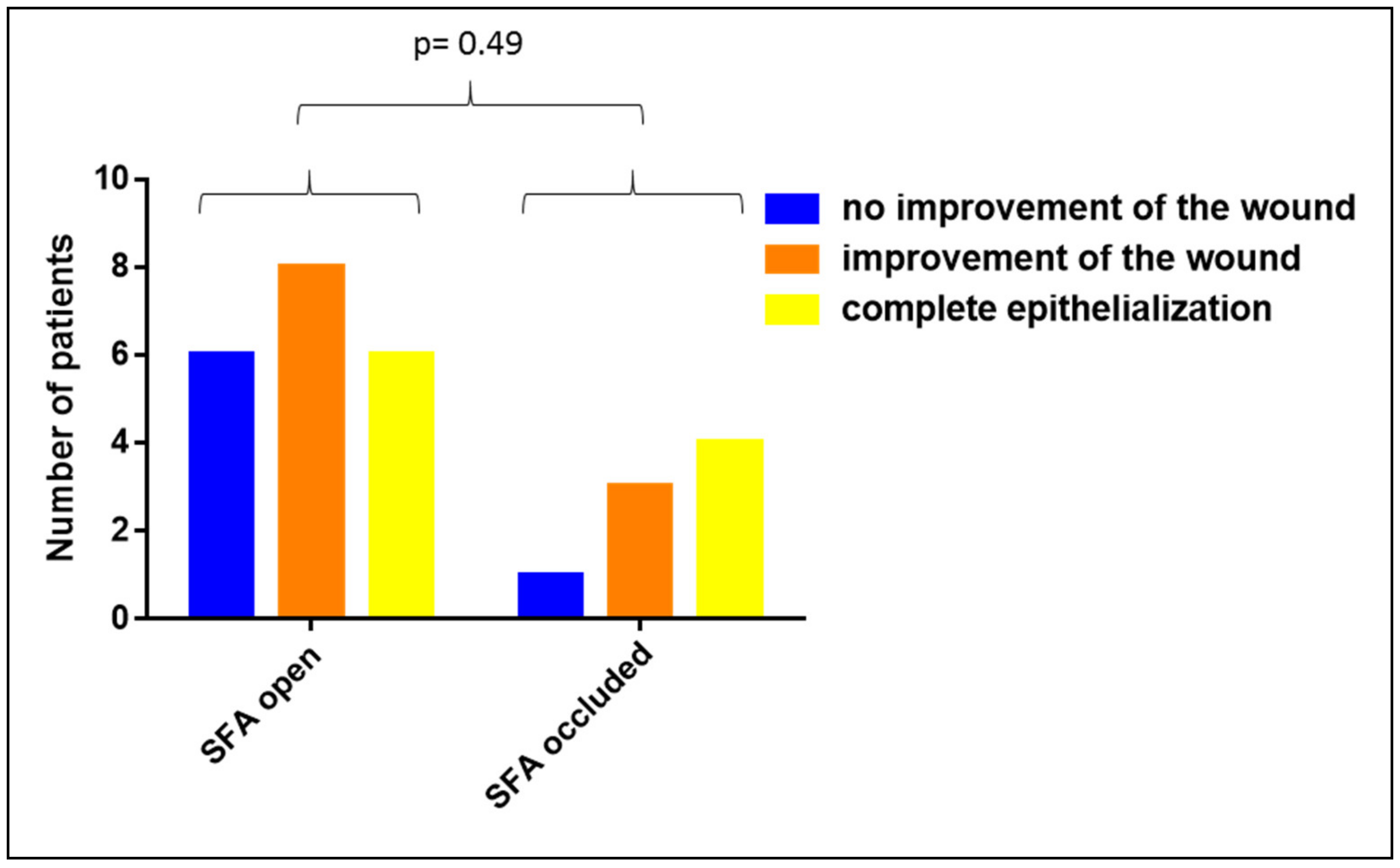
3.7. Impact of the Initial Wound Size on the Clinical Efficacy of Vivostat PRF®
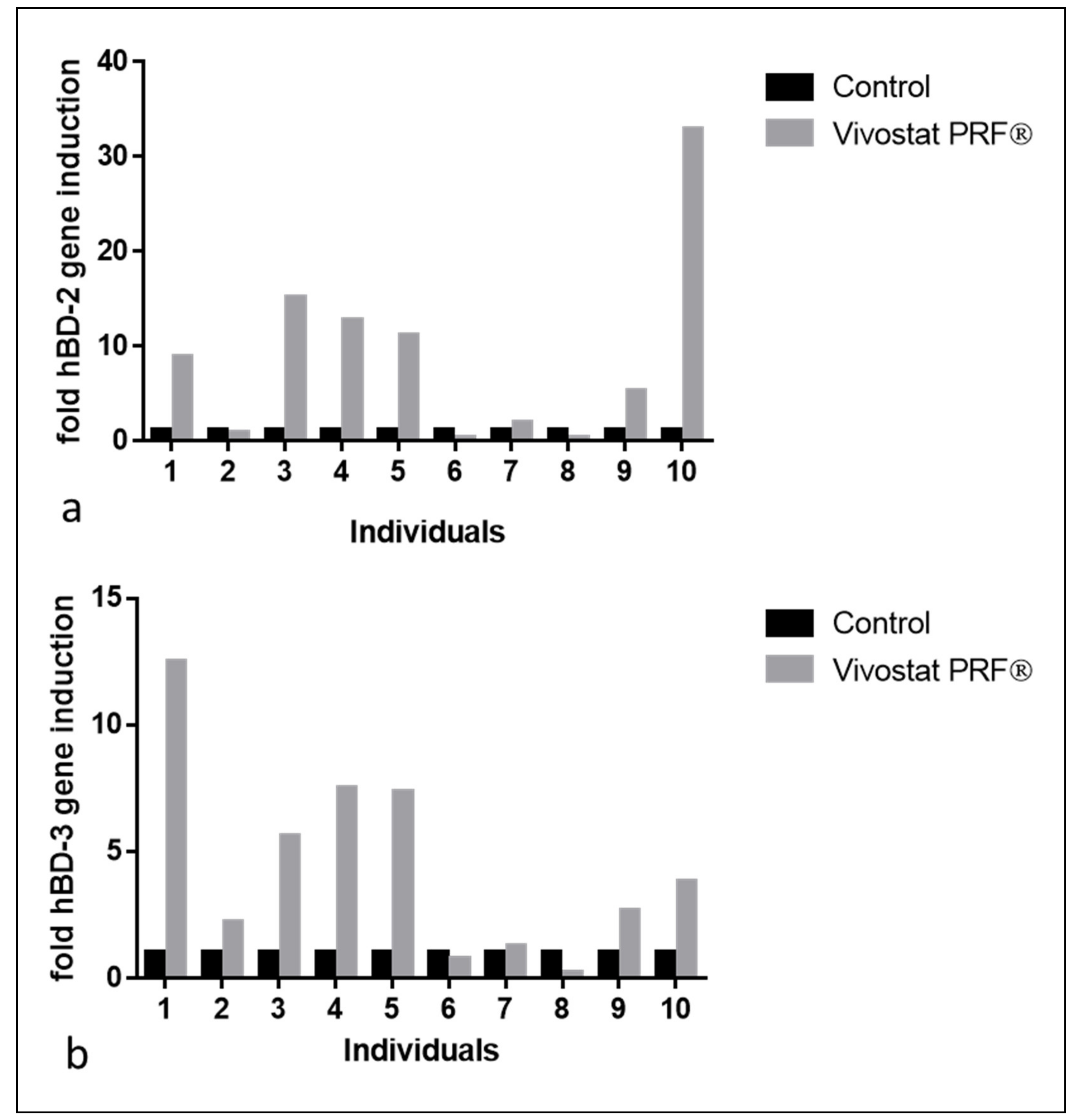
4. Results in the Experimental Study Population
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heyer, K.; Herberger, K.; Protz, K.; Glaeske, G.; Augustin, M. Epidemiology of chronic wounds in Germany: Analysis of statutory health insurance data. Wound Repair Regen. 2016, 24, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Jester, R.; McKinley, R.; Pooler, A. The impact of chronic venous leg ulcers: A systematic review. J. Wound Care 2014, 23, 601–612. [Google Scholar] [CrossRef]
- Phillips, P.; Lumley, E.; Duncan, R.; Aber, A.; Woods, H.B.; Jones, G.L.; Michaels, J. A systematic review of qualitative research into people’s experiences of living with venous leg ulcers. J. Adv. Nurs. 2018, 74, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Furtado, K.; Pina, E.; Moffatt, C.J.; Franks, P.J. Leg ulceration in Portugal: Quality of life. Int. Wound J. 2007, 5, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Purwins, S.; Herberger, K.; Debus, E.S.; Rustenbach, S.J.; Pelzer, P.; Rabe, E.; Schäfer, E.; Stadler, R.; Augustin, M. Cost-of-illness of chronic leg ulcers in Germany. Int. Wound J. 2010, 7, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Hersant, B.; Bosc, R.; Meningaud, J.-P. The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: A review and a proposal for a new standard care. Wound Repair Regen. 2015, 23, 638–643. [Google Scholar] [CrossRef]
- Alsousou, J.; Ali, A.; Willett, K.; Harrison, P. The role of platelet-rich plasma in tissue regeneration. Platelets 2013, 24, 173–182. [Google Scholar] [CrossRef]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef]
- Eppley, B.L.; Woodell, J.E.; Higgins, J. Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast. Reconstr. Surg. 2004, 114, 1502–1508. [Google Scholar] [CrossRef]
- Weibric, G.; Buch, R.S.R.; Kleis, W.K.G.; Hafner, G.; Hitzler, W.E.; Wagner, W. Quantification of thrombocyte growth factors in platelet concentrates produced by discontinuous cell separation. Growth Factors 2002, 20, 93–97. [Google Scholar] [CrossRef]
- Ågren, M.S.; Rasmussen, K.; Pakkenberg, B.; Jørgensen, B. Growth factor and proteinase profile of Vivostat ® platelet-rich fibrin linked to tissue repair. Vox Sang. 2014, 107, 37–43. [Google Scholar] [CrossRef]
- Steenvoorde, P.; van Doorn, L.P.; Naves, C.; Oskam, J. Use of autologous platelet-rich fibrin on hard-to-heal wounds. J. Wound Care 2008, 17, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zapata, M.J.; Martí-Carvajal, A.J.; Solà, I.; Expósito, J.A.; Bolíbar, I.; Rodríguez, L.; Garcia, J.; Zaror, C. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst. Rev. 2016, 25, CD006899. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Reffat, S.A.; Hassan, A.; Eskander, F. Platelet-Rich Plasma for the Treatment of Clean Diabetic Foot Ulcers. Ann. Vasc. Surg. 2017, 38, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.-Q.; Zhang, Y.-N.; Ma, J.; Li, Y.-H.; Zuo, D.-M.; Qiu, J.-L.; Cheng, B.; Chen, Z. Liang Evaluation of the effects of homologous platelet gel on healing lower extremity wounds in patients with diabetes. Int. J. Lower Extrem. Wounds 2013, 12, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.L.; Ågren, M.S.; Jorgensen, L.N. Platelet-Rich Fibrin Versus Albumin in Surgical Wound Repair. Ann. Surg. 2010, 251, 825–831. [Google Scholar] [CrossRef]
- Somani, A.; Rai, R. Comparison of Efficacy of Autologous Platelet-rich Fibrin versus Saline Dressing in Chronic Venous Leg Ulcers: A Randomised Controlled Trial. J. Cutan. Aesthetic Surg. 2017, 10, 8–12. [Google Scholar]
- Little, C.; McDonald, J.; Jenkins, M.G.; McCarron, P. An overview of techniques used to measure wound area and volume. J. Wound Care 2009, 18, 250–253. [Google Scholar] [CrossRef]
- Jørgensen, L.B.; Sørensen, J.A.; Jemec, G.B.; Yderstraede, K.B. Methods to assess area and volume of wounds—A systematic review. Int. Wound J. 2016, 13, 540–553. [Google Scholar] [CrossRef]
- Bayer, A.; Lammel, J.; Rademacher, F.; Groß, J.; Siggelkow, M.; Lippross, S.; Klüter, T.; Varoga, D.; Tohidnezhad, M.; Pufe, T.; et al. Platelet-released growth factors induce the antimicrobial peptide human beta-defensin-2 in primary keratinocytes. Exp. Dermatol. 2016, 25, 460–465. [Google Scholar] [CrossRef]
- Bayer, A.; Lammel, J.; Tohidnezhad, M.; Lippross, S.; Behrendt, P.; Klüter, T.; Pufe, T.; Cremer, J.; Jahr, H.; Rademacher, F.; et al. The Antimicrobial Peptide Human Beta-Defensin-3 Is Induced by Platelet-Released Growth Factors in Primary Keratinocytes. Mediat. Inflamm. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinke, J.M.; Sorg, H. European surgical research Europaische chirurgische Forschung Recherches chirurgicales europeennes. Wound Repair Regen. 2012, 49, 35–43. [Google Scholar]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Badran, K.W.; Sand, J.P. Platelet-Rich Plasma for Hair Loss. Facial Plast. Surg. Clin. N. Am. 2018, 26, 469–485. [Google Scholar] [CrossRef]
- Yazawa, M.; Ogata, H.; Nakajima, T.; Mori, T.; Watanabe, N.; Handa, M. Basic studies on the clinical applications of platelet-rich plasma. Cell Transplant. 2003, 12, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Alsousou, J.; Thompson, M.; Hulley, P.; Noble, A.; Willett, K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: A review of the literature. J. Bone Jt. Surg. Br. Vol. 2009, 91, 987–996. [Google Scholar] [CrossRef]
- Mohammadi, S.; Nasiri, S.; Mohammadi, M.H.; Malek Mohammadi, A.; Nikbakht, M.; Zahed Panah, M.; Safar, H.; Mostafaei, S.; Norooznezhad, A.H.; Soroosh, A.R.; et al. Evaluation of platelet-rich plasma gel potential in acceleration of wound healing duration in patients underwent pilonidal sinus surgery: A randomized controlled parallel clinical trial. Transfus. Apher. Sci. 2017. [Google Scholar] [CrossRef]
- Mohammadi, M.H.; Molavi, B.; Mohammadi, S.; Nikbakht, M.; Mohammadi, A.M.; Mostafaei, S.; Norooznezhad, A.H.; Ghorbani Abdegah, A.; Ghavamzadeh, A. Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel: A single-arm clinical trial. Transfus. Apher. Sci. 2016. [Google Scholar] [CrossRef]
- Xian, L.J.; Roy Chowdhury, S.; Bin Saim, A.; Bt Hj Idrus, R. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy 2015, 17, 293–300. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Terashima, H.; Yoneyama, S.; Tadano, S.; Ohkohchi, N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J. Surg. Res. 2012, 173, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Kawasumi, M.; Kitoh, H.; Siwicka, K.A.; Ishiguro, N. The effect of the platelet concentration in platelet-rich plasma gel on the regeneration of bone. J. Bone Jt. Surg. Br. Vol. 2008, 90, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Lammel, J.; Lippross, S.; Klüter, T.; Behrendt, P.; Tohidnezhad, M.; Pufe, T.; Cremer, J.; Jahr, H.; Rademacher, F.; et al. Platelet-released growth factors induce psoriasin in keratinocytes: Implications for the cutaneous barrier. Ann. Anat. 2017, 213. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Tohidnezhad, M.; Lammel, J.; Lippross, S.; Behrendt, P.; Klüter, T.; Varoga, D.; Tohidnezhad, M.; Pufe, T.; Cremer, J.; et al. Platelet-Released Growth Factors Induce Differentiation of Primary Keratinocytes. Mediat. Inflamm. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, S.M.; Impeduglia, T.; Hessler, K.; Wang, X.-J.; Carroll, R.J.; Dardik, H. Autologous platelet-rich fibrin matrix as cell therapy in the healing of chronic lower-extremity ulcers. Wound Repair Regen. 2008, 16, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, D.; Wang, C.; Yuan, N.; Wang, Y.; He, L.; Yang, Y.; Chen, L.; Liu, G.; Li, X.; et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regen. 2015, 23, 495–505. [Google Scholar] [CrossRef]
- Liu, T.; Yang, F.; Li, Z.; Yi, C.; Bai, X. A prospective pilot study to evaluate wound outcomes and levels of serum C-reactive protein and interleukin-6 in the wound fluid of patients with trauma-related chronic wounds. Ostomy Wound Manag. 2014, 60, 30–37. [Google Scholar]
- Kushnir, I.; Kushnir, A.; Serena, T.E.; Garfinkel, D. Efficacy and Safety of a Novel Autologous Wound Matrix in the Management of Complicated, Chronic Wounds: A Pilot Study. Wounds Compend. Clin. Res. Pract. 2016, 28, 317–327. [Google Scholar]
- Nakano, M.; Kamada, N.; Suehiro, K.; Oikawa, A.; Shibata, C.; Nakamura, Y.; Matsue, H.; Sasahara, Y.; Hosokawa, H.; Nakayama, T.; et al. Establishment of a new three-dimensional human epidermal model reconstructed from plucked hair follicle-derived keratinocytes. Exp. Dermatol. 2016, 25, 903–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Diagnoses and Concomitant Diseases of Analyzed Patients | n (%) |
|---|---|
| Peripheral arterial occlusive disease (PAOD) | 29 (82.85%) |
| Chronic venous insufficiency (CVI) | 12 (34.29%) |
| Diabetes mellitus | 17 (48.57%) |
| Diabetic foot syndrome (DFS) | 6 (17.14%) |
| Obesity | 8 (22.86%) |
| Arterial hypertension | 27 (77.14%) |
| Renal insufficiency | 17 (48.57%) |
| Hyperlipidemia | 15 (42.86%) |
| Nicotine abuse | 11 (31.43%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayer, A.; Höntsch, G.; Kaschwich, M.; Dell, A.; Siggelkow, M.; Berndt, R.; Rusch, R.; Harder, J.; Gläser, R.; Cremer, J. Vivostat Platelet-Rich Fibrin® for Complicated or Chronic Wounds—A Pilot Study. Biomedicines 2020, 8, 276. https://doi.org/10.3390/biomedicines8080276
Bayer A, Höntsch G, Kaschwich M, Dell A, Siggelkow M, Berndt R, Rusch R, Harder J, Gläser R, Cremer J. Vivostat Platelet-Rich Fibrin® for Complicated or Chronic Wounds—A Pilot Study. Biomedicines. 2020; 8(8):276. https://doi.org/10.3390/biomedicines8080276
Chicago/Turabian StyleBayer, Andreas, Gesa Höntsch, Mark Kaschwich, Annika Dell, Markus Siggelkow, Rouven Berndt, Rene Rusch, Jürgen Harder, Regine Gläser, and Jochen Cremer. 2020. "Vivostat Platelet-Rich Fibrin® for Complicated or Chronic Wounds—A Pilot Study" Biomedicines 8, no. 8: 276. https://doi.org/10.3390/biomedicines8080276
APA StyleBayer, A., Höntsch, G., Kaschwich, M., Dell, A., Siggelkow, M., Berndt, R., Rusch, R., Harder, J., Gläser, R., & Cremer, J. (2020). Vivostat Platelet-Rich Fibrin® for Complicated or Chronic Wounds—A Pilot Study. Biomedicines, 8(8), 276. https://doi.org/10.3390/biomedicines8080276





