Incomplete Pattern of Steroidogenic Protein Expression in Functioning Adrenocortical Carcinomas
Abstract
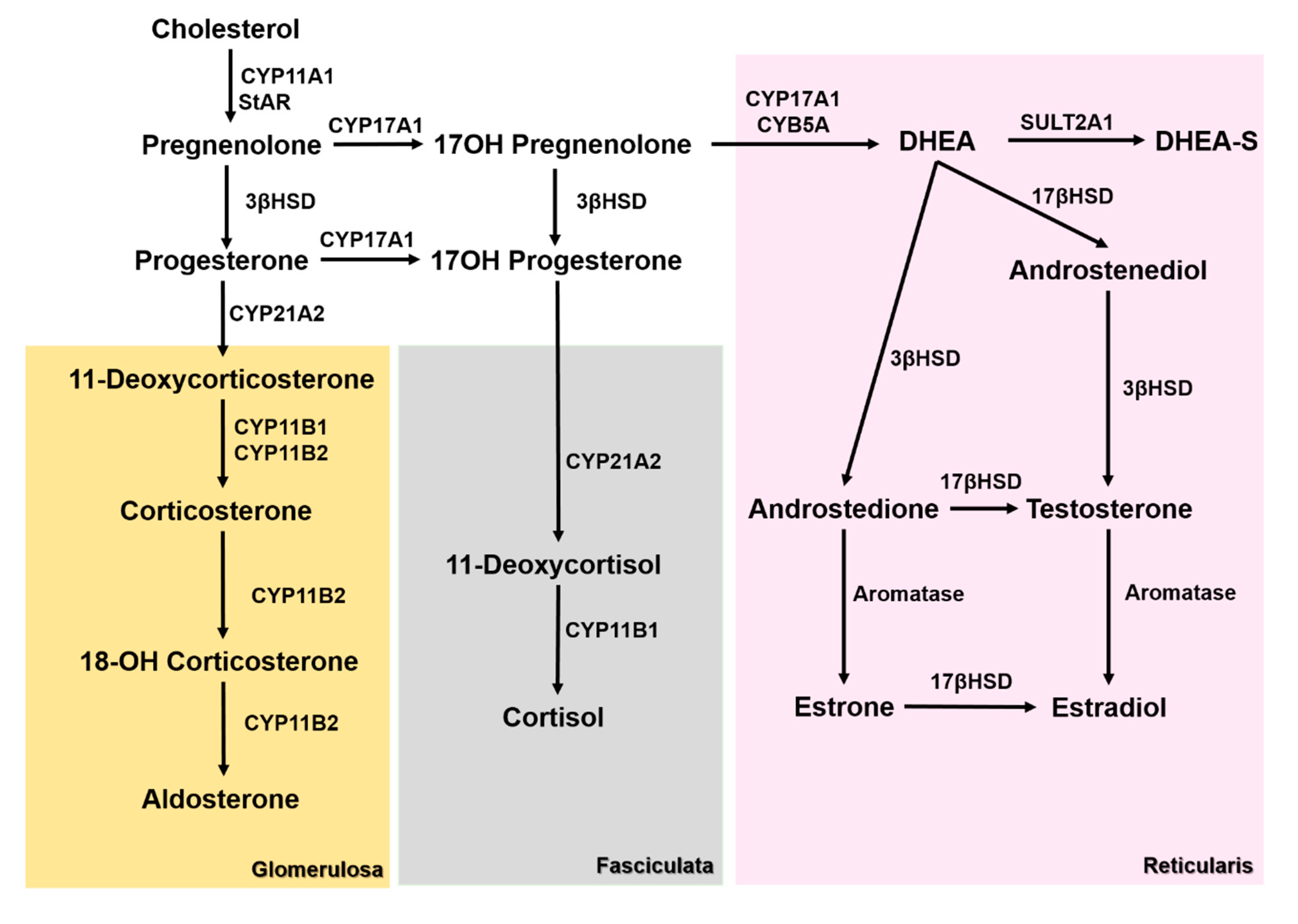
1. Introduction
2. Experimental Section
2.1. Case Selection
2.2. Immunohistochemistry and Analysis
2.3. Immunofluorescence
2.4. Statistical Analysis
3. Results
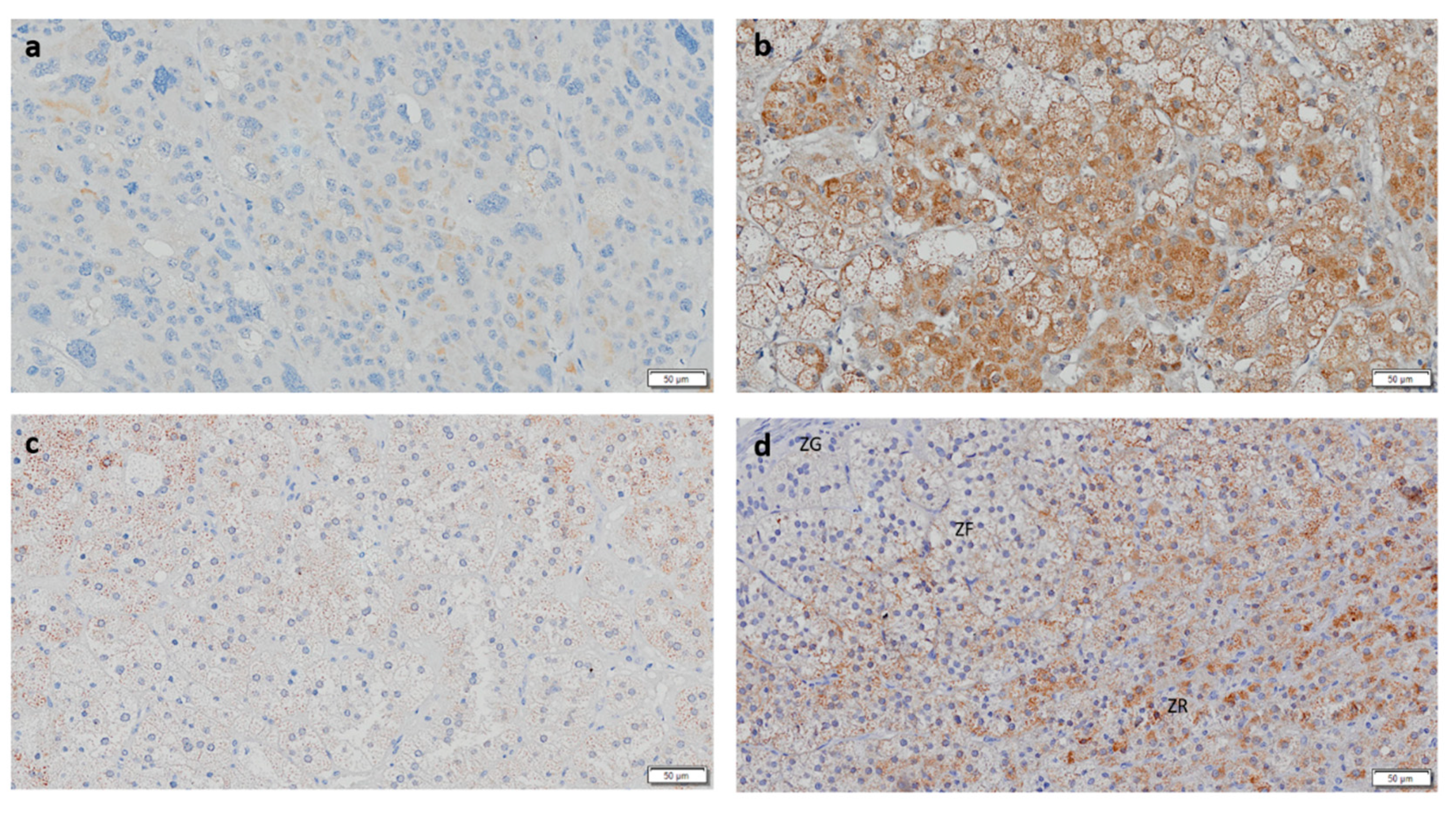
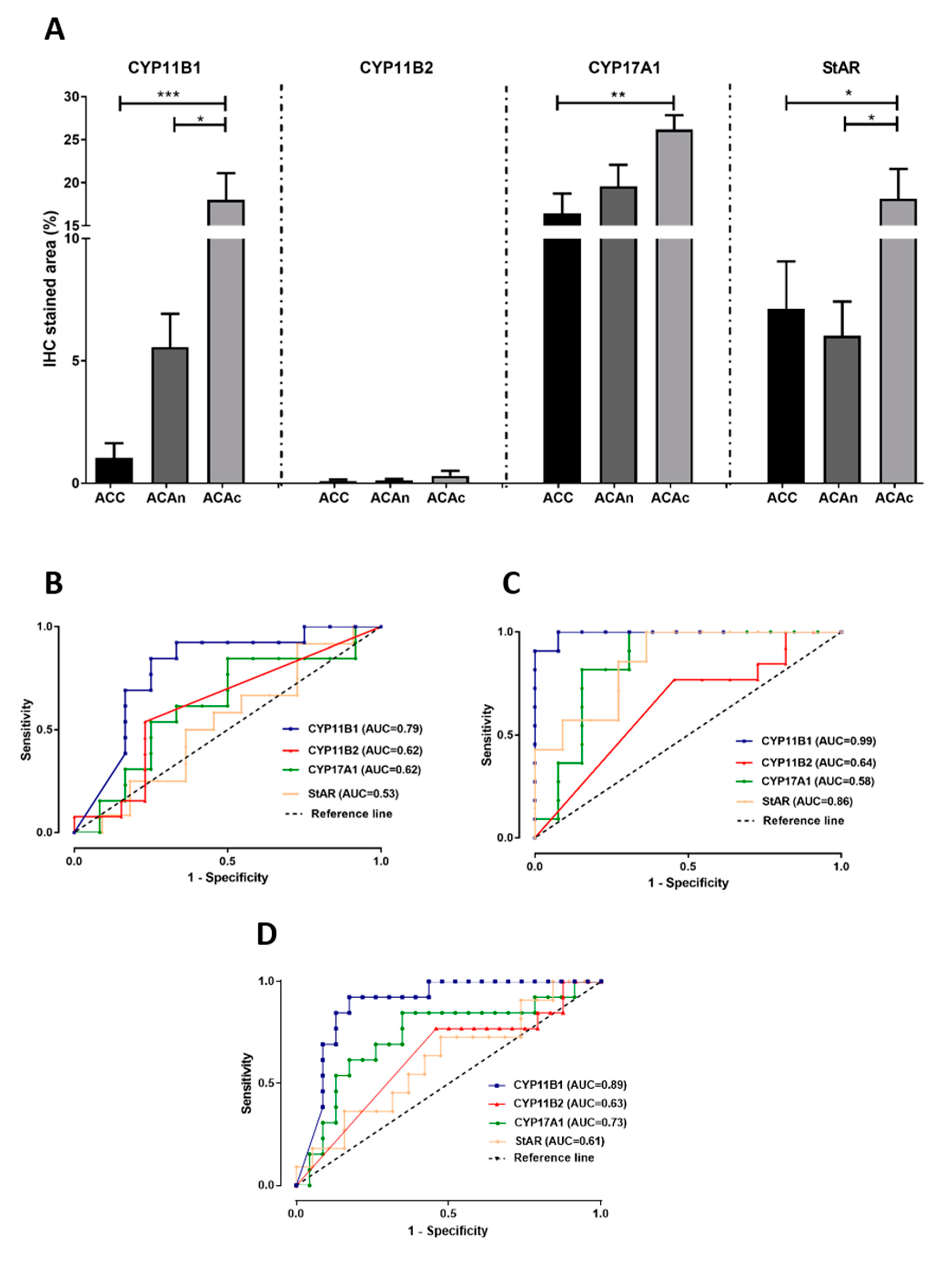
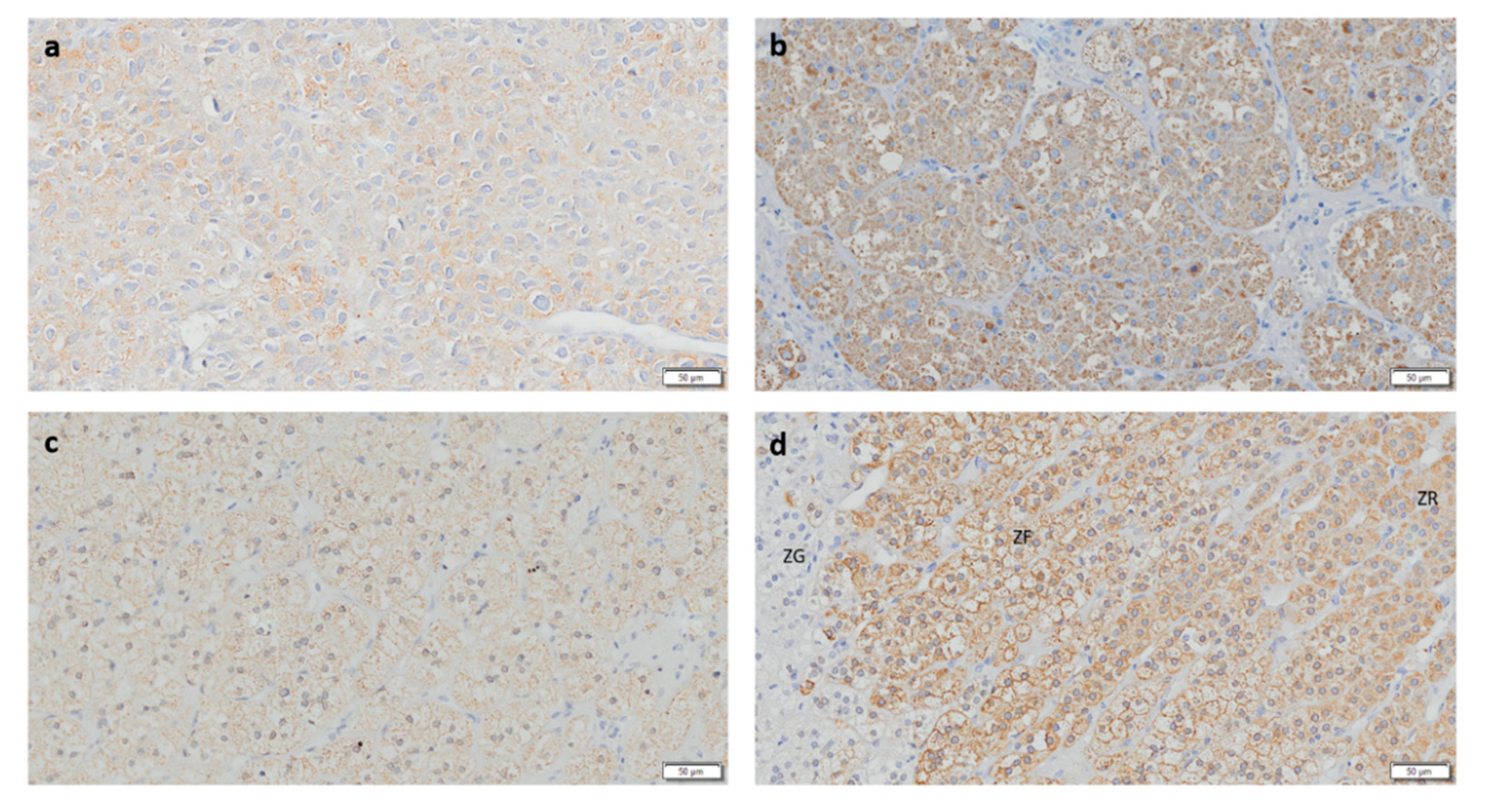
3.1. CYP11B1 Expression in ACC Is Low
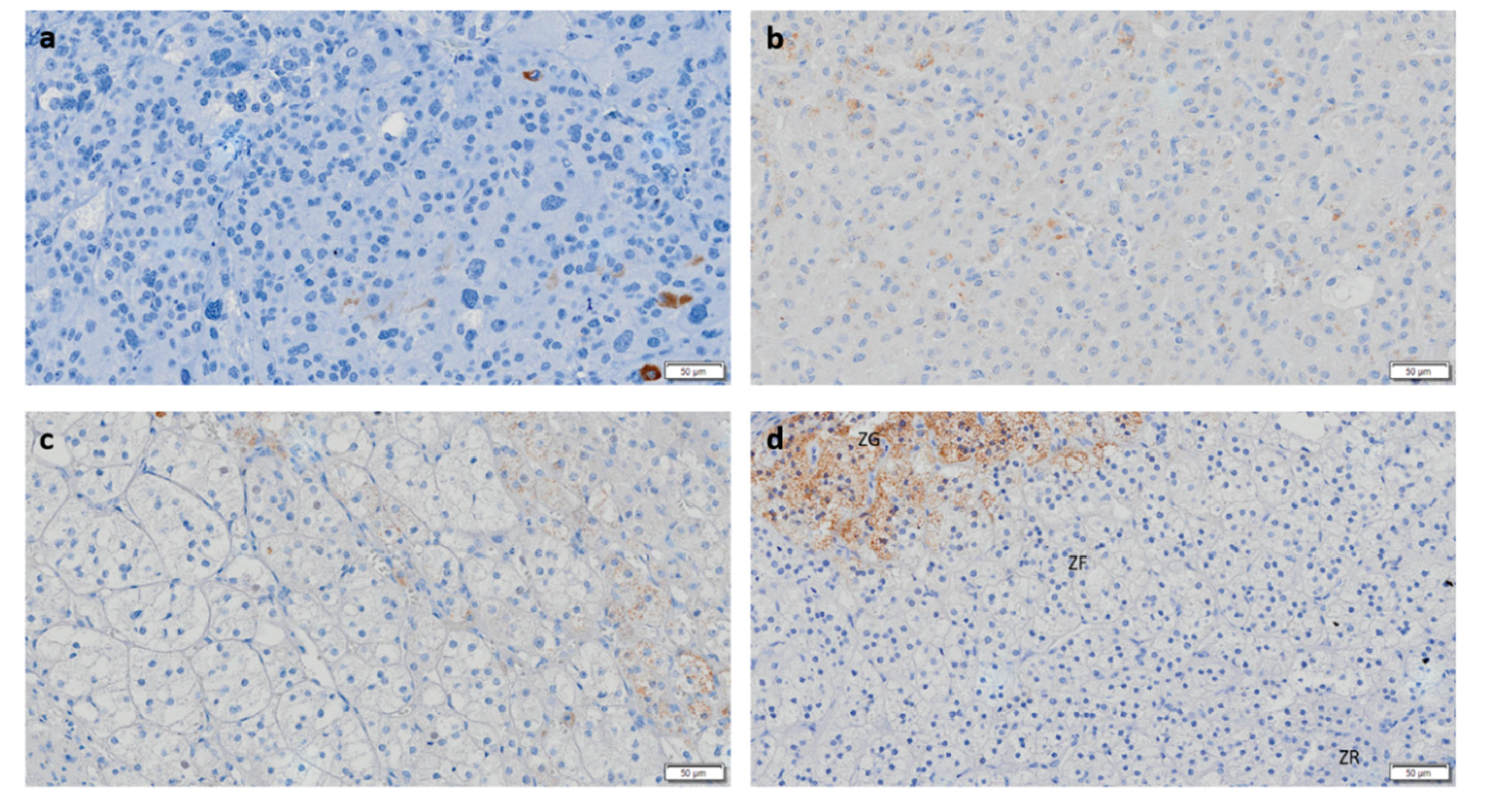
3.2. CYP11B2 and CYP11B1 Dual Negativity Is Highly Suggestive of Malignant Adrenocortical Tumors
3.3. CYP17A1 Expression in ACC Is Low
3.4. StAR Expression Is Decreased in ACC and ACAn
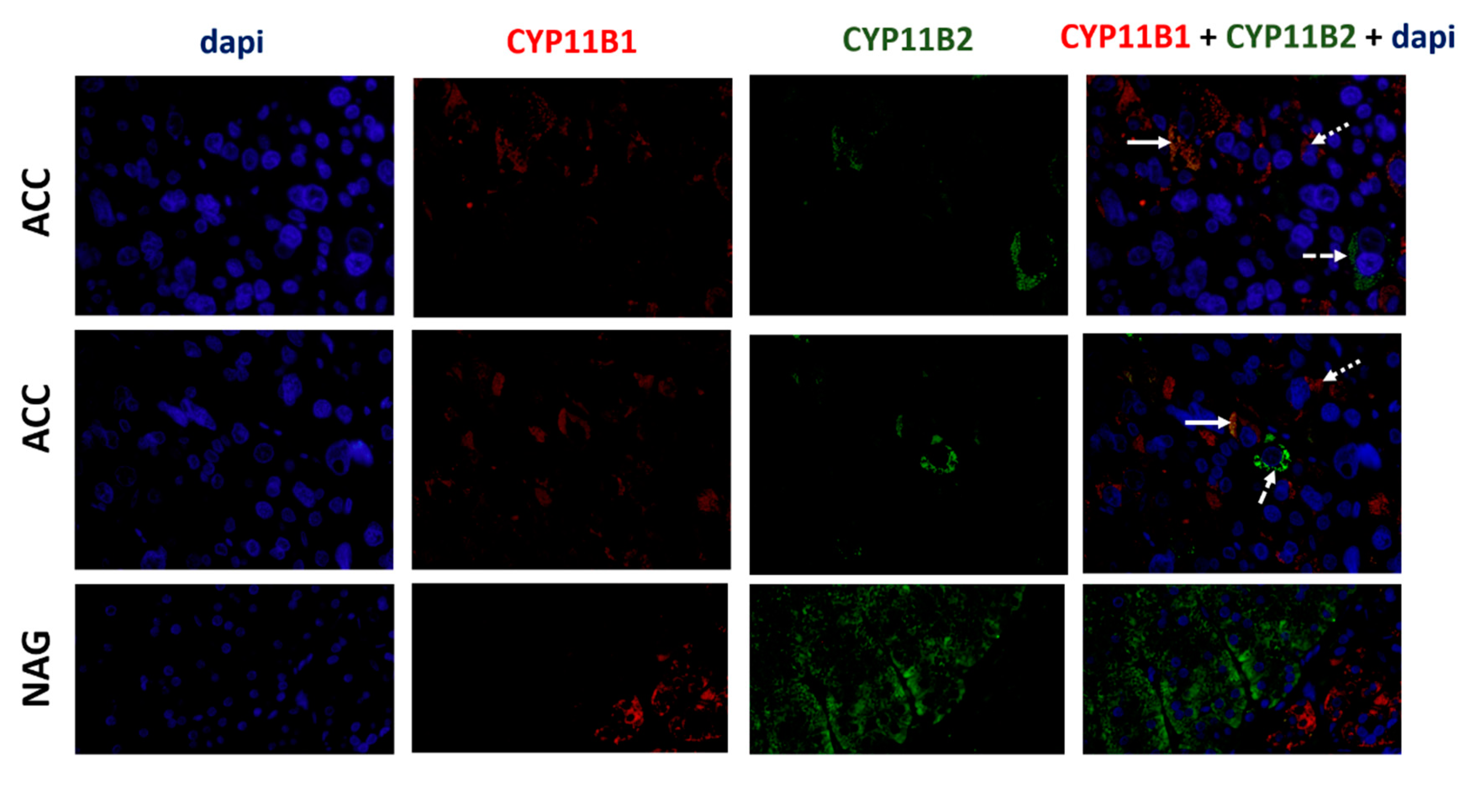
3.5. ACCs Present Cells That Express Both CYP11B1 and CYP11B2
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef] [PubMed]
- Allolio, B.; Fassnacht, M. Clinical review: Adrenocortical carcinoma: Clinical update. J. Clin. Endocrinol. Metab. 2006, 91, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Allolio, B. Clinical management of adrenocortical carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Creemers, S.G.; Hofland, L.J.; Korpershoek, E.; Franssen, G.J.; van Kemenade, F.J.; de Herder, W.W.; Feelders, R.A. Future directions in the diagnosis and medical treatment of adrenocortical carcinoma. Endocr. Relat. Cancer 2016, 23, R43–R69. [Google Scholar] [CrossRef] [PubMed]
- Grondal, S.; Eriksson, B.; Hagenas, L.; Werner, S.; Curstedt, T. Steroid profile in urine: A useful tool in the diagnosis and follow up of adrenocortical carcinoma. Acta Endocrinol. 1990, 122, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Arlt, W.; Biehl, M.; Taylor, A.E.; Hahner, S.; Libe, R.; Hughes, B.A.; Schneider, P.; Smith, D.J.; Stiekema, H.; Krone, N.; et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 2011, 96, 3775–3784. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, E.; Yanaihara, H.; Nakashima, J.; Homma, K.; Ohigashi, T.; Asakura, H.; Tachibana, M.; Shibata, H.; Saruta, T.; Murai, M. Urinary steroid profile in adrenocortical tumors. Biomed. Pharmacother. 2000, 54, 194s–197s. [Google Scholar] [CrossRef]
- Kerkhofs, T.M.; Kerstens, M.N.; Kema, I.P.; Willems, T.P.; Haak, H.R. Diagnostic Value of Urinary Steroid Profiling in the Evaluation of Adrenal Tumors. Horm. Cancer 2015, 6, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Chortis, V.; Bancos, I.; Nijman, T.; Gilligan, L.C.; Taylor, A.E.; Ronchi, C.L.; O’Reilly, M.W.; Schreiner, J.; Asia, M.; Riester, A.; et al. Urine steroid metabolomics as a novel tool for detection of recurrent adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2019. [Google Scholar] [CrossRef]
- Hodgson, A.; Pakbaz, S.; Mete, O. A Diagnostic Approach to Adrenocortical Tumors. Surg. Pathol. Clin. 2019, 12, 967–995. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, C.E.; Qi, X.; Velarde-Miranda, C.; Plonczynski, M.W.; Parker, C.R.; Rainey, W.; Satoh, F.; Maekawa, T.; Nakamura, Y.; Sasano, H.; et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol. Cell Endocrinol. 2014, 383, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.S.; Morais, T.; Costa, M.M.; Monteiro, M.P.; Pignatelli, D. The emerging role of the molecular marker p27 in the differential diagnosis of adrenocortical tumors. Endocr. Connect. 2013, 2, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Pereira, S.S.; Mesquita, M.; Morais, T.; Costa, M.M.; Quelhas, P.; Lopes, C.; Monteiro, M.P.; Leite, V. Lymph Node Metastases in Papillary and Medullary Thyroid Carcinoma Are Independent of Intratumoral Lymphatic Vessel Density. Eur. Thyroid. J. 2017, 6, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Upadhye, S.; Worster, A. Understanding receiver operating characteristic (ROC) curves. Can. J. Emerg. Med. 2006, 8, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef] [PubMed]
- Sasano, H.; Suzuki, T.; Nagura, H.; Nishikawa, T. Steroidogenesis in human adrenocortical carcinoma: Biochemical activities, immunohistochemistry, and in situ hybridization of steroidogenic enzymes and histopathologic study in nine cases. Hum. Pathol. 1993, 24, 397–404. [Google Scholar] [CrossRef]
- Uchida, T.; Nishimoto, K.; Fukumura, Y.; Asahina, M.; Goto, H.; Kawano, Y.; Shimizu, F.; Tsujimura, A.; Seki, T.; Mukai, K.; et al. Disorganized Steroidogenesis in Adrenocortical Carcinoma, a Case Study. Endocr. Pathol. 2017, 28, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Fishman, L. Biosynthesis and metabolism of steroid hormones by human adrenal carcinomas. Braz. J. Med. Biol. Res. 2000, 33, 1235–1244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gazdar, A.F.; Oie, H.K.; Shackleton, C.H.; Chen, T.R.; Triche, T.J.; Myers, C.E.; Chrousos, G.P.; Brennan, M.F.; Stein, C.A.; La Rocca, R.V. Establishment and Characterization of a Human Adrenocortical Carcinoma Cell Line That Expresses Multiple Pathways of Steroid Biosynthesis. Cancer Res. 1990, 50, 5488–5496. [Google Scholar] [PubMed]

| Adrenocortical Tumors | Steroidogenic Proteins | Negative | Positive |
|---|---|---|---|
| ACC (n = 14) | CYP11B1 | 5 (35.7%) | 9 (64.3%) |
| CYP11B2 | 11 (78.6%) | 3 (21.4%) | |
| CYP17A1 | 0 (0.00%) | 14 (100.0%) | |
| StAR | 0 (0.00%) | 14 (100.0%) | |
| ACAc (n = 11) | CYP11B1 | 0 (0.00%) | 11 (100.0%) |
| CYP11B2 | 5 (45.5%) | 6 (54.5%) | |
| CYP17A1 | 0 (0.00%) | 11 (100.0%) | |
| StAR | 0 (0.00%) | 11 (100.0%) | |
| ACAn (n = 15) | CYP11B1 | 2 (13.3%) | 13 (86.7%) |
| CYP11B2 | 7 (46.7%) | 8 (53.3%) | |
| CYP17A1 | 1 (6.7%) | 14 (93.3%) | |
| StAR | 0 (0.00%) | 15 (100.0%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.S.; Costa, M.M.; Gomez-Sanchez, C.E.; Monteiro, M.P.; Pignatelli, D. Incomplete Pattern of Steroidogenic Protein Expression in Functioning Adrenocortical Carcinomas. Biomedicines 2020, 8, 256. https://doi.org/10.3390/biomedicines8080256
Pereira SS, Costa MM, Gomez-Sanchez CE, Monteiro MP, Pignatelli D. Incomplete Pattern of Steroidogenic Protein Expression in Functioning Adrenocortical Carcinomas. Biomedicines. 2020; 8(8):256. https://doi.org/10.3390/biomedicines8080256
Chicago/Turabian StylePereira, Sofia S., Madalena M. Costa, Celso E. Gomez-Sanchez, Mariana P. Monteiro, and Duarte Pignatelli. 2020. "Incomplete Pattern of Steroidogenic Protein Expression in Functioning Adrenocortical Carcinomas" Biomedicines 8, no. 8: 256. https://doi.org/10.3390/biomedicines8080256
APA StylePereira, S. S., Costa, M. M., Gomez-Sanchez, C. E., Monteiro, M. P., & Pignatelli, D. (2020). Incomplete Pattern of Steroidogenic Protein Expression in Functioning Adrenocortical Carcinomas. Biomedicines, 8(8), 256. https://doi.org/10.3390/biomedicines8080256





