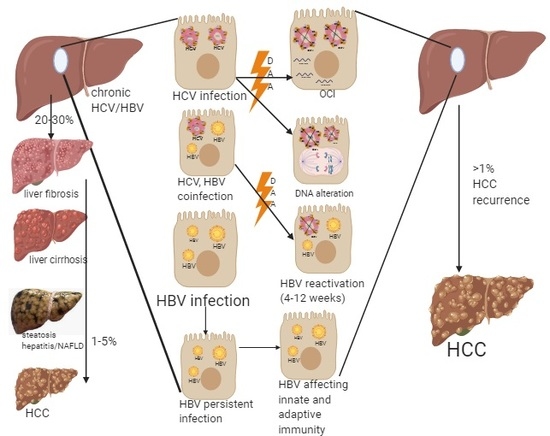
Risk Factors Contributing to the Occurrence and Recurrence of Hepatocellular Carcinoma in Hepatitis C Virus Patients Treated with Direct-Acting Antivirals
Abstract
1. Introduction
2. Possible Risk Factors for HCC Development
2.1. Risk of DNA Methylation in the Progression of HCC
2.2. Occult HCV
2.3. Immunity and Cytokines
2.3.1. HCV
2.3.2. HBV
2.4. Liver Cirrhosis and DNA Alteration
2.4.1. HBV
2.4.2. HCV
3. Patients with HCV, HBV Hepatitis and Without Liver Diseases Progression
4. Patients with HCV or/and HBV Hepatitis with Liver Diseases Progression
5. Patients with Non-Viral Hepatitis with Liver Diseases Progression
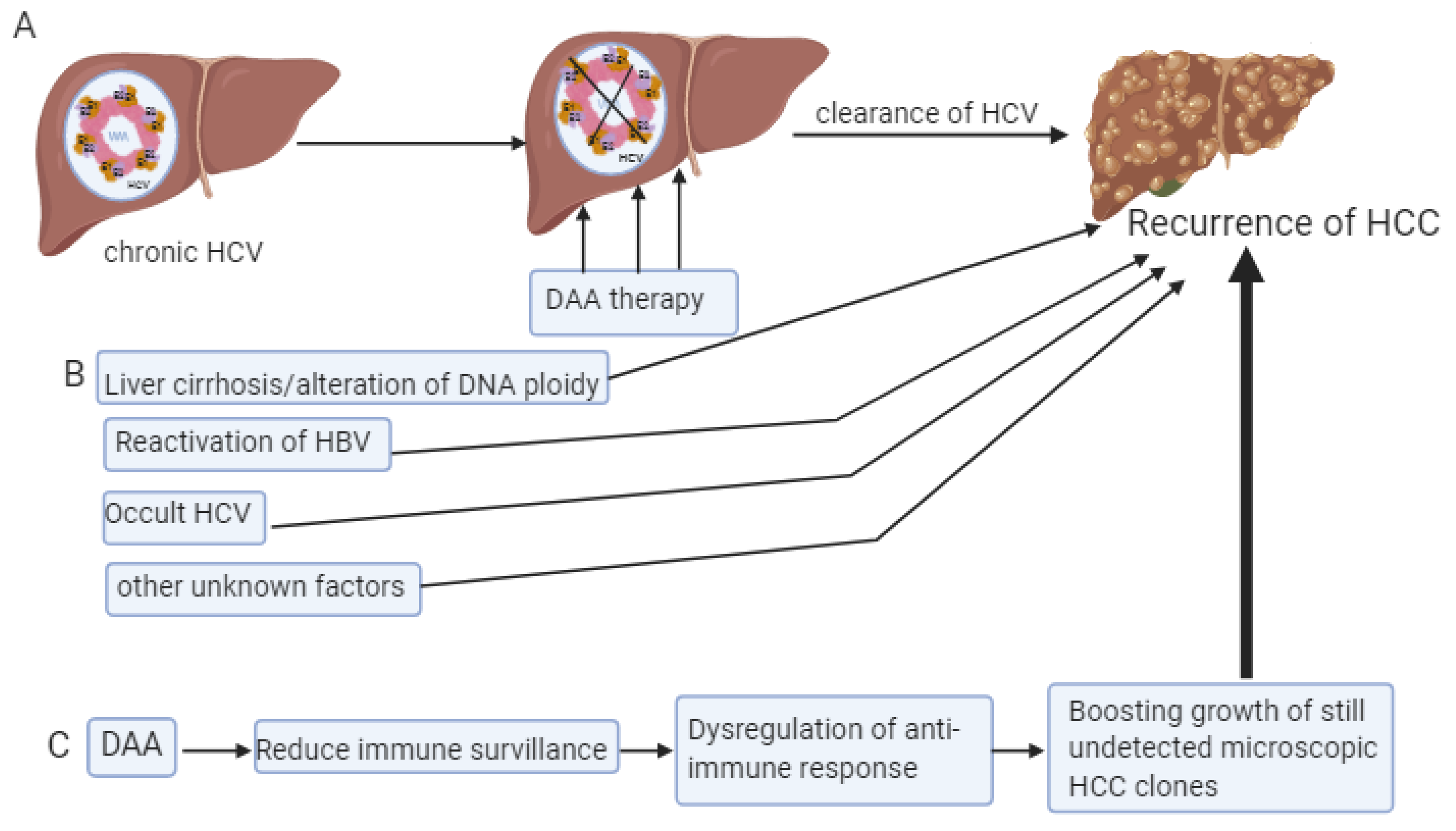
6. Recurrence of HCC Despite the DAA Therapy
7. Other Factors
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Serag, H.B.; Kramer, J.R.; Chen, G.J.; Duan, Z.; Richardson, P.A.; Davila, J.A. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut 2011, 60, 992–997. [Google Scholar] [CrossRef]
- Von Köckritz, L.; Dufour, J.F. Management of chronic hepatitis C in 2017. Hamostaseologie 2017, 37, 186–195. [Google Scholar] [CrossRef]
- Aly, A.M.; Adel, A.; El-Gendy, A.O.; Essam, T.M.; Aziz, R.K. Gut microbiome alterations in patients with stage 4 hepatitis C. Gut Pathog. 2016, 8, 42. [Google Scholar] [CrossRef]
- Bandiera, S.; Billie Bian, C.; Hoshida, Y.; Baumert, T.F.; Zeisel, M.B. Chronic hepatitis C virus infection and pathogenesis of hepatocellular carcinoma. Curr. Opin. Virol. 2016, 20, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.H.; Thimme, R. Innate and adaptive immune responses in HCV infections. J. Hepatol. 2014, 61, S14–S25. [Google Scholar] [CrossRef] [PubMed]
- Aziz, B.; Nazar, T.; Akhlaq, S. The frequency of occurrence of Hepatocellular Carcinoma after direct antiviral therapy in Hepatitis C virus patients. Pak. J. Med. Sci. 2019, 35, 101–105. [Google Scholar] [CrossRef]
- Kishta, S.S.; Kishta, S.A.; El-Shenawy, R. Statin (3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor)-based therapy for hepatitis C virus (HCV) infection-related diseases in the era of direct-acting antiviral agents. F1000Research 2016, 5, 223. [Google Scholar] [CrossRef]
- Gigi, E.; Lagopoulos, V.I.; Bekiari, E. Hepatocellular carcinoma occurrence in DAA-treated hepatitis C virus patients: Correlated or incidental? A brief review. World J. Hepatol. 2018, 10, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hanafiah, K.; Groeger, J.; Flaxman, A.D.; Wiersma, S.T. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013, 57, 1333–1342. [Google Scholar] [CrossRef]
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61, S45–S57. [Google Scholar] [CrossRef]
- Perz, J.F.; Armstrong, G.L.; Farrington, L.A.; Hutin, Y.J.; Bell, B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006, 45, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Shiha, G.; Soliman, R.; Mikhail, N.N.H.; Easterbrook, P. An educate, test and treat model towards elimination of hepatitis C infection in Egypt: Feasibility and effectiveness in 73 villages. J. Hepatol. 2020, 72, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, A.; Genedy, M.; El-Refai, S.; Funk, A.L.; Fontanet, A.; Talaat, M. The prevalence of hepatitis C virus infection in Egypt 2015: implications for future policy on prevention and treatment. Liver Int. 2017, 37, 45–53. [Google Scholar] [CrossRef]
- El Batae, H.; Amer, I.; Kobtan, A.; Saied, S.M.; Ghazy, A.; Elkalla, F.; El Sharawy, S. Seroprevalence of hepatitis C virus among the newcomer students, Kafrelsheikh University, Egypt. J. Med. Virol. 2018, 90, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Breban, R.; Doss, W.; Esmat, G.; Elsayed, M.; Hellard, M.; Ayscue, P.; Albert, M.; Fontanet, A.; Mohamed, M.K. Towards realistic estimates of HCV incidence in Egypt. J. Viral Hepat. 2013, 20, 294–296. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis C. Available online: http://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 12 May 2020).
- Chonprasertsuk, S.; Vilaichone, R.K. Epidemiology and treatment of hepatocellular carcinoma in Thailand. Jpn. J. Clin. Oncol. 2017, 47, 294–297. [Google Scholar] [CrossRef][Green Version]
- Ferlay, J.; Randi, G.; Bosetti, C.; Levi, F.; Negri, E.; Boyle, P.; La Vecchia, C. Declining mortality from bladder cancer in Europe. BJU Int. 2008, 101, 11–19. [Google Scholar] [CrossRef]
- Matsuda, F.; Torii, Y.; Enomoto, H.; Kuga, C.; Aizawa, N.; Iwata, Y.; Saito, M.; Imanishi, H.; Shimomura, S.; Nakamura, H.; et al. Anti-interferon-α neutralizing antibody is associated with nonresponse to pegylated interferon-α plus ribavirin in chronic hepatitis C. J. Viral Hepat. 2012, 19, 694–703. [Google Scholar] [CrossRef]
- Altekruse, S.F.; McGlynn, K.A.; Reichman, M.E. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009, 27, 1485–1491. [Google Scholar] [CrossRef]
- Yang, X.; Shi, J.; Chen, X.; Jiang, Y.; Zhao, H. Efficacy of Cabozantinib and Nivolumab in Treating Hepatocellular Carcinoma with RET Amplification, High Tumor Mutational Burden, and PD-L1 Expression. Oncologist 2020. [Google Scholar] [CrossRef]
- Budny, A.; Kozłowski, P.; Kamińska, M.; Jankiewicz, M.; Kolak, A.; Budny, B.; Budny, W.; Niemunis-Sawicka, J.; Szczypiór, G.; Kurniawka, B.; et al. Epidemiology and risk factors of hepatocellular carcinoma. Pol Merkur Lek. 2017, 43, 133–139. [Google Scholar]
- Waziry, R.; Gomaa, A.; Waked, I.; Dore, G.J. Determinants of survival following hepatocellular carcinoma in Egyptian patients with untreated chronic HCV infection in the pre-DAA era. Arab J. Gastroenterol. 2018, 19, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, Y.; Fuchs, B.C.; Bardeesy, N.; Baumert, T.F.; Chung, R.T. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J. Hepatol. 2014, 61, S79–S90. [Google Scholar] [CrossRef] [PubMed]
- Pisaturo, M.; Macera, M.; Alessio, L.; Calò, F.; Coppola, N. Hepatitis B Virus (HBV) Reactivation Following Pharmacological Eradication of Hepatitis C Virus (HCV). Viruses 2019, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yu, M.L. Unmet needs of chronic hepatitis C in the era of direct-acting antiviral therapy. Clin. Mol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Kanda, T.; Lau, G.K.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL HCV guidelines of virus-eradicated patients by DAA on how to monitor HCC occurrence and HBV reactivation. Hepatol. Int. 2019, 13, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Serper, M.; Forde, K.A.; Kaplan, D.E. Rare clinically significant hepatic events and hepatitis B reactivation occur more frequently following rather than during direct-acting antiviral therapy for chronic hepatitis C: Data from a national US cohort. J. Viral Hepat. 2018, 25, 187–197. [Google Scholar] [CrossRef]
- Pawłowska, M.; Flisiak, R.; Gil, L.; Horban, A.; Hus, I.; Jaroszewicz, J.; Lech-Marańda, E.; Styczyński, J. Prophylaxis of hepatitis B virus (HBV) infection reactivation - recommendations of the Working Group for prevention of HBV reactivation. Clin. Exp. Hepatol. 2019, 5, 195–202. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131.e1. [Google Scholar] [CrossRef]
- Okamoto, Y.; Shinjo, K.; Shimizu, Y.; Sano, T.; Yamao, K.; Gao, W.; Fujii, M.; Osada, H.; Sekido, Y.; Murakami, S.; et al. Hepatitis virus infection affects DNA methylation in mice with humanized livers. Gastroenterology 2014, 146, 562–572. [Google Scholar] [CrossRef]
- Nishida, N.; Nagasaka, T.; Nishimura, T.; Ikai, I.; Boland, C.R.; Goel, A. Aberrant methylation of multiple tumor suppressor genes in aging liver, chronic hepatitis, and hepatocellular carcinoma. Hepatology 2008, 47, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.Y.; Zhang, X.X.; Hou, G.; Jin, G.D.; Deng, Q.; Kong, X.F.; Zhang, D.H.; Ling, Y.; Yu, D.M.; Gong, Q.M.; et al. Assessment of specific antibodies to F protein in serum samples from Chinese hepatitis C patients treated with interferon plus ribavarin. J. Clin. Microbiol. 2008, 46, 3746–3751. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Hiramatsu, N.; Mizuki, M.; Nagatomo, I.; Kida, H.; Tazumi, K.; Shinzaki, S.; Miyazaki, M.; Yakushijin, T.; Tatsumi, T.; et al. Managing hepatitis B virus carriers with systemic chemotherapy or biologic therapy in the outpatient clinic. Hepatol. Res. 2013, 43, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Toraih, E.A.; Ellawindy, A.; Fala, S.Y.; Al Ageeli, E.; Gouda, N.S.; Fawzy, M.S.; Hosny, S. Oncogenic long noncoding RNA MALAT1 and HCV-related hepatocellular carcinoma. Biomed. Pharm. 2018, 102, 653–669. [Google Scholar] [CrossRef]
- Kuramoto, J.; Arai, E.; Tian, Y.; Funahashi, N.; Hiramoto, M.; Nammo, T.; Nozaki, Y.; Takahashi, Y.; Ito, N.; Shibuya, A.; et al. Genome-wide DNA methylation analysis during non-alcoholic steatohepatitis-related multistage hepatocarcinogenesis: comparison with hepatitis virus-related carcinogenesis. Carcinogenesis 2017, 38, 261–270. [Google Scholar] [CrossRef]
- Austria, A.; Wu, G.Y. Occult Hepatitis C Virus Infection: A Review. J. Clin. Transl. Hepatol. 2018, 6, 155–160. [Google Scholar] [CrossRef]
- Yousif, M.M.; Elsadek Fakhr, A.; Morad, E.A.; Kelani, H.; Hamed, E.F.; Elsadek, H.M.; Zahran, M.H.; Fahmy Afify, A.; Ismail, W.A.; Elagrody, A.I.; et al. Prevalence of occult hepatitis C virus infection in patients who achieved sustained virologic response to direct-acting antiviral agents. Infez. Med. 2018, 26, 237–243. [Google Scholar]
- Manickam, C.; Reeves, R.K. Silent damage? Occult HCV replication and histological disease may occur following apparent HCV clearance. EBioMedicine 2019, 47, 12–13. [Google Scholar] [CrossRef]
- Roche, B.; Coilly, A.; Duclos-Vallee, J.C.; Samuel, D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018, 38 (Suppl. 1), 139–145. [Google Scholar] [CrossRef]
- El Kassas, M.; Shimakawa, Y.; Ali-Eldin, Z.; Funk, A.L.; Wifi, M.N.; Zaky, S.; El-Raey, F.; Esmat, G.; Fontanet, A. Risk of hepatitis B virus reactivation with direct-acting antivirals against hepatitis C virus: A cohort study from Egypt and meta-analysis of published data. Liver Int. 2018, 38, 2159–2169. [Google Scholar] [CrossRef]
- Warzyszyńska, K.; Jonas, M.; Wasiak, D.; Kosieradzki, M.; Małkowski, P. Accelerated hepatocellular carcinoma recurrence rate after postoperative direct-acting antivirals treatment—Preliminary report. Clin. Exp. Hepatol. 2017, 3, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Hengst, J.; Strunz, B.; Deterding, K.; Ljunggren, H.G.; Leeansyah, E.; Manns, M.P.; Cornberg, M.; Sandberg, J.K.; Wedemeyer, H.; Björkström, N.K. Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur. J. Immunol. 2016, 46, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Serti, E.; Park, H.; Keane, M.; O’Keefe, A.C.; Rivera, E.; Liang, T.J.; Ghany, M.; Rehermann, B. Rapid decrease in hepatitis C viremia by direct acting antivirals improves the natural killer cell response to IFNα. Gut 2017, 66, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Yoneda, Y.; Kuriyama, M.; Kubota, T. IFN-gamma- and cell-to-cell contact-dependent cytotoxicity of allograft-induced macrophages against syngeneic tumor cells and cell lines: an application of allografting to cancer treatment. J. Immunol. 1999, 163, 148–154. [Google Scholar]
- Cardoso, A.C.; Moucari, R.; Figueiredo-Mendes, C.; Ripault, M.P.; Giuily, N.; Castelnau, C.; Boyer, N.; Asselah, T.; Martinot-Peignoux, M.; Maylin, S.; et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J. Hepatol. 2010, 52, 652–657. [Google Scholar] [CrossRef]
- Hiramatsu, N.; Oze, T.; Takehara, T. Suppression of hepatocellular carcinoma development in hepatitis C patients given interferon-based antiviral therapy. Hepatol. Res. 2015, 45, 152–161. [Google Scholar] [CrossRef]
- Schietroma, I.; Scheri, G.C.; Pinacchio, C.; Statzu, M.; Petruzziello, A.; Vullo, V. Hepatitis C Virus and Hepatocellular Carcinoma: Pathogenetic Mechanisms and Impact of Direct-Acting Antivirals. Open Virol. J. 2018, 12, 16–25. [Google Scholar] [CrossRef]
- Toyoda, H.; Bregerie, O.; Vallet, A.; Nalpas, B.; Pivert, G.; Brechot, C.; Desdouets, C. Changes to hepatocyte ploidy and binuclearity profiles during human chronic viral hepatitis. Gut 2005, 54, 297–302. [Google Scholar] [CrossRef]
- Irshad, M.; Gupta, P.; Irshad, K. Immunopathogenesis of Liver Injury During Hepatitis C Virus Infection. Viral Immunol. 2019, 32, 112–120. [Google Scholar] [CrossRef]
- Lapa, D.; Garbuglia, A.R.; Capobianchi, M.R.; Del Porto, P. Hepatitis C Virus Genetic Variability, Human Immune Response, and Genome Polymorphisms: Which Is the Interplay? Cells 2019, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.R.; Depla, M.; Freije, C.A.; Gaucher, D.; Mazouz, S.; Boisvert, M.; Bédard, N.; Bruneau, J.; Rice, C.M.; Shoukry, N.H. Longitudinal transcriptomic characterization of the immune response to acute hepatitis C virus infection in patients with spontaneous viral clearance. PLoS Pathog. 2018, 14, e1007290. [Google Scholar] [CrossRef] [PubMed]
- Dustin, L.B. Innate and Adaptive Immune Responses in Chronic HCV Infection. Curr. Drug Targets 2017, 18, 826–843. [Google Scholar] [CrossRef]
- Barjon, C.; Dahlqvist, G.; Calmus, Y.; Conti, F. Role of regulatory T-cells during hepatitis C infection: From the acute phase to post-transplantation recurrence. Dig. Liver Dis. 2015, 47, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Villani, R.; Vendemiale, G.; Serviddio, G. Molecular Mechanisms Involved in HCC Recurrence after Direct-Acting Antiviral Therapy. Int. J. Mol. Sci. 2018, 20, 49. [Google Scholar] [CrossRef]
- Missale, G.; Pilli, M.; Zerbini, A.; Penna, A.; Ravanetti, L.; Barili, V.; Orlandini, A.; Molinari, A.; Fasano, M.; Santantonio, T.; et al. Lack of full CD8 functional restoration after antiviral treatment for acute and chronic hepatitis C virus infection. Gut 2012, 61, 1076–1084. [Google Scholar] [CrossRef]
- Caja, L.; Dituri, F.; Mancarella, S.; Caballero-Diaz, D.; Moustakas, A.; Giannelli, G.; Fabregat, I. TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 1294. [Google Scholar] [CrossRef]
- Alter, H.J.; Chisari, F.V. Is Elimination of Hepatitis B and C a Pipe Dream or Reality? Gastroenterology 2019, 156, 294–296. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Z. HBV-Induced Immune Imbalance in the Development of HCC. Front. Immunol. 2019, 10, 2048. [Google Scholar] [CrossRef]
- An, P.; Xu, J.; Yu, Y.; Winkler, C.A. Host and Viral Genetic Variation in HBV-Related Hepatocellular Carcinoma. Front. Genet. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, F.; Belloni, L.; Pediconi, N.; Levrero, M. Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin. Liver Dis. 2013, 33, 147–156. [Google Scholar] [CrossRef]
- Gramantieri, L.; Melchiorri, C.; Chieco, P.; Gaiani, S.; Stecca, B.; Casali, A.; Bolondi, L. Alteration of DNA ploidy and cell nuclearity in human hepatocellular carcinoma associated with HBV infection. J. Hepatol. 1996, 25, 848–853. [Google Scholar] [CrossRef]
- Wang, B.; Mufti, G.; Agarwal, K. Reactivation of hepatitis B virus infection in patients with hematologic disorders. Haematologica 2019, 104, 435–443. [Google Scholar] [CrossRef]
- Sohn, W.; Paik, Y.H.; Cho, J.Y.; Ahn, J.M.; Choi, G.S.; Kim, J.M.; Kwon, C.H.; Joh, J.W.; Sinn, D.H.; Gwak, G.Y.; et al. Influence of hepatitis B virus reactivation on the recurrence of HBV-related hepatocellular carcinoma after curative resection in patients with low viral load. J. Viral Hepat. 2015, 22, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, E.; D’Ambrosio, R.; Iavarone, M.; Sangiovanni, A.; Aghemo, A.; Soffredini, R.; Borghi, M.; Lunghi, G.; Colombo, M.; Lampertico, P. Factors Associated With Increased Risk of De Novo or Recurrent Hepatocellular Carcinoma in Patients With Cirrhosis Treated With Direct-Acting Antivirals for HCV Infection. Clin. Gastroenterol. Hepatol. 2018. [Google Scholar] [CrossRef]
- Suit, P.F.; Bauer, T.W. DNA quantitation by image cytometry of touch preparations from fresh and frozen tissue. Am. J. Clin. Pathol. 1990, 94, 49–53. [Google Scholar] [CrossRef]
- Linder, M.E.; Ewald, D.A.; Miller, R.J.; Gilman, A.G. Purification and characterization of Go alpha and three types of Gi alpha after expression in Escherichia coli. J. Biol. Chem. 1990, 265, 8243–8251. [Google Scholar]
- El-Sayed, S.S.; El-Sadany, M.; Tabll, A.A.; Soltan, A.; El-Dosoky, I.; Attallah, A.M. DNA ploidy and liver cell dysplasia in liver biopsies from patients with liver cirrhosis. Can. J. Gastroenterol. 2004, 18, 87–91. [Google Scholar] [CrossRef]
- Sanad, S.M.; Mangoud, A.M.; Shalabi, A.A.; Saber, M.; Fouad, M.A. DNA ploidy and S-phase fraction in the patients of chronic HCV and hepatocellular carcinomas. J. Egypt Soc. Parasitol. 2004, 34, 483–500. [Google Scholar]
- Danque, P.O.; Chen, H.B.; Patil, J.; Jagirdar, J.; Orsatti, G.; Paronetto, F. Image analysis versus flow cytometry for DNA ploidy quantitation of solid tumors: a comparison of six methods of sample preparation. Mod. Pathol. 1993, 6, 270–275. [Google Scholar] [PubMed]
- Attallah, A.M.; Tabll, A.A.; El-Nashar, E.; El-Bakry, K.A.; El-Sadany, M.; Ibrahim, T.; El-Dosoky, I. AgNORs count and DNA ploidy in liver biopsies from patients with schistosomal liver cirrhosis and hepatocellular carcinoma. Clin. Biochem. 2009, 42, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Wied, G.L.; Bartels, P.H.; Bibbo, M.; Dytch, H.E. Image analysis in quantitative cytopathology and histopathology. Hum. Pathol. 1989, 20, 549–571. [Google Scholar] [CrossRef]
- Tabll, A.A.; Kishta, S.S.; Farrag, A.R.; El Din, N.G.; Mohamed, M.S.; El Abd, Y.S.; El Esawy, B.H.; Kishta, S.A.; Dawood, R.M.; Ismail, A.; et al. Alteration of the Total Nuclear DNA Ploidy in Different Histopathological Liver Tissues Negative and Positive for HCV RNA. Clin. Lab. 2015, 61, 1247–1256. [Google Scholar] [CrossRef]
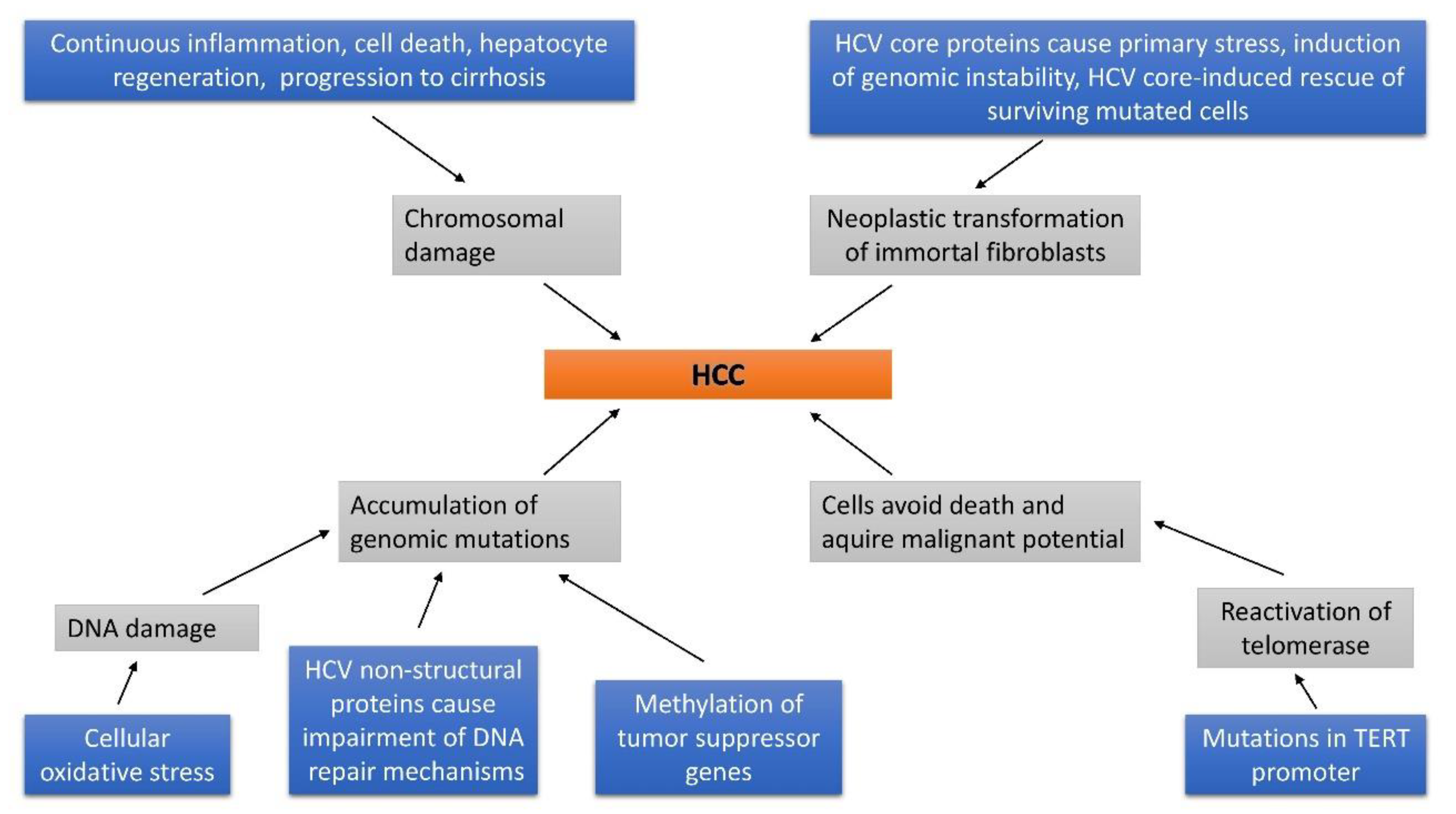
- Ke, P.Y.; Chen, S.S. Hepatitis C virus and cellular stress response: implications to molecular pathogenesis of liver diseases. Viruses 2012, 4, 2251–2290. [Google Scholar] [CrossRef]
- Machida, K.; Liu, J.C.; McNamara, G.; Levine, A.; Duan, L.; Lai, M.M. Hepatitis C virus causes uncoupling of mitotic checkpoint and chromosomal polyploidy through the Rb pathway. J. Virol. 2009, 83, 12590–12600. [Google Scholar] [CrossRef]
- Werling, K.; Szepesi, A.; Szentirmay, Z.; Schaff, Z.; Tulassay, Z.; Szalay, F. Effect of hepatitis C virus on hepatocyte proliferation and DNA ploidy in patients with chronic hepatitis C. Z Gastroenterol. 2000, 38, 553–554, 556–558. [Google Scholar] [CrossRef]
- Smirnova, I.S.; Aksenov, N.D.; Kashuba, E.V.; Payakurel, P.; Grabovetsky, V.V.; Zaberezhny, A.D.; Vonsky, M.S.; Buchinska, L.; Biberfeld, P.; Hinkula, J.; et al. Hepatitis C virus core protein transforms murine fibroblasts by promoting genomic instability. Cell Oncol. 2006, 28, 177–190. [Google Scholar]
- Suhail, M.; Sohrab, S.S.; Qureshi, A.; Tarique, M.; Abdel-Hafiz, H.; Al-Ghamdi, K.; Qadri, I. Association of HCV mutated proteins and host SNPs in the development of hepatocellular carcinoma. Infect. Genet. Evol. 2018, 60, 160–172. [Google Scholar] [CrossRef]
- Esaki, T.; Suzuki, N.; Yokoyama, K.; Iwata, K.; Irie, M.; Anan, A.; Nakane, H.; Yoshikane, M.; Nishizawa, S.; Ueda, S.; et al. Hepatocellular carcinoma in a patient with liver cirrhosis associated with negative serum HCV tests but positive liver tissue HCV RNA. Intern. Med. 2004, 43, 279–282. [Google Scholar] [CrossRef]
- Das, U.; Kar, P.; Gopalkrishna, V.; Sharma, J.K.; Madan, K.; Das, B.C. Comparative evaluation of hepatitis C virus infection in serum and liver tissue of patients with chronic liver disease by reverse transcription-polymerase chain reaction. Clin. Microbiol. Infect. 1999, 5, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.K.; Jeng, K.S.; Machida, K.; Cheng, Y.S.; Lai, M.M. Hepatitis C virus NS3/4A protein interacts with ATM, impairs DNA repair and enhances sensitivity to ionizing radiation. Virology 2008, 370, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Machida, K.; McNamara, G.; Cheng, K.T.; Huang, J.; Wang, C.H.; Comai, L.; Ou, J.H.; Lai, M.M. Hepatitis C virus inhibits DNA damage repair through reactive oxygen and nitrogen species and by interfering with the ATM-NBS1/Mre11/Rad50 DNA repair pathway in monocytes and hepatocytes. J. Immunol. 2010, 185, 6985–6998. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Polyak, S.J.; Bano, N.; Qiu, W.C.; Carithers, R.L.; Shuhart, M.; Gretch, D.R.; Das, A. Hepatitis C virus induces oxidative stress, DNA damage and modulates the DNA repair enzyme NEIL1. J. Gastroenterol. Hepatol. 2010, 25, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Sugimoto, R.; Ma, N.; Tanaka, H.; Iwasa, M.; Kobayashi, Y.; Kawanishi, S.; Watanabe, S.; Kaito, M.; Takei, Y. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. J. Viral Hepat. 2008, 15, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.; Allam, N.; Elsharkawy, A.; El Kassas, M.; Waked, I. Hepatitis C infection in Egypt: prevalence, impact and management strategies. Hepat. Med. 2017, 9, 17–25. [Google Scholar] [CrossRef]
- Gehrau, R.C.; Archer, K.J.; Mas, V.R.; Maluf, D.G. Molecular profiles of HCV cirrhotic tissues derived in a panel of markers with clinical utility for hepatocellular carcinoma surveillance. PLoS ONE 2012, 7, e40275. [Google Scholar] [CrossRef]
- Saad, Y.; Awad, A.; Alakel, W.; Doss, W.; Awad, T.; Mabrouk, M. Data mining of routine laboratory tests can predict liver disease progression in Egyptian diabetic patients with hepatitis C virus (G4) infection: A cohort study of 71 806 patients. Eur. J. Gastroenterol. Hepatol. 2018, 30, 201–206. [Google Scholar] [CrossRef]
- Milovanova, S.Y.; Lysenko Kozlovskaya, L.V.; Milovanova, L.Y.; Mrykhin, N.N.; Russkih, A.V.; Muchin, N.A. HCV-associated mixed cryoglobulinemia and b-cell non-Hodgkin’s lymphoma—Pathogenetically related problems. Ter Arkh 2018, 90, 112–120. [Google Scholar] [CrossRef]
- Bartosiewicz, A.J.; Mikuła, T. Hepatocellular carcinoma after direct-acting antivirals: an unresolved problem. Review of five cases. Clin. Exp. Hepatol. 2019, 5, 88–92. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Asch, S.M.; Cao, Y.; Li, L.; El-Serag, H.B. Long-Term Risk of Hepatocellular Carcinoma in HCV Patients Treated With Direct Acting Antiviral Agents. Hepatology 2020, 71, 44–55. [Google Scholar] [CrossRef]
- Takeda, H.; Takai, A.; Inuzuka, T.; Marusawa, H. Genetic basis of hepatitis virus-associated hepatocellular carcinoma: linkage between infection, inflammation, and tumorigenesis. J. Gastroenterol. 2017, 52, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Furusyo, N.; Nakamuta, M.; Kajiwara, E.; Nomura, H.; Dohmen, K.; Takahashi, K.; Satoh, T.; Azuma, K.; Kawano, A.; et al. Telaprevir-based triple therapy for chronic hepatitis C patients with advanced fibrosis: a prospective clinical study. Aliment. Pharmacol. Ther. 2013, 38, 1076–1085. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chacko, S.; Samanta, S. Hepatocellular carcinoma: A life-threatening disease. Biomed. Pharm. 2016, 84, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef]
- Donato, M.F.; Arosio, E.; Monti, V.; Fasani, P.; Prati, D.; Sangiovanni, A.; Ronchi, G.; Colombo, M. Proliferating cell nuclear antigen assessed by a computer-assisted image analysis system in patients with chronic viral hepatitis and cirrhosis. Dig. Liver Dis. 2002, 34, 197–203. [Google Scholar] [CrossRef]
| HBV/HCV Infection Without Cirrhosis/Fibrosis | HBV/HCV Infection With Cirrhosis/Fibrosis | Cirrhosis/Fibrosis Without HBV/HCV Infection | ||
|---|---|---|---|---|
| HCV | Core | mitotic checkpoint induces Polyploidy leading to neoplastic transformation [77] | cofactor-induced chronicity and cirrhosis [78] | - |
| neoplastic transformation-driven Genomic instability, due to preservation of surviving mutated cells and initiation of primary stress [79] | long term expression of HCV core protein transforms immortal fibroblasts leading to HCC [79] | - | ||
| firm correlation with SNP within NS and NS5A leads to HCC [80] | - | - | ||
| RNA | 50% positive sera and 69% positive in tissue | - | - | |
| DNA content | significant amount of HCV RNA in the liver without DNA ploidy [75] | - | Alteration in DNA ploidy: 80% in HCC, 0% in cirrhotic, 20% in fibrotic and 4% in normal participants [75] | |
| 80% more tetra and aneuploidy as compared to HCV-negative-cirrhotic and fibrotic liver [75] | - | - | ||
| HBV and HCV | - | mononuclear DNA polyploidy in hepatocyte increases with fibrosis | - | |
| S phase fraction | - | - | Increased in cirrhosis and chronic liver disease [71] | |
| - | - | significantly decreases in HCV compared to chronic non- HCV | ||
| Cyclin A | - | continuous inflammation | significant decrease in HCV than chronic non-HCV | |
| - | Causes chromosomal damage leading to HCC | |||
| Inflammation | - | hepatocyte regeneration | - | |
| - | cell death | - | ||
| - | Non-cancerous HCV cirrhotic accelerate HCC | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishta, S.; Tabll, A.; Omanovic Kolaric, T.; Smolic, R.; Smolic, M. Risk Factors Contributing to the Occurrence and Recurrence of Hepatocellular Carcinoma in Hepatitis C Virus Patients Treated with Direct-Acting Antivirals. Biomedicines 2020, 8, 175. https://doi.org/10.3390/biomedicines8060175
Kishta S, Tabll A, Omanovic Kolaric T, Smolic R, Smolic M. Risk Factors Contributing to the Occurrence and Recurrence of Hepatocellular Carcinoma in Hepatitis C Virus Patients Treated with Direct-Acting Antivirals. Biomedicines. 2020; 8(6):175. https://doi.org/10.3390/biomedicines8060175
Chicago/Turabian StyleKishta, Sara, Ashraf Tabll, Tea Omanovic Kolaric, Robert Smolic, and Martina Smolic. 2020. "Risk Factors Contributing to the Occurrence and Recurrence of Hepatocellular Carcinoma in Hepatitis C Virus Patients Treated with Direct-Acting Antivirals" Biomedicines 8, no. 6: 175. https://doi.org/10.3390/biomedicines8060175
APA StyleKishta, S., Tabll, A., Omanovic Kolaric, T., Smolic, R., & Smolic, M. (2020). Risk Factors Contributing to the Occurrence and Recurrence of Hepatocellular Carcinoma in Hepatitis C Virus Patients Treated with Direct-Acting Antivirals. Biomedicines, 8(6), 175. https://doi.org/10.3390/biomedicines8060175