8-Hydroxydaidzein, an Isoflavone from Fermented Soybean, Induces Autophagy, Apoptosis, Differentiation, and Degradation of Oncoprotein BCR-ABL in K562 Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of 8-Hydroxydaidzein (8-OHD, 7,8,4′-trihydoxyisoflavone, NSC-678112)
2.2. Cell Culture
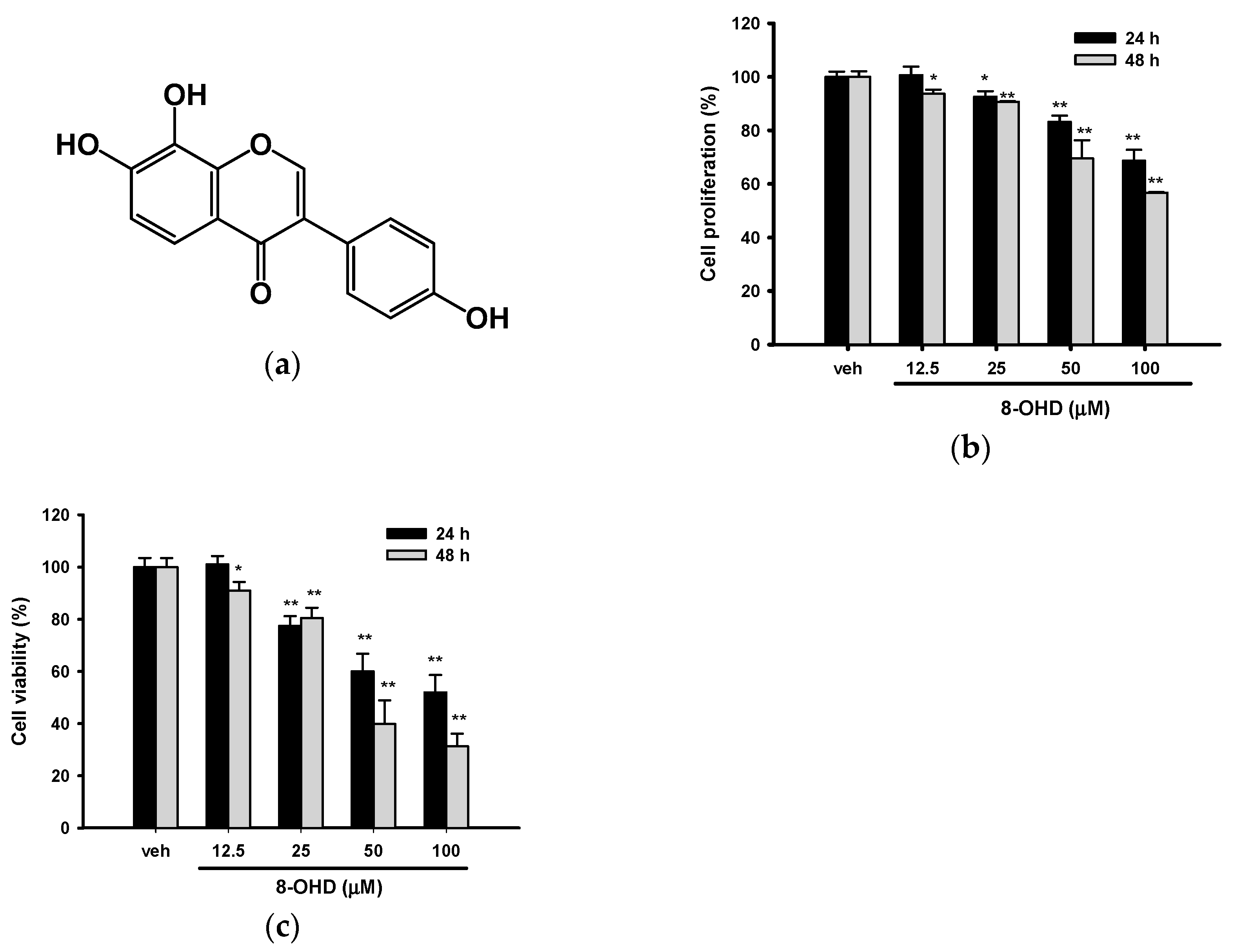
2.3. Cell Proliferation and Viability Analysis
2.4. Cell Cycle Analysis
2.5. Intracellular Reactive Oxygen Species (ROS) Assay
2.6. Western Blot Analysis
2.7. RNA Extraction and Reverse Transcription Quantitative PCR
2.8. Analysis of Cell Morphology
2.9. Microarray Analysis
2.10. Gene Ontology, KEGG, and Biocarta Pathways and Protein–Protein Interaction Analysis
2.11. Pathway Enrichment and Process Network Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. 8-OHD Reduces the Proliferation and Viability of K562 Cells
3.2. 8-OHD Causes Cell Cycle Arrest at S phase
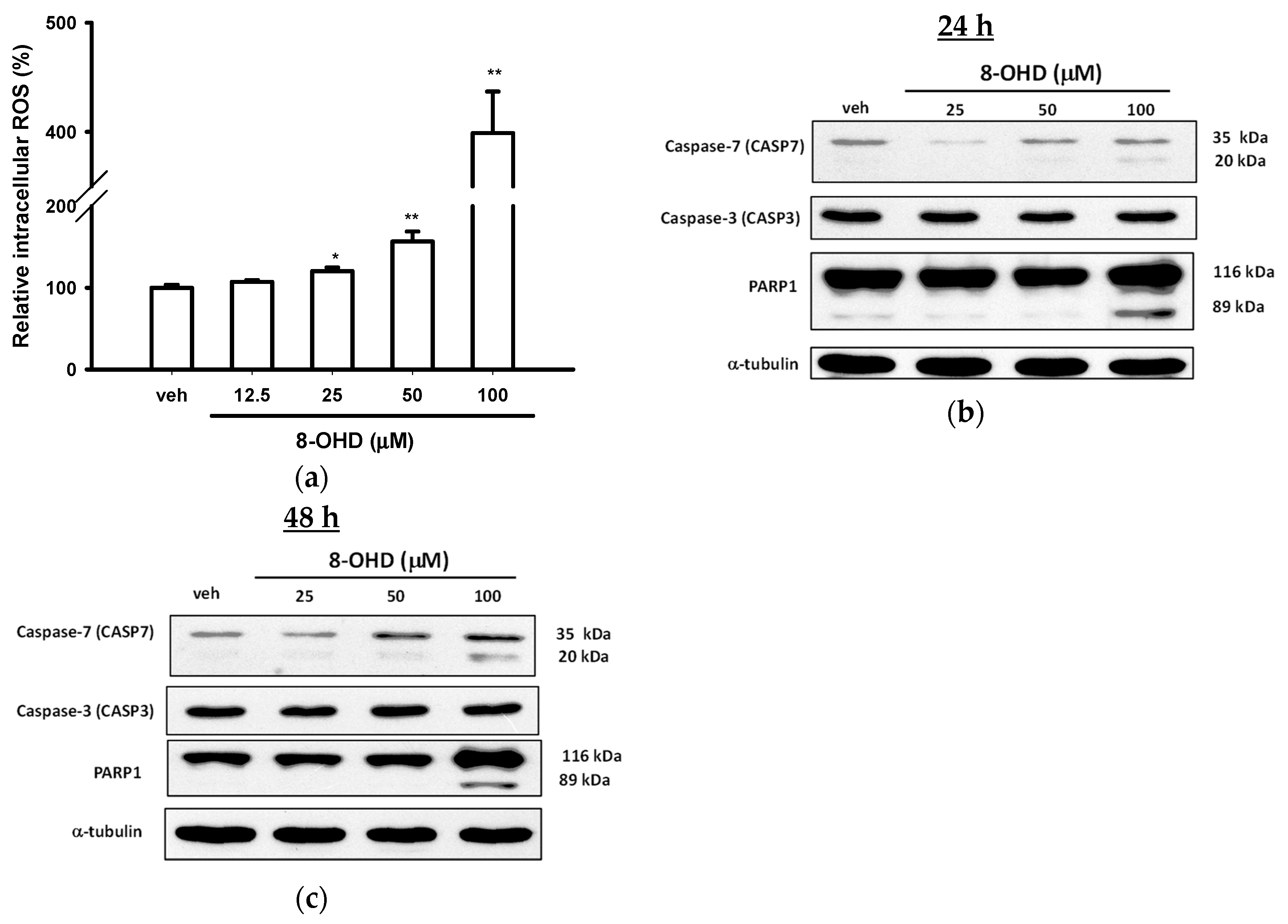
3.3. 8-OHD Causes ROS Production and Apoptosis in K562 Cells
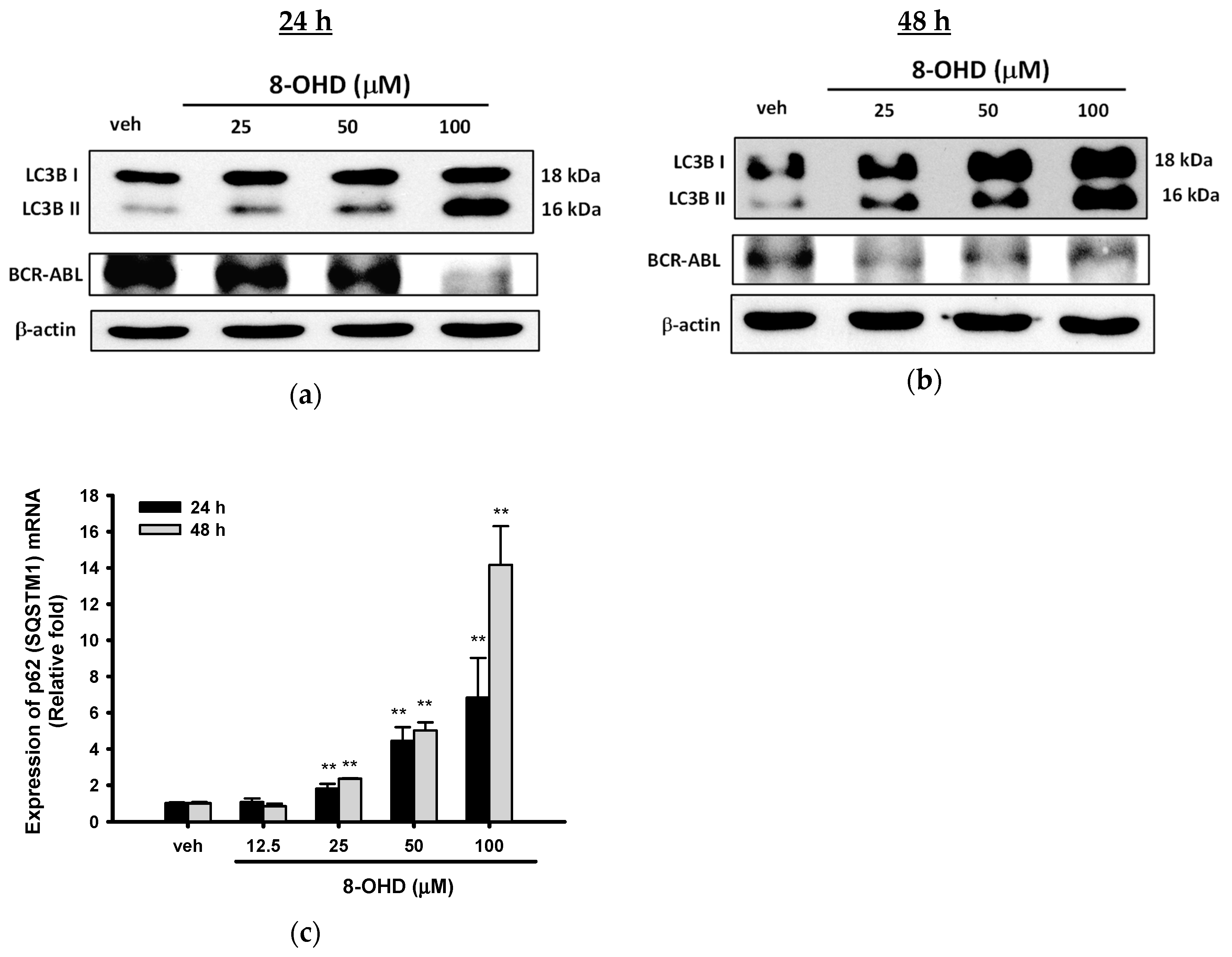
3.4. 8-OHD Causes Autophagy and Decreases BCR-ABL Oncoprotein in K562 Cells
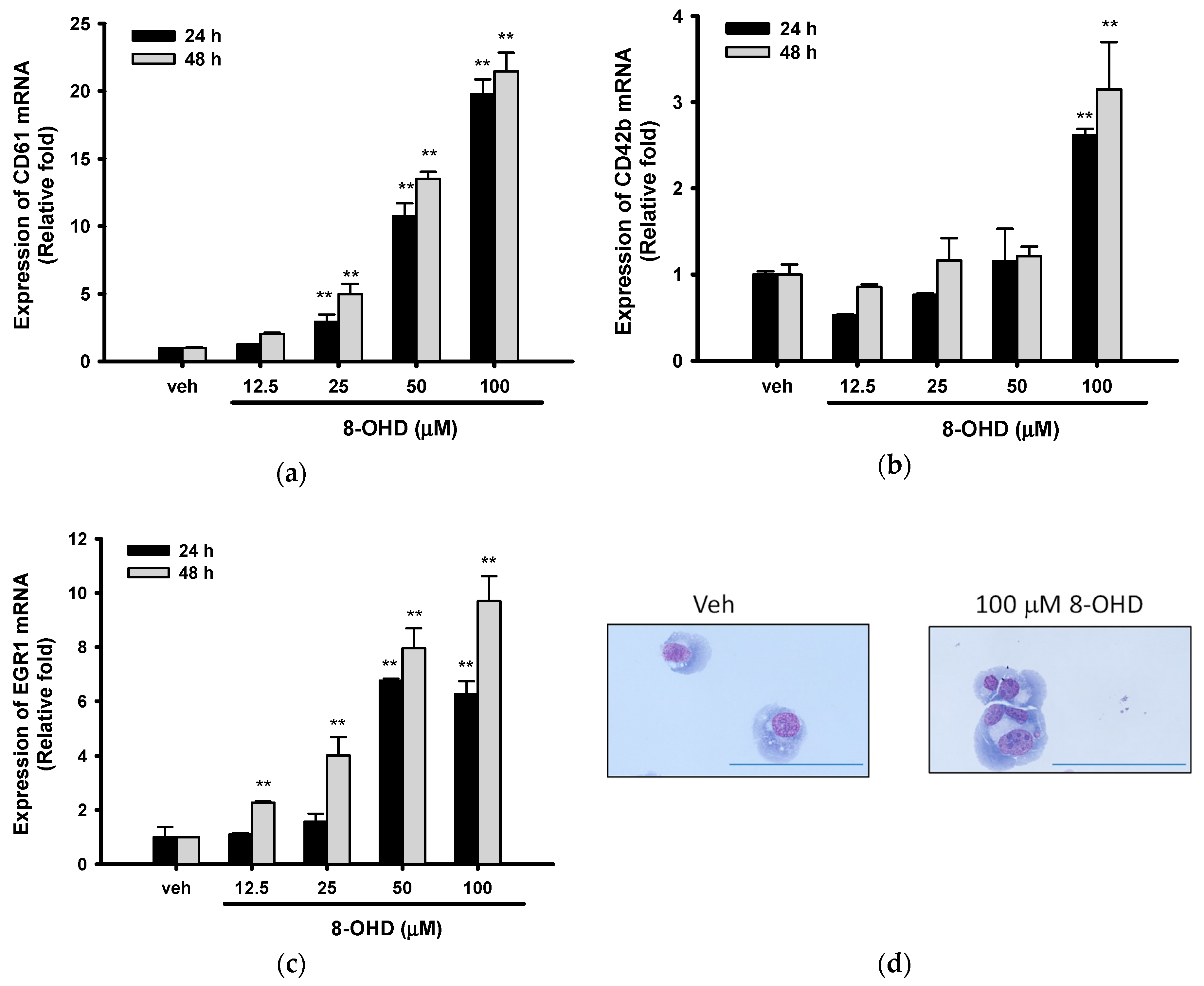
3.5. 8-OHD Promotes EGR1-Induced K562 Cell Differentiation
3.6. Analysis of 8-OHD-Modulated Gene Expression
3.7. Analysis of 8-OHD-Modulated Biological Processes
3.8. Analysis of Signaling Pathways and Network Processes Using KEGG, BioCarta, and MetaCore
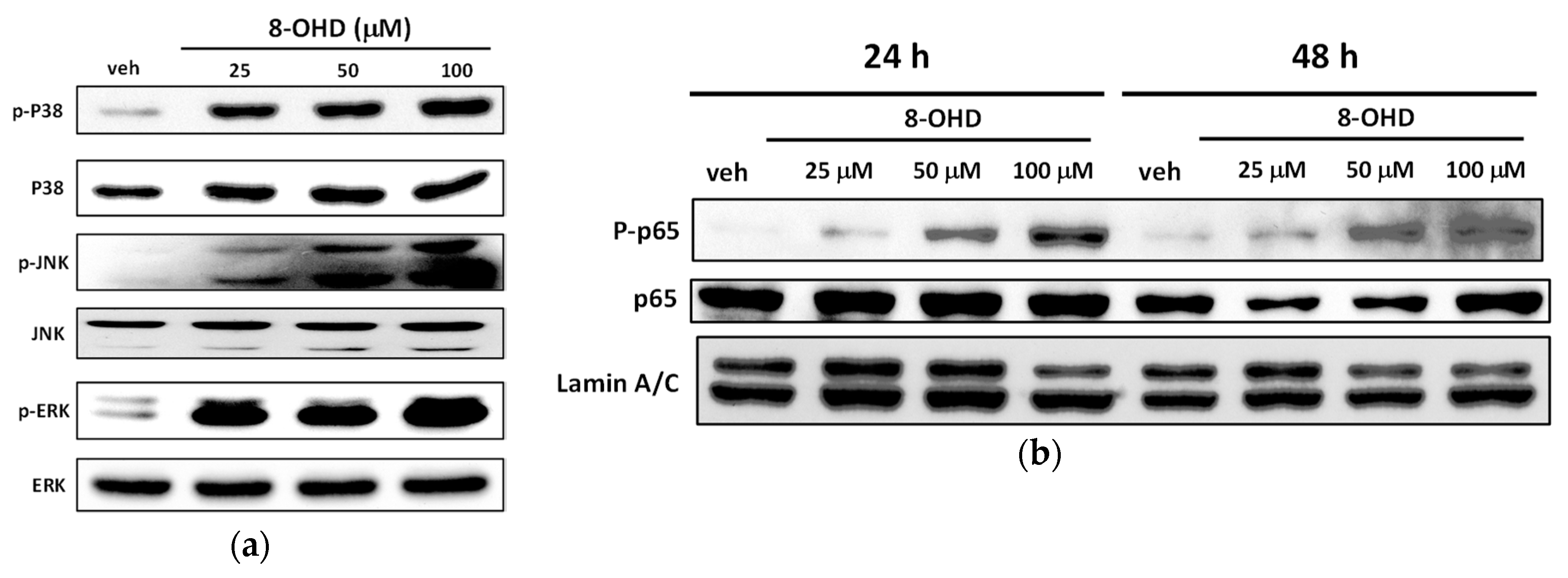
3.9. 8-OHD Modulates MAPK Pathways and NF-kB Activation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rowley, J.D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef]
- Jabbour, E.; Cortes, J.E.; Giles, F.J.; O’Brien, S.; Kantarjian, H.M. Current and emerging treatment options in chronic myeloid leukemia. Cancer 2007, 109, 2171–2181. [Google Scholar] [CrossRef]
- Westerweel, P.E.; Te Boekhorst, P.A.W.; Levin, M.D.; Cornelissen, J.J. New Approaches and Treatment Combinations for the Management of Chronic Myeloid Leukemia. Front. Oncol. 2019, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, B.C.; Possick, J.; Fradley, M. Cardiovascular, pulmonary, and metabolic toxicities complicating tyrosine kinase inhibitor therapy in chronic myeloid leukemia: Strategies for monitoring, detecting, and managing. Blood Rev. 2018, 32, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Q.; Gao, F.H. Regulatory Molecules and Corresponding Processes of BCR-ABL Protein Degradation. J. Cancer 2019, 10, 2488–2500. [Google Scholar] [CrossRef] [PubMed]
- Ablain, J.; Nasr, R.; Bazarbachi, A.; de The, H. The drug-induced degradation of oncoproteins: An unexpected Achilles’ heel of cancer cells? Cancer Discov. 2011, 1, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, J.H.; Sun, X.Y.; Liu, J.X.; Zhang, Q.Y.; Liu, P.; Huang, Q.H.; Li, K.K.; Chen, Q.; Chen, Z.; Chen, S.J. As4S4 targets RING-type E3 ligase c-CBL to induce degradation of BCR-ABL in chronic myelogenous leukemia. Proc. Natl. Acad. Sci. USA 2010, 107, 21683–21688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Weng, H.; Dong, B.; Zhao, P.; Zhou, H.; Qu, L. Oridonin Triggers Chaperon-mediated Proteasomal Degradation of BCR-ABL in Leukemia. Sci. Rep. 2017, 7, 41525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.H.; Lin, C.H.; Liang, F.P.; Chen, P.F.; Kuo, C.D.; Alam, M.M.; Maiti, B.; Hung, S.K.; Chi, C.W.; Sun, C.M.; et al. Andrographolide downregulates the v-Src and Bcr-Abl oncoproteins and induces Hsp90 cleavage in the ROS-dependent suppression of cancer malignancy. Biochem. Pharmacol. 2014, 87, 229–242. [Google Scholar] [CrossRef]
- Nimmanapalli, R.; O’Bryan, E.; Bhalla, K. Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res. 2001, 61, 1799–1804. [Google Scholar]
- Goussetis, D.J.; Gounaris, E.; Wu, E.J.; Vakana, E.; Sharma, B.; Bogyo, M.; Altman, J.K.; Platanias, L.C. Autophagic degradation of the BCR-ABL oncoprotein and generation of antileukemic responses by arsenic trioxide. Blood 2012, 120, 3555–3562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, X.; Zhao, C.; Chen, X.; Zhang, P.; Zang, D.; Wu, J.; Chen, J.; Long, H.; Yang, L.; Huang, H.; et al. Platinum pyrithione induces apoptosis in chronic myeloid leukemia cells resistant to imatinib via DUB inhibition-dependent caspase activation and Bcr-Abl downregulation. Cell Death Dis. 2017, 8, e2913. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Geng, J.; Zhang, J.; Miao, J.; Liu, M. Xanthohumol, a Prenylated Flavonoid from Hops, Induces Caspase-Dependent Degradation of Oncoprotein BCR-ABL in K562 Cells. Antioxidants 2019, 8, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Wang, Q.; Ge, X.; Tian, X.; Zhang, Y.; Zhang, J.; Zhang, P. Soy isoflavone: The multipurpose phytochemical (Review). Biomed. Rep. 2013, 1, 697–701. [Google Scholar] [CrossRef]
- Mann, G.E.; Bonacasa, B.; Ishii, T.; Siow, R.C. Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: Protection afforded by dietary isoflavones. Curr. Opin. Pharmacol. 2009, 9, 139–145. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Yan, G.R.; Xiao, C.L.; He, G.W.; Yin, X.F.; Chen, N.P.; Cao, Y.; He, Q.Y. Global phosphoproteomic effects of natural tyrosine kinase inhibitor, genistein, on signaling pathways. Proteomics 2010, 10, 976–986. [Google Scholar] [CrossRef]
- Chang, T.S. Isolation, bioactivity, and production of ortho-hydroxydaidzein and ortho-hydroxygenistein. Int. J. Mol. Sci. 2014, 15, 5699–5716. [Google Scholar] [CrossRef] [Green Version]
- Esaki, H.; Onozaki, H.; Morimitsu, Y.; Kawakishi, S.; Osawa, T. Potent Antioxidative Isoflavones Isolated from Soybeans Fermented with Aspergillus saitoi. Biosci. Biotechnol. Biochem. 1998, 62, 740–746. [Google Scholar] [CrossRef]
- Fujita, T.; Funako, T.; Hayashi, H. 8-Hydroxydaidzein, an aldose reductase inhibitor from okara fermented with Aspergillus sp. HK-388. Biosci. Biotechnol. Biochem. 2004, 68, 1588–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Park, H.Y.; Kim, D.H.; Kim, D.H.; Kim, H.K. ortho-dihydroxyisoflavone derivatives from aged Doenjang (Korean fermented soypaste) and its radical scavenging activity. Bioorg. Med. Chem. Lett. 2008, 18, 5006–5009. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, D.H.; Lee, J.K.; Lee, J.Y.; Kim, D.H.; Kim, H.K.; Lee, H.J.; Kim, H.C. Natural ortho-dihydroxyisoflavone derivatives from aged Korean fermented soybean paste as potent tyrosinase and melanin formation inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.S.; Ding, H.Y.; Yen, J.H.; Chen, S.F.; Lee, K.H.; Wu, M.J. Anti-inflammatory Activity of 8-Hydroxydaidzein in LPS-Stimulated BV2 Microglial Cells via Activation of Nrf2-Antioxidant and Attenuation of Akt/NF-kappaB-Inflammatory Signaling Pathways, as Well As Inhibition of COX-2 Activity. J. Agric. Food Chem. 2018, 66, 5790–5801. [Google Scholar] [CrossRef] [PubMed]
- Hirota, A.; Taki, S.; Kawaii, S.; Yano, M.; Abe, N. 1,1-Diphenyl-2-picrylhydrazyl radical-scavenging compounds from soybean miso and antiproliferative activity of isoflavones from soybean miso toward the cancer cell lines. Biosci. Biotechnol. Biochem. 2000, 64, 1038–1040. [Google Scholar] [CrossRef] [Green Version]
- Lozzio, C.B.; Lozzio, B.B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 1975, 45, 321–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbist, B.; Klambauer, G.; Vervoort, L.; Talloen, W.; Consortium, Q.; Shkedy, Z.; Thas, O.; Bender, A.; Gohlmann, H.W.; Hochreiter, S. Using transcriptomics to guide lead optimization in drug discovery projects: Lessons learned from the QSTAR project. Drug Discov. Today 2015, 20, 505–513. [Google Scholar] [CrossRef] [Green Version]
- The Gene Ontology, C. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, D. BioCarta. Biotech Softw. Internet Rep. 2001, 2, 117–120. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3 B 1–A3 B 3. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Zhai, J.; Jiang, H.; Zhang, Y.; Wu, M.; Qiu, Y.; Fan, C.; Yu, L.; Bai, S.; Sun, L.; et al. Acetylshikonin induces apoptosis of human leukemia cell line K562 by inducing S phase cell cycle arrest, modulating ROS accumulation, depleting Bcr-Abl and blocking NF-kappaB signaling. Biomed. Pharmacother. 2020, 122, 109677. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.H.; Lin, C.Y.; Chuang, C.H.; Chin, H.K.; Wu, M.J.; Chen, P.Y. Nobiletin Promotes Megakaryocytic Differentiation through the MAPK/ERK-Dependent EGR1 Expression and Exerts Anti-Leukemic Effects in Human Chronic Myeloid Leukemia (CML) K562 Cells. Cells 2020, 9, 877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.Y.; Chen, Y.T.; Gao, W.Y.; Wu, M.J.; Yen, J.H. Nobiletin Down-Regulates c-KIT Gene Expression and Exerts Antileukemic Effects on Human Acute Myeloid Leukemia Cells. J. Agric. Food Chem. 2018, 66, 13423–13434. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Lin, Y.-C.; Cheng, Y.-H.; Tung, C.-W. Profiling transcriptomes of human SH-SY5Y neuroblastoma cells exposed to maleic acid. PeerJ 2017, 5, e3175. [Google Scholar] [CrossRef] [Green Version]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.M.; Kim, J.H.; Kim, J.K. Evaluation of MTT and Trypan Blue assays for radiation-induced cell viability test in HepG2 cells. Int. J. Radiat. Res. 2015, 13, 331–335. [Google Scholar] [CrossRef]
- Goh, M.J.; Park, J.S.; Bae, J.H.; Kim, D.H.; Kim, H.K.; Na, Y.J. Effects of ortho-dihydroxyisoflavone derivatives from Korean fermented soybean paste on melanogenesis in B16 melanoma cells and human skin equivalents. Phytother. Res. 2012, 26, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L. A potential daidzein derivative enhances cytotoxicity of epirubicin on human colon adenocarcinoma Caco-2 cells. Int. J. Mol. Sci. 2013, 14, 158–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.W.; Yen, J.H.; Shen, Y.T.; Wu, K.Y.; Wu, M.J. Luteolin modulates 6-hydroxydopamine-induced transcriptional changes of stress response pathways in PC12 cells. PLoS ONE 2014, 9, e97880. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, L.; Wu, S.; Teraishi, F.; Davis, J.J.; Jacob, D.; Fang, B. Induction of S-phase arrest and p21 overexpression by a small molecule 2[[3-(2,3-dichlorophenoxy)propyl] amino]ethanol in correlation with activation of ERK. Oncogene 2004, 23, 4984–4992. [Google Scholar] [CrossRef] [Green Version]
- Parry, D.; Mahony, D.; Wills, K.; Lees, E. Cyclin D-CDK subunit arrangement is dependent on the availability of competing INK4 and p21 class inhibitors. Mol. Cell. Biol. 1999, 19, 1775–1783. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.G.; Cullen, S.P.; Sheridan, C.; Luthi, A.U.; Gerner, C.; Martin, S.J. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. USA 2008, 105, 12815–12819. [Google Scholar] [CrossRef] [Green Version]
- McComb, S.; Chan, P.K.; Guinot, A.; Hartmannsdottir, H.; Jenni, S.; Dobay, M.P.; Bourquin, J.P.; Bornhauser, B.C. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci. Adv. 2019, 5, eaau9433. [Google Scholar] [CrossRef] [Green Version]
- D’Amours, D.; Sallmann, F.R.; Dixit, V.M.; Poirier, G.G. Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: Implications for apoptosis. J. Cell Sci. 2001, 114, 3771–3778. [Google Scholar]
- Germain, M.; Affar, E.B.; D’Amours, D.; Dixit, V.M.; Salvesen, G.S.; Poirier, G.G. Cleavage of automodified poly(ADP-ribose) polymerase during apoptosis. Evidence for involvement of caspase-7. J. Biol. Chem. 1999, 274, 28379–28384. [Google Scholar] [CrossRef] [Green Version]
- Los, M.; Mozoluk, M.; Ferrari, D.; Stepczynska, A.; Stroh, C.; Renz, A.; Herceg, Z.; Wang, Z.Q.; Schulze-Osthoff, K. Activation and caspase-mediated inhibition of PARP: A molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 2002, 13, 978–988. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, D.P.; Militao, G.C.G.; de Morais, M.C.; de Sousa, D.P. The Dual Antioxidant/Prooxidant Effect of Eugenol and Its Action in Cancer Development and Treatment. Nutrients 2017, 9, 1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain Yar, K.; Haseeb, Z.; Mohd Fahad, U.; Aamir, A.; Sheikh Mumtaz, H. A Prooxidant Mechanism for the Anticancer and Chemopreventive Properties of Plant Polyphenols. Curr. Drug Targets 2012, 13, 1738–1749. [Google Scholar] [CrossRef]
- Schwartz, J.L. The dual roles of nutrients as antioxidants and prooxidants: Their effects on tumor cell growth. J. Nutr. 1996, 126, 1221S–1227S. [Google Scholar] [CrossRef] [Green Version]
- Koukourakis, M.I.; Kalamida, D.; Giatromanolaki, A.; Zois, C.E.; Sivridis, E.; Pouliliou, S.; Mitrakas, A.; Gatter, K.C.; Harris, A.L. Autophagosome Proteins LC3A, LC3B and LC3C Have Distinct Subcellular Distribution Kinetics and Expression in Cancer Cell Lines. PLoS ONE 2015, 10, e0137675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohsumi, Y. Molecular dissection of autophagy: Two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol. 2001, 2, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Diaz-Meco, M.T. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 2009, 137, 1001–1004. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Ma, Y.; Sun, J.; Shen, Q.; Liu, L.; Lu, H.; Wang, F.; Yue, Y.; Li, J.; Zhang, S.; et al. YY1-MIR372-SQSTM1 regulatory axis in autophagy. Autophagy 2014, 10, 1442–1453. [Google Scholar] [CrossRef] [Green Version]
- Colosetti, P.; Puissant, A.; Robert, G.; Luciano, F.; Jacquel, A.; Gounon, P.; Cassuto, J.P.; Auberger, P. Autophagy is an important event for megakaryocytic differentiation of the chronic myelogenous leukemia K562 cell line. Autophagy 2009, 5, 1092–1098. [Google Scholar] [CrossRef] [Green Version]
- Elzinga, B.M.; Nyhan, M.J.; Crowley, L.C.; O’Donovan, T.R.; Cahill, M.R.; McKenna, S.L. Induction of autophagy by Imatinib sequesters Bcr-Abl in autophagosomes and down-regulates Bcr-Abl protein. Am. J. Hematol. 2013, 88, 455–462. [Google Scholar] [CrossRef]
- Sutherland, J.A.; Turner, A.R.; Mannoni, P.; McGann, L.E.; Turc, J.M. Differentiation of K562 leukemia cells along erythroid, macrophage, and megakaryocyte lineages. J. Biol. Response Mod. 1986, 5, 250–262. [Google Scholar]
- Wang, H.; Hu, H.; Zhang, Q.; Yang, Y.; Li, Y.; Hu, Y.; Ruan, X.; Yang, Y.; Zhang, Z.; Shu, C.; et al. Dynamic transcriptomes of human myeloid leukemia cells. Genomics 2013, 102, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Puerto, M.C.; Folkerts, H.; Wierenga, A.T.; Schepers, K.; Schuringa, J.J.; Coffer, P.J.; Vellenga, E. Autophagy Proteins ATG5 and ATG7 Are Essential for the Maintenance of Human CD34(+) Hematopoietic Stem-Progenitor Cells. Stem Cells 2016, 34, 1651–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, G.; Kim, C.N.; Perkins, C.L.; Ramadevi, N.; Winton, E.; Wittmann, S.; Bhalla, K.N. CGP57148B (STI-571) induces differentiation and apoptosis and sensitizes Bcr-Abl-positive human leukemia cells to apoptosis due to antileukemic drugs. Blood 2000, 96, 2246–2253. [Google Scholar] [CrossRef]
- Gibbs, J.D.; Liebermann, D.A.; Hoffman, B. Egr-1 abrogates the E2F-1 block in terminal myeloid differentiation and suppresses leukemia. Oncogene 2008, 27, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, T.; Wang, Y.; Dai, W. Transcription factor egr-1 is involved in phorbol 12-myristate 13-acetate-induced megakaryocytic differentiation of K562 cells. J. Biol. Chem. 1994, 269, 30848–30853. [Google Scholar] [PubMed]
- Kitatani, K.; Taniguchi, M.; Okazaki, T. Role of Sphingolipids and Metabolizing Enzymes in Hematological Malignancies. Mol. Cells 2015, 38, 482–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hantschel, O.; Gstoettenbauer, A.; Colinge, J.; Kaupe, I.; Bilban, M.; Burkard, T.R.; Valent, P.; Superti-Furga, G. The chemokine interleukin-8 and the surface activation protein CD69 are markers for Bcr-Abl activity in chronic myeloid leukemia. Mol. Oncol. 2008, 2, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.Y.; Liu, Y.H.; Chen, Y.J.; Yeh, Y.Y.; Huang, H.M. CD69 partially inhibits apoptosis and erythroid differentiation via CD24, and their knockdown increase imatinib sensitivity in BCR-ABL-positive cells. J. Cell. Physiol. 2018, 233, 7467–7479. [Google Scholar] [CrossRef]
- Payne, S.L.; Hendrix, M.J.; Kirschmann, D.A. Paradoxical roles for lysyl oxidases in cancer—A prospect. J. Cell. Biochem. 2007, 101, 1338–1354. [Google Scholar] [CrossRef]
- Supek, F.; Bosnjak, M.; Skunca, N.; Smuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef] [Green Version]
- Zambon, A.C.; Gaj, S.; Ho, I.; Hanspers, K.; Vranizan, K.; Evelo, C.T.; Conklin, B.R.; Pico, A.R.; Salomonis, N. GO-Elite: A flexible solution for pathway and ontology over-representation. Bioinformatics 2012, 28, 2209–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberzon, A.; Birger, C.; Thorvaldsdottir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enright, A.J.; Van Dongen, S.; Ouzounis, C.A. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002, 30, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Feng, P.; Ku, B.; Dotan, I.; Canaani, D.; Oh, B.H.; Jung, J.U. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat. Cell Biol. 2006, 8, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; He, Y.; Qiu, X.; Yang, W.; Liu, W.; Li, X.; Li, Y.; Shen, H.M.; Wang, R.; Yue, Z.; et al. Targeting the potent Beclin 1-UVRAG coiled-coil interaction with designed peptides enhances autophagy and endolysosomal trafficking. Proc. Natl. Acad. Sci. USA 2018, 115, E5669–E5678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Li, Y.; Shou, L.; Li, L.; Chen, Z.; Ye, X.; Qian, W. The molecular mechanisms underlying BCR/ABL degradation in chronic myeloid leukemia cells promoted by Beclin1-mediated autophagy. Cancer Manag. Res. 2019, 11, 5197–5208. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.; Scherr, M.; Breyer, B.; Mancini, A.; Kardinal, C.; Battmer, K.; Eder, M.; Tamura, T. Inhibition of Abl tyrosine kinase enhances nerve growth factor-mediated signaling in Bcr–Abl transformed cells via the alteration of signaling complex and the receptor turnover. Oncogene 2008, 27, 4678–4689. [Google Scholar] [CrossRef] [Green Version]
- Luo, N.; Balko, J.M. Role of JAK-STAT Pathway in Cancer Signaling. In Predictive Biomarkers in Oncology: Applications in Precision Medicine; Badve, S., Kumar, G.L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 311–319. [Google Scholar] [CrossRef]
- Rajabi, S.; Shojaee, M.; Malmir, A.; Rezaei Tavirani, M.; Noori, S. Anti-Breast Cancer Activities of 8-Hydroxydaidzein by Targeting Breast Cancer Stem-Like Cells. J. Pharm. Pharm. Sci. 2020, 23, 47–57. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Z.; Li, X.; Zhong, J.; Bi, Y.; Li, R.; Zhao, Q.; Yu, X. Pristimerin Induces Autophagy-Mediated Cell Death in K562 Cells through the ROS/JNK Signaling Pathway. Chem. Biodivers. 2019, 16, e1900325. [Google Scholar] [CrossRef]
- Nica, A.F.; Tsao, C.C.; Watt, J.C.; Jiffar, T.; Kurinna, S.; Jurasz, P.; Konopleva, M.; Andreeff, M.; Radomski, M.W.; Ruvolo, P.P. Ceramide promotes apoptosis in chronic myelogenous leukemia-derived K562 cells by a mechanism involving caspase-8 and JNK. Cell Cycle 2008, 7, 3362–3370. [Google Scholar] [CrossRef]
- Wu, X.P.; Xiong, M.; Xu, C.S.; Duan, L.N.; Dong, Y.Q.; Luo, Y.; Niu, T.H.; Lu, C.R. Resveratrol induces apoptosis of human chronic myelogenous leukemia cells in vitro through p38 and JNK-regulated H2AX phosphorylation. Acta Pharmacol. Sin. 2015, 36, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Copetti, T.; Bertoli, C.; Dalla, E.; Demarchi, F.; Schneider, C. p65/RelA modulates BECN1 transcription and autophagy. Mol. Cell. Biol. 2009, 29, 2594–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaltschmidt, B.; Kaltschmidt, C.; Hofmann, T.G.; Hehner, S.P.; Droge, W.; Schmitz, M.L. The pro- or anti-apoptotic function of NF-kappaB is determined by the nature of the apoptotic stimulus. Eur. J. Biochem. 2000, 267, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-D.; Han, C.-S.; Kim, K.-W.; Do, I.-R.; Kim, C.-M.; Kim, S.-H.; Lee, E.-Y.; Chung, B.-S. Activation of NF-κB mediates the PMA-induced differentiation of K562 cells. Cancer Lett. 1998, 132, 99–106. [Google Scholar] [CrossRef]
- Basile, J.R.; Eichten, A.; Zacny, V.; Münger, K. NF-κB-Mediated Induction of p21Cip1/Waf1 by Tumor Necrosis Factor α Induces Growth Arrest and Cytoprotection in Normal Human Keratinocytes1 1 R01 CA81135 (KM), K16DE00275 (JRB), DAAD Doktorandenstipendium im Rahmen des gemeinsamen HSP III von Bund und Ländern (AE) and a Senior Postdoctoral Fellowship from the New England Division of the American Cancer Society (VZ). Mol. Cancer Res. 2003, 1, 262–270. [Google Scholar]

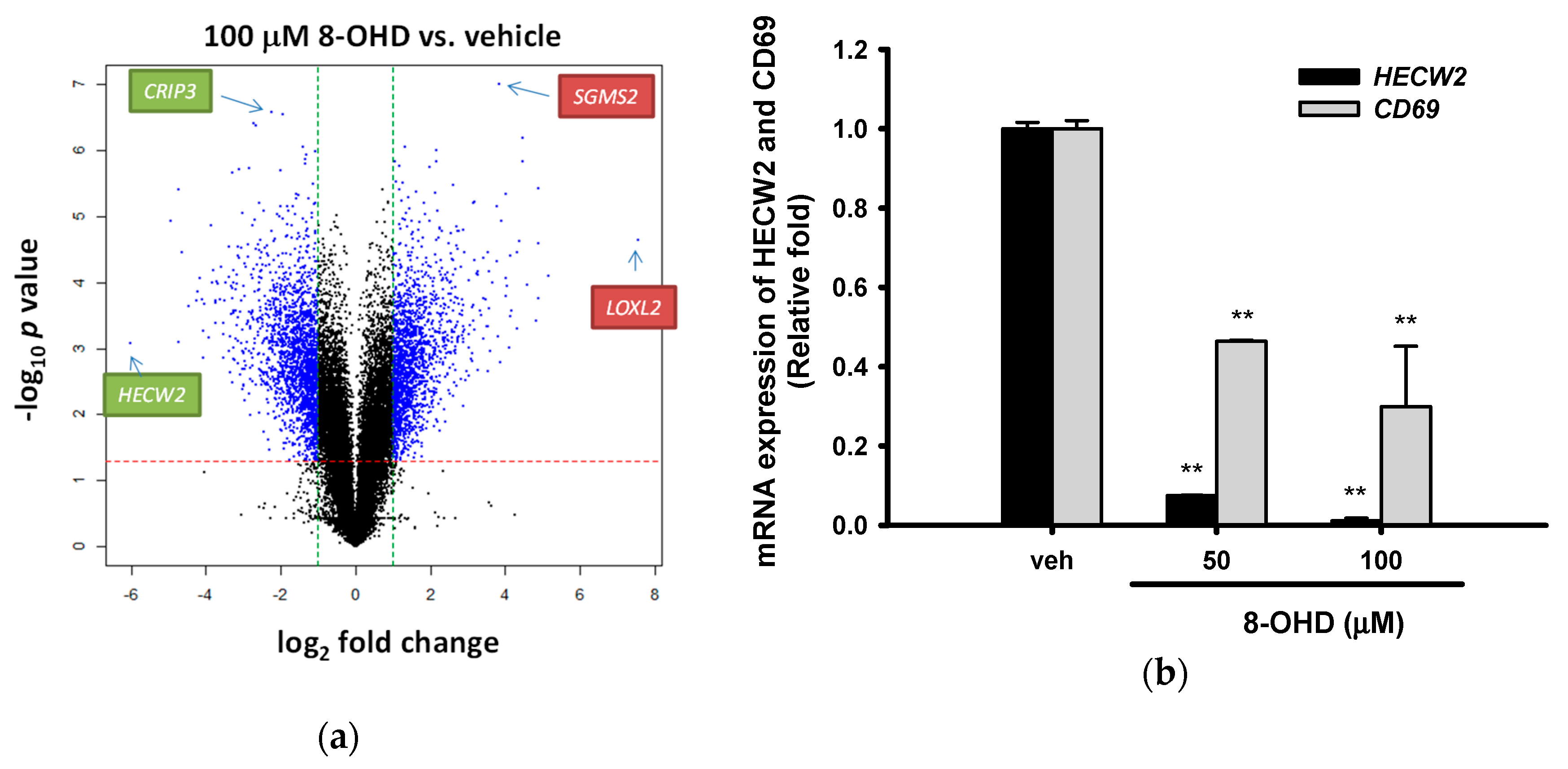
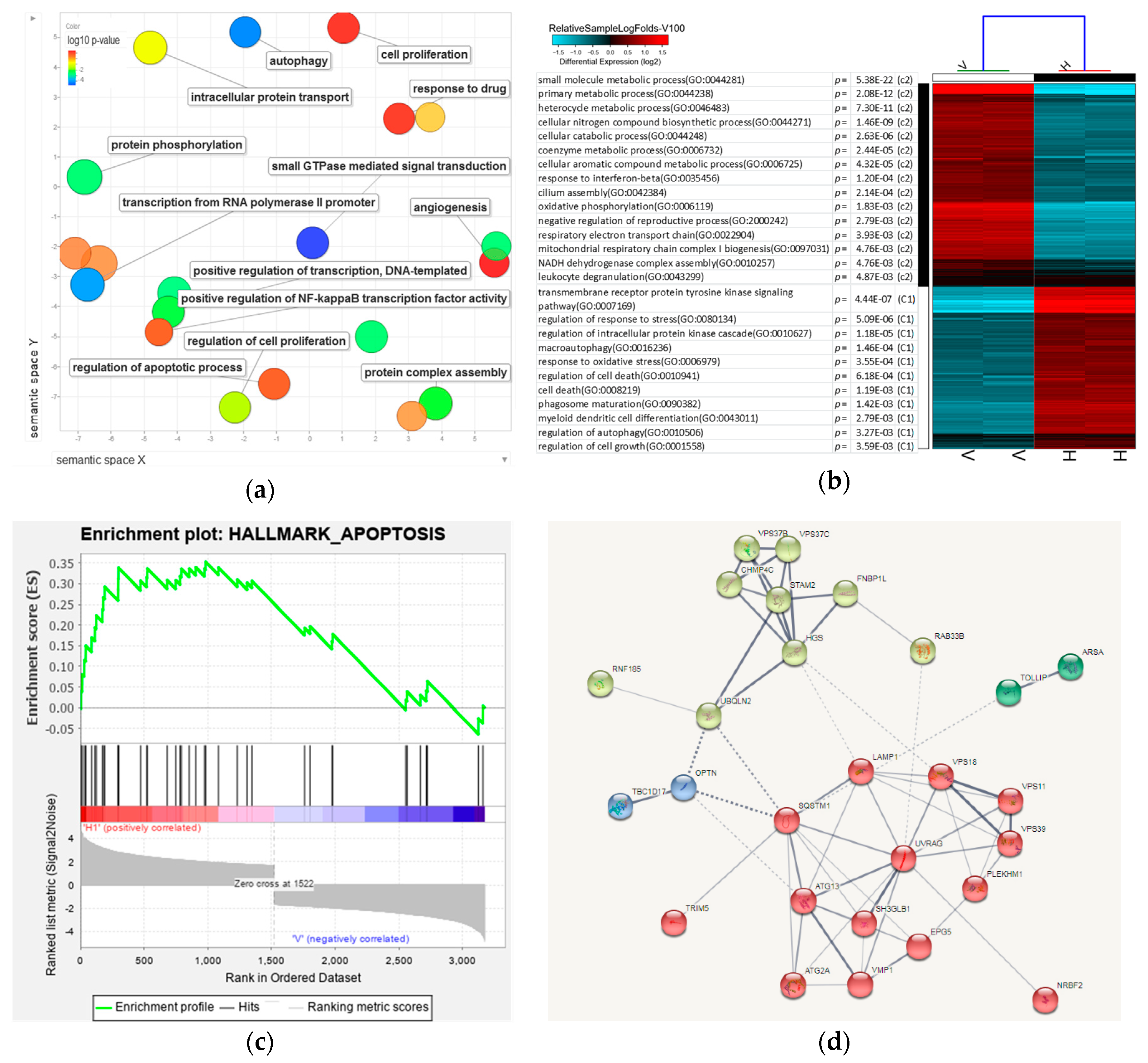
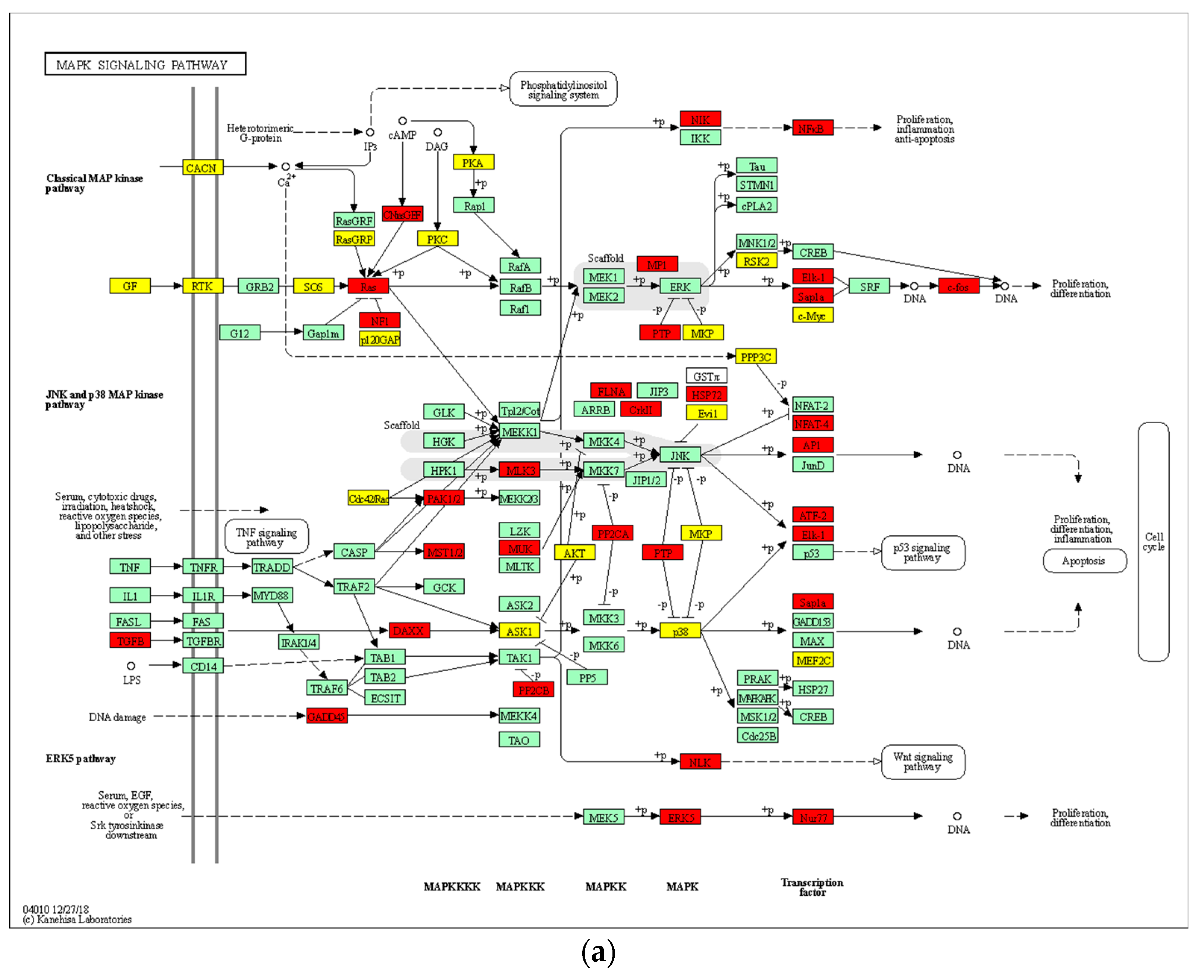
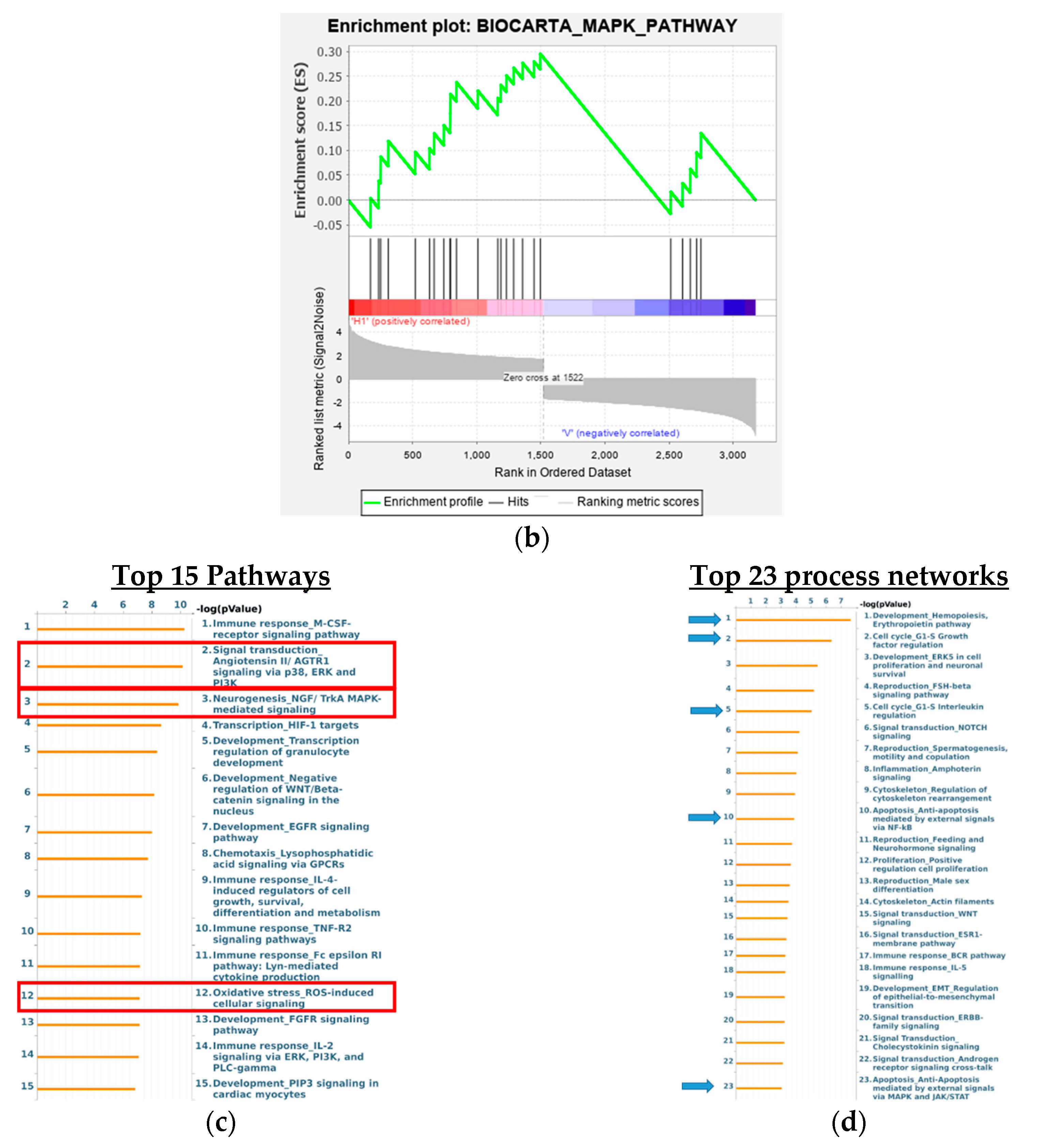

| Antibody | Company | Catalog Number |
|---|---|---|
| α-Tubulin | Sigma | T6199 |
| β-Actin | Genetex | GTX629630 |
| c-ABL | Cell Signaling | 2862 |
| Caspase-3 | Cell Signaling | 9662 |
| Caspase-7 | Cell Signaling | 9492 |
| CDK6 | Cell Signaling | 13331 |
| Cyclin D2 | Cell Signaling | 3741 |
| JNK2 | Cell Signaling | 9258 |
| Lamin A/C | Genetex | GTX101127 |
| LC3B | Genetex | GTX127375 |
| P21 | Cell Signaling | 2947 |
| p38 MAPK | Cell Signaling | 9212 |
| p44/42 MAP Kinase | Cell Signaling | 4695 |
| p65 NF-κB | Cell Signaling | 8242 |
| PARP1 | Santa Cruz | Sc-7150 |
| Phoshpo-p38 MAPK | Cell Signaling | 9215 |
| Phoshpo-p44/42 MAPK | Cell Signaling | 4370 |
| Phospho-JNK1/2 | Cell Signaling | 4668 |
| Phospho-p65 NF-κB | Cell Signaling | 3033 |
| Gene | Primer Sequence (5′-3′) | Amplicon (bp) |
|---|---|---|
| β-actin | CATGTACGTTGCTATCCAGGC | 250 |
| CTCCTTAATGTCACGCACGAT | ||
| ITGB3 (CD61) | TGTATGGGACTCAAGATTGGA | 187 |
| AGCGATGGCTATTAGGTTCA | ||
| GP1BA (CD42b) | TCTGTATCAGAAGCCCTGTCTTCAC | 112 |
| GCATCGGGAGCTTTGTCTTG | ||
| EGR1 | CCGCAGAGTCTTTTCCTGAC | 200 |
| TGGGTTGGTCATGCTCACTA | ||
| CD69 | GCTGGACTTCAGCCCAAAATGC | 121 |
| AGTCCAACCCAGTGTTCCTCTC | ||
| HECW2 | GAGTATCCGCAGAACCATGACC | 133 |
| GGTAGAGTGAGGCAGGATGTTC | ||
| SQSTM1 (P62) | AAGCCGGGTGGGAATGTTG | 116 |
| CCTGAACAGTTATCCGACTCCAT |
| ID | Symbol | Description | log2FC | p Value | q Value |
|---|---|---|---|---|---|
| 8878 | SQSTM1 | autophagy receptor that interacts directly with both the cargo to become degraded and an autophagy modifier of the MAP1 LC3 family | 3.48 | 1.79 × 10−4 | 7.76 × 10−3 |
| 54472 | TOLLIP | component of the signaling pathway of IL-1 and Toll-like receptors. | 2.37 | 7.55 × 10−4 | 1.20 × 10−2 |
| 55823 | VPS11 | vacuolar protein sorting-associated protein 11 homolog; plays a role in vesicle-mediated protein trafficking to lysosomal compartments including the endocytic membrane transport and autophagic pathway | 2.3 | 3.22 × 10−4 | 9.01×10−3 |
| 10254 | STAM2 | signal transducing adapter molecule 2; Involved in intracellular signal transduction mediated by cytokines and growth factors. | 2.15 | 1.61 × 10−3 | 1.62 × 10−2 |
| 3916 | LAMP1 | lysosomal associated membrane protein 1 | 1.98 | 7.97 × 10−4 | 1.21 × 10−2 |
| 51100 | SH3GLB1 | SH3 domain containing GRB2 like endophilin B1 | 1.87 | 5.95 × 10−3 | 3.06 × 10−2 |
| 81671 | VMP1 | vacuole membrane protein 1 | 1.8 | 8.26 × 10−5 | 6.26 × 10−3 |
| 85363 | TRIM5 | tripartite motif containing 5 | 1.75 | 1.77 × 10−2 | 6.08 × 10−2 |
| 23130 | ATG2A | autophagy related 2A | 1.74 | 1.07 × 10−3 | 1.36 × 10−2 |
| 91445 | RNF185 | ring finger protein 185 | 1.64 | 2.70 × 10−4 | 8.72 × 10−3 |
| 9146 | HGS | hepatocyte growth factor-regulated tyrosine kinase substrate | 1.62 | 1.18 × 10−3 | 1.42 × 10−2 |
| 9842 | PLEKHM1 | pleckstrin homology and RUN domain containing M1 | 1.61 | 1.70 × 10−3 | 1.67 × 10−2 |
| 79735 | TBC1D17 | TBC1 domain family member 17 | 1.57 | 1.47 × 10−4 | 7.34 × 10−3 |
| 83452 | RAB33B | RAB33B, member RAS oncogene family | 1.54 | 3.33 × 10−3 | 2.26 × 10−2 |
| 7405 | UVRAG | UV radiation resistance associated | 1.47 | 8.62 × 10−3 | 6.33 × 10−3 |
| 9776 | ATG13 | autophagy related 13 | 1.41 | 4.72 × 10−3 | 2.70 × 10−2 |
| 3428 | IFI16 | interferon gamma inducible protein 16 | 1.35 | 9.18 × 10−3 | 3.97 × 10−2 |
| 55048 | VPS37C | VPS37C, ESCRT-I subunit | 1.31 | 5.69 × 10−3 | 2.99 × 10−2 |
| 57617 | VPS18 | VPS18, CORVET/HOPS core subunit | 1.31 | 2.87 × 10−4 | 8.81 × 10−3 |
| 79142 | PHF23 | PHD finger protein 23 | 1.3 | 2.16 × 10−3 | 1.85 × 10−2 |
| 54874 | FNBP1L | formin binding protein 1 like | 1.3 | 6.60 × 10−3 | 3.25 × 10−2 |
| 57724 | EPG5 | ectopic P-granules autophagy protein 5 homolog | 1.26 | 1.06 × 10−3 | 1.35 × 10−2 |
| 79720 | VPS37B | VPS37B, ESCRT-I subunit | 1.25 | 3.11 × 10−6 | 3.66 × 10−3 |
| 29978 | UBQLN2 | ubiquilin 2 | 1.23 | 4.25 × 10−3 | 2.55 × 10−2 |
| 23339 | VPS39 | VPS39, HOPS complex subunit | 1.23 | 1.29 × 10−3 | 1.47 × 10−2 |
| 92421 | CHMP4C | charged multivesicular body protein 4C | 1.18 | 1.76 × 10−2 | 6.05 × 10−2 |
| 410 | ARSA | arylsulfatase A | 1.18 | 9.04 × 10−3 | 3.94 × 10−2 |
| 10133 | OPTN | optineurin | 1.04 | 2.95 × 10−3 | 2.14 × 10−2 |
| 29982 | NRBF2 | nuclear receptor binding factor 2 | 1.01 | 5.32 × 10−4 | 1.07 × 10−2 |
| 7957 | EPM2A | epilepsy, progressive myoclonus type 2A, Lafora disease (laforin) | −1.02 | 4.22 × 10−4 | 9.84 × 10−3 |
| 83734 | ATG10 | autophagy related 10 | −1.29 | 8.40 × 10−5 | 6.28 × 10−3 |
| 51160 | VPS28 | VPS28, ESCRT-I subunit | −1.46 | 7.59 × 10−3 | 3.52 × 10−2 |
| 51028 | VPS36 | vacuolar protein sorting 36 homolog | −1.53 | 1.26 × 10−2 | 4.84 × 10−2 |
| 93343 | MVB12A | multivesicular body subunit 12A | −1.86 | 1.28 × 10−4 | 7.06 × 10−3 |
| 84938 | ATG4C | autophagy related 4C cysteine peptidase | −2.05 | 8.90 × 10−4 | 1.26 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-S.; Yen, J.-H.; Wang, C.-Y.; Chen, P.-Y.; Hung, J.-H.; Wu, M.-J. 8-Hydroxydaidzein, an Isoflavone from Fermented Soybean, Induces Autophagy, Apoptosis, Differentiation, and Degradation of Oncoprotein BCR-ABL in K562 Cells. Biomedicines 2020, 8, 506. https://doi.org/10.3390/biomedicines8110506
Wu P-S, Yen J-H, Wang C-Y, Chen P-Y, Hung J-H, Wu M-J. 8-Hydroxydaidzein, an Isoflavone from Fermented Soybean, Induces Autophagy, Apoptosis, Differentiation, and Degradation of Oncoprotein BCR-ABL in K562 Cells. Biomedicines. 2020; 8(11):506. https://doi.org/10.3390/biomedicines8110506
Chicago/Turabian StyleWu, Pei-Shan, Jui-Hung Yen, Chih-Yang Wang, Pei-Yi Chen, Jui-Hsiang Hung, and Ming-Jiuan Wu. 2020. "8-Hydroxydaidzein, an Isoflavone from Fermented Soybean, Induces Autophagy, Apoptosis, Differentiation, and Degradation of Oncoprotein BCR-ABL in K562 Cells" Biomedicines 8, no. 11: 506. https://doi.org/10.3390/biomedicines8110506
APA StyleWu, P.-S., Yen, J.-H., Wang, C.-Y., Chen, P.-Y., Hung, J.-H., & Wu, M.-J. (2020). 8-Hydroxydaidzein, an Isoflavone from Fermented Soybean, Induces Autophagy, Apoptosis, Differentiation, and Degradation of Oncoprotein BCR-ABL in K562 Cells. Biomedicines, 8(11), 506. https://doi.org/10.3390/biomedicines8110506






