SEZ6L2 Is an Important Regulator of Drug-Resistant Cells and Tumor Spheroid Cells in Lung Adenocarcinoma
Abstract
:1. Introduction
2. Experimental Section
2.1. Cell Culture and Reagents
2.2. RNA Sequencing and Data Analysis
2.3. Flow Cytometry
2.4. TS Formation and Antibody Treatment
2.5. Statistical Analysis
3. Results
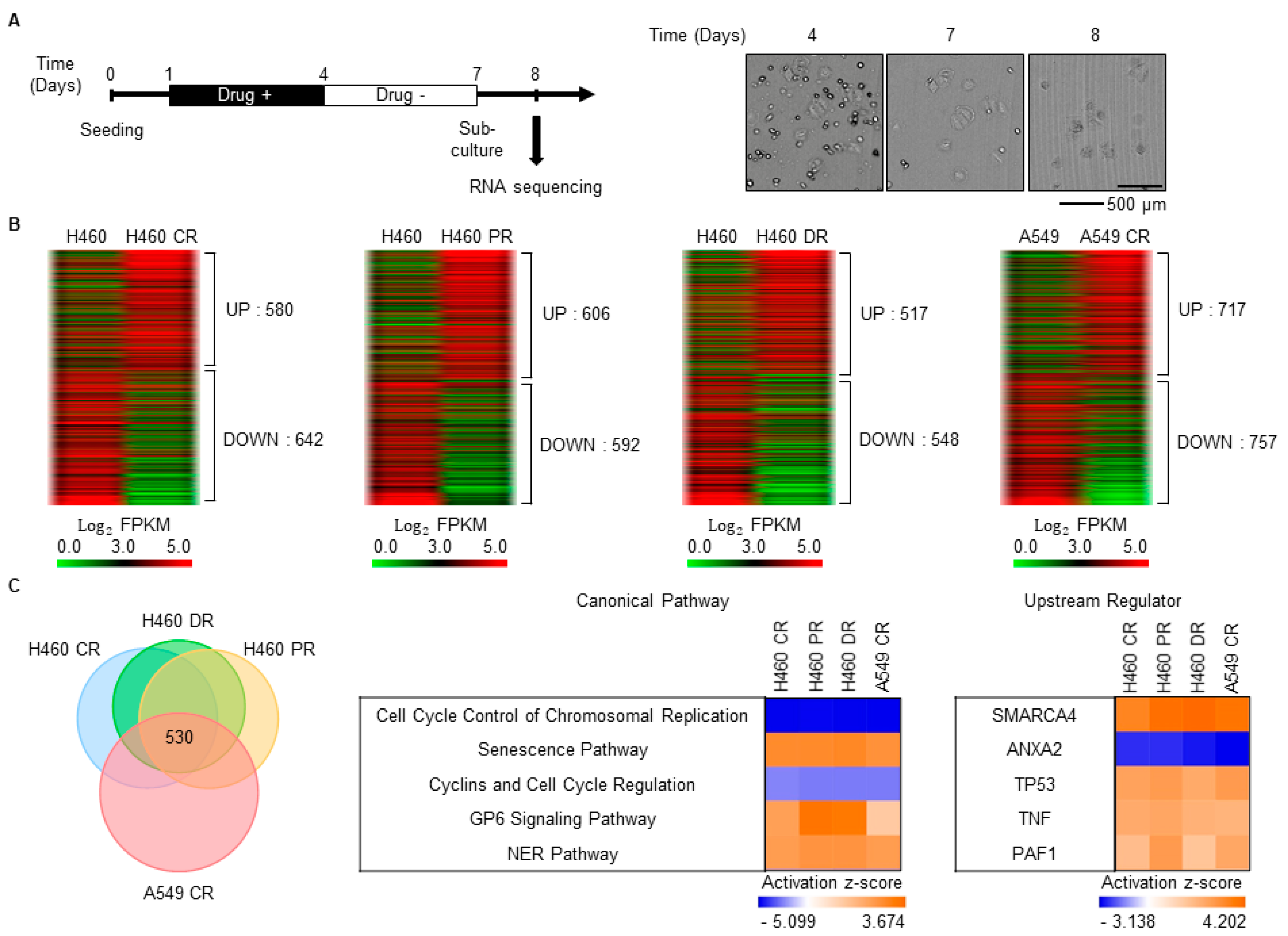
3.1. Characterization of Gene Expression Changes in Drug-Resistant Cells
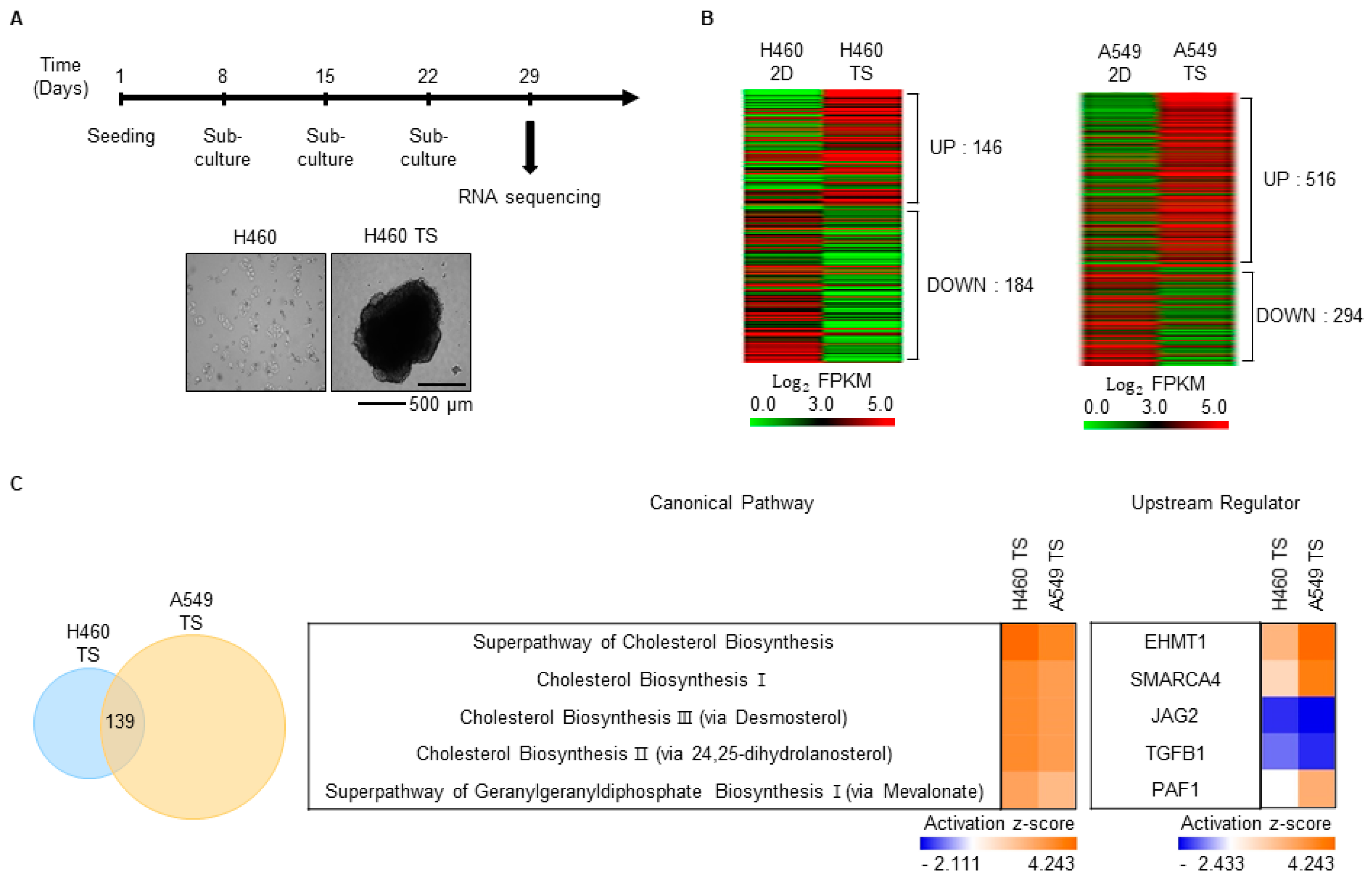
3.2. Characterization of Gene Expression Changes in TS Cells
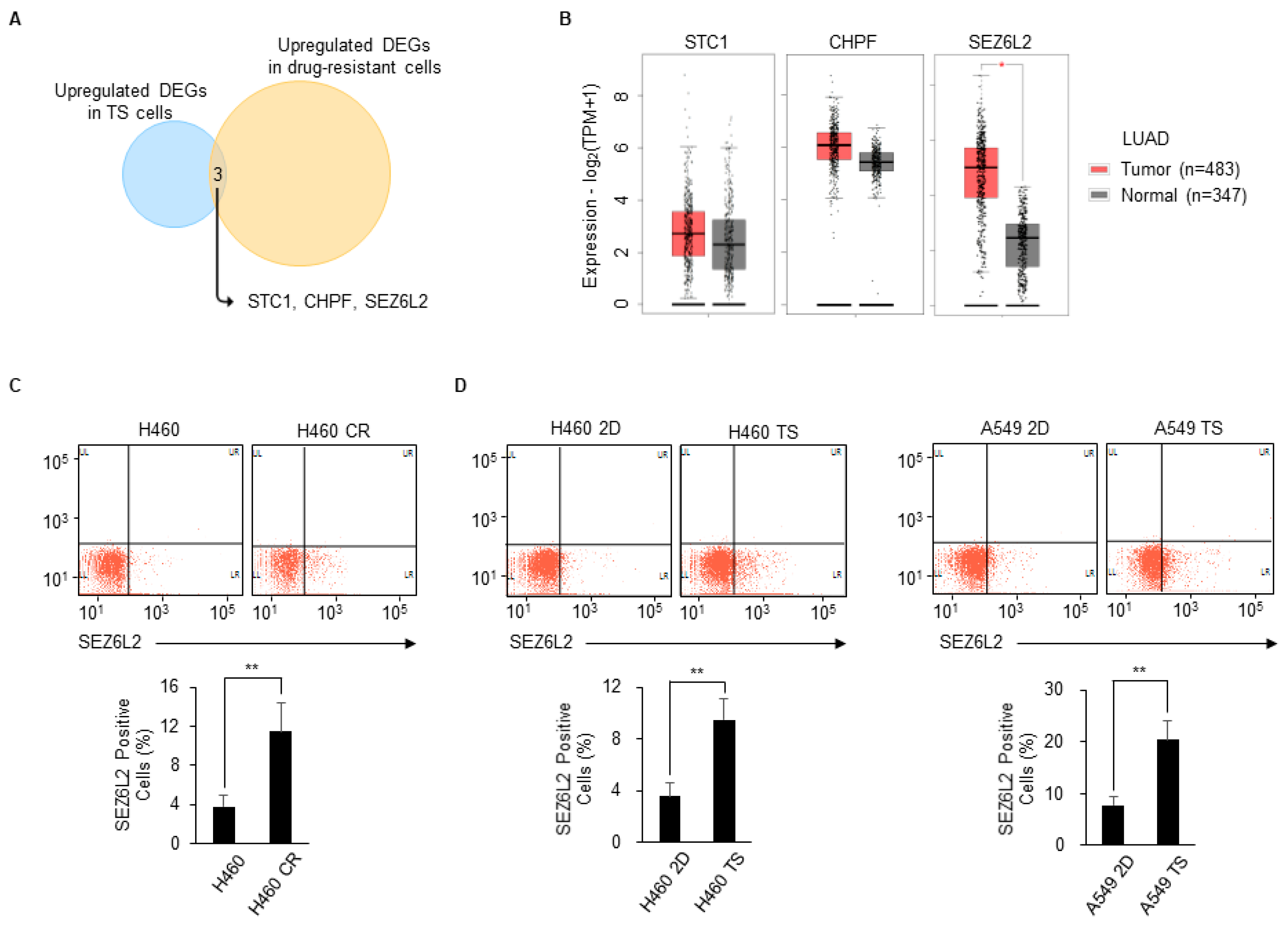
3.3. SEZ6L2 Is Commonly Upregulated in Drug-Resistant Cells and TS Cells
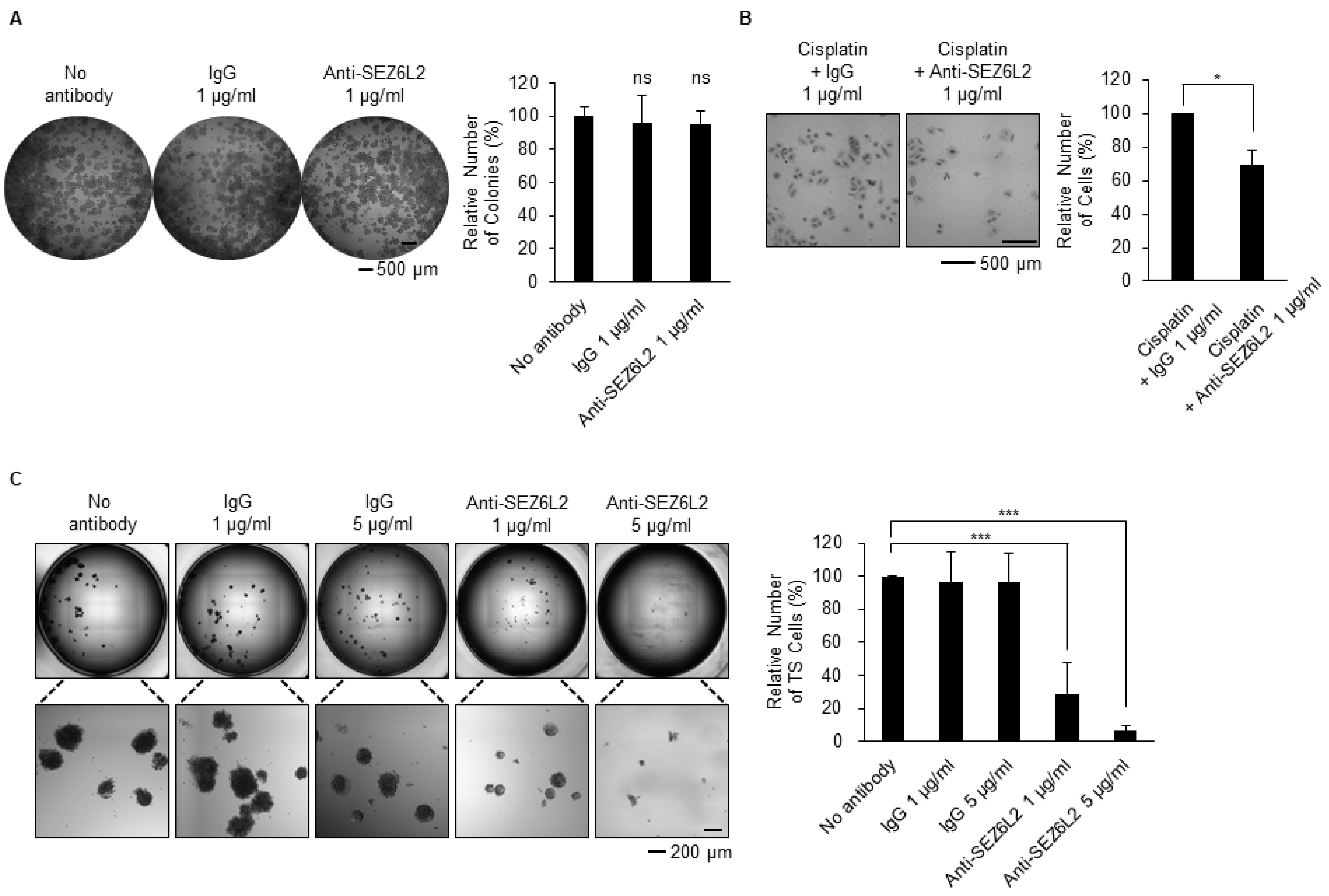
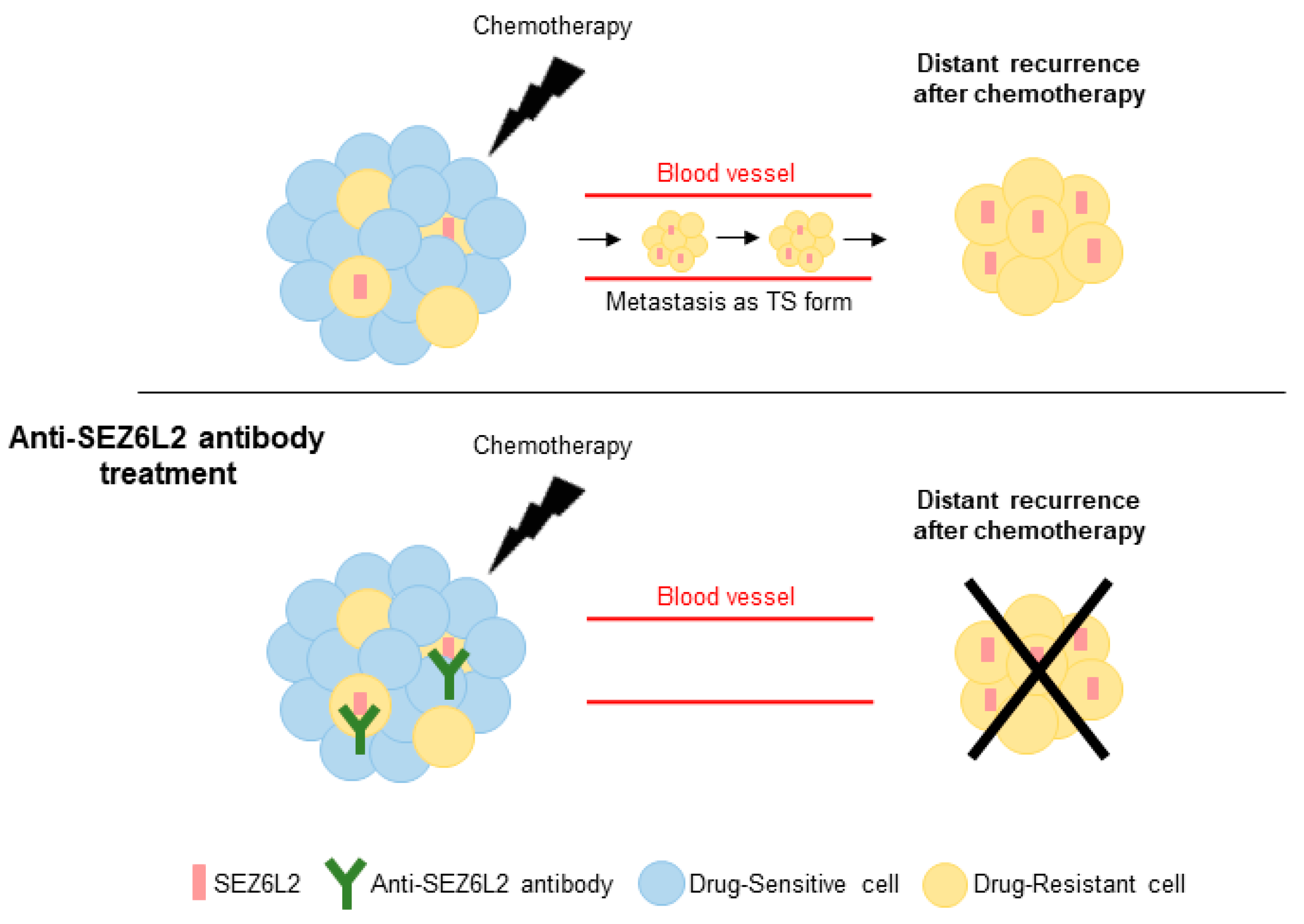
3.4. Anti-SEZ6L2 Antibody Treatment Inhibits Drug Resistance and TS Formation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Heymach, J.V.; Lippman, S.M. Lung cancer. N. Engl. J. Med. 2008, 359, 1367–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relli, V.; Trerotola, M.; Guerra, E.; Alberti, S. Distinct lung cancer subtypes associate to distinct drivers of tumor progression. Oncotarget 2018, 9, 35528–35540. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef]
- Valdes, M.; Nicholas, G.; Goss, G.D.; Wheatley-Price, P. Chemotherapy in recurrent advanced non-small-cell lung cancer after adjuvant chemotherapy. Curr. Oncol. 2016, 23, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Hotta, K.; Matsuo, K.; Ueoka, H.; Kiura, K.; Tabata, M.; Tanimoto, M. Meta-analysis of randomized clinical trials comparing Cisplatin to Carboplatin in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2004, 22, 3852–3859. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.J.; Yeh, Y.C.; Jeng, W.J.; Chien, H.C.; Wu, Y.C.; Chou, T.Y.; Hsu, W.H. Prognostic Factors of Survival after Recurrence in Patients with Resected Lung Adenocarcinoma. J. Thorac. Oncol. 2015, 10, 1328–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wu, Y.; Shao, J.; Liu, D.; Li, W. Clinicopathological variables influencing overall survival, recurrence and post-recurrence survival in resected stage I non-small-cell lung cancer. BMC Cancer 2020, 20, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Jiao, S.C.; Zhang, G.Q.; Guan, Y.; Wang, J.L. Tumor-associated immune factors are associated with recurrence and metastasis in non-small cell lung cancer. Cancer Gene Ther. 2017, 24, 57–63. [Google Scholar] [CrossRef]
- Shah, N.; Liu, Z.; Tallman, R.M.; Mohammad, A.; Sprowls, S.A.; Saralkar, P.A.; Vickers, S.D.; Pinti, M.V.; Gao, W.; Lockman, P.R. Drug resistance occurred in a newly characterized preclinical model of lung cancer brain metastasis. BMC Cancer 2020, 20, 292. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.; Lotey, N. Role of circulating tumor cells in future diagnosis and therapy of cancer. J. Cancer Metastasis Treat. 2015, 1. [Google Scholar] [CrossRef] [Green Version]
- Weiswald, L.B.; Bellet, D.; Dangles-Marie, V. Spherical cancer models in tumor biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, G.; Rath, B. Role of circulating tumor cell spheroids in drug resistance. Cancer Drug Resist. 2019. [Google Scholar] [CrossRef] [Green Version]
- Menyailo, M.E.; Tretyakova, M.S.; Denisov, E.V. Heterogeneity of Circulating Tumor Cells in Breast Cancer: Identifying Metastatic Seeds. Int. J. Mol. Sci. 2020, 21, 1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Shueng, P.W.; Chou, C.M.; Lin, B.X.; Lin, M.H.; Kuo, D.Y.; Tsai, I.L.; Wu, S.M.; Lin, C.W. Elevation of CD109 promotes metastasis and drug resistance in lung cancer via activation of EGFR-AKT-mTOR signaling. Cancer Sci. 2020, 111, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Zugazagoitia, J.; Herbertz, S.; John, W.; Paz-Ares, L.; Schmid-Bindert, G. KRAS-Mutant non-small cell lung cancer: From biology to therapy. Lung Cancer 2018, 124, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Kastelijn, E.A.; de Langen, A.J.; Peters, B.J.M. Treatment of oncogene-driven non-small cell lung cancer. Curr. Opin. Pulm. Med. 2019, 25, 300–307. [Google Scholar] [CrossRef]
- Wang, L.; Ling, X.; Zhu, C.; Zhang, Z.; Wang, Z.; Huang, S.; Tang, Y.; He, S.; Guo, Z.; He, X. Upregulated Seizure-Related 6 Homolog-Like 2 Is a Prognostic Predictor of Hepatocellular Carcinoma. Dis. Markers 2020, 2020, 7318703. [Google Scholar] [CrossRef]
- Dufresne, J.; Bowden, P.; Thavarajah, T.; Florentinus-Mefailoski, A.; Chen, Z.Z.; Tucholska, M.; Norzin, T.; Ho, M.T.; Phan, M.; Mohamed, N.; et al. The plasma peptides of ovarian cancer. Clin. Proteomics 2018, 15, 41. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, N.; Daigo, Y.; Takano, A.; Taniwaki, M.; Kato, T.; Tanaka, S.; Yasui, W.; Takeshima, Y.; Inai, K.; Nishimura, H.; et al. Characterization of SEZ6L2 cell-surface protein as a novel prognostic marker for lung cancer. Cancer Sci. 2006, 97, 737–745. [Google Scholar] [CrossRef]
- Bong, S.M.; Bae, S.H.; Song, B.; Gwak, H.; Yang, S.W.; Kim, S.; Nam, S.; Rajalingam, K.; Oh, S.J.; Kim, T.W.; et al. Regulation of mRNA export through API5 and nuclear FGF2 interaction. Nucleic Acids Res. 2020, 48, 6340–6352. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Aceto, N.; Bersani, F.; Madden, M.W.; Donaldson, M.C.; Desai, R.; Zhu, H.; Comaills, V.; Zheng, Z.; et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 2014, 345, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Kim, D.K.; Bae, S.H.; Gwak, H.; Jeon, J.H.; Kim, J.K.; Lee, B.I.; You, H.J.; Shin, D.H.; Kim, Y.H.; et al. Farnesyl diphosphate synthase is important for the maintenance of glioblastoma stemness. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Poojan, S.; Bae, S.H.; Min, J.W.; Lee, E.Y.; Song, Y.; Kim, H.Y.; Sim, H.W.; Kang, E.K.; Kim, Y.H.; Lee, H.O.; et al. Cancer cells undergoing epigenetic transition show short-term resistance and are transformed into cells with medium-term resistance by drug treatment. Exp. Mol. Med. 2020, 52, 1102–1115. [Google Scholar] [CrossRef]
- Kim, D.-K.; Song, B.; Han, S.; Jang, H.; Bae, S.-H.; Kim, H.Y.; Lee, S.-H.; Lee, S.; Kim, J.K.; Kim, H.-S.; et al. Phosphorylation of OCT4 Serine 236 Inhibits Germ Cell Tumor Growth by Inducing Differentiation. Cancers 2020, 12, 2601. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, B.I.; Jeon, J.H.; Kim, D.K.; Kang, S.G.; Shim, J.K.; Kim, S.Y.; Kang, S.W.; Jang, H. Gossypol Suppresses Growth of Temozolomide-Resistant Glioblastoma Tumor Spheres. Biomolecules 2019, 9, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, J.H.; Kim, D.K.; Shin, Y.; Kim, H.Y.; Song, B.; Lee, E.Y.; Kim, J.K.; You, H.J.; Cheong, H.; Shin, D.H.; et al. Migration and invasion of drug-resistant lung adenocarcinoma cells are dependent on mitochondrial activity. Exp. Mol. Med. 2016, 48, e277. [Google Scholar] [CrossRef] [Green Version]
- Rosell, R.; Taron, M.; Barnadas, A.; Scagliotti, G.; Sarries, C.; Roig, B. Nucleotide excision repair pathways involved in Cisplatin resistance in non-small-cell lung cancer. Cancer Control. 2003, 10, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Shimizu-Nishikawa, K.; Kajiwara, K.; Kimura, M.; Katsuki, M.; Sugaya, E. Cloning and expression of SEZ-6, a brain-specific and seizure-related cDNA. Brain Res. Mol. Brain Res. 1995, 28, 201–210. [Google Scholar] [CrossRef]
- Gunnersen, J.M.; Kim, M.H.; Fuller, S.J.; De Silva, M.; Britto, J.M.; Hammond, V.E.; Davies, P.J.; Petrou, S.; Faber, E.S.; Sah, P.; et al. Sez-6 proteins affect dendritic arborization patterns and excitability of cortical pyramidal neurons. Neuron 2007, 56, 621–639. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.A.; Schwartz, G.K. Cell cycle-mediated drug resistance: An emerging concept in cancer therapy. Clin. Cancer Res. 2001, 7, 2168–2181. [Google Scholar] [PubMed]
- Guillon, J.; Petit, C.; Toutain, B.; Guette, C.; Lelievre, E.; Coqueret, O. Chemotherapy-induced senescence, an adaptive mechanism driving resistance and tumor heterogeneity. Cell Cycle 2019, 18, 2385–2397. [Google Scholar] [CrossRef]
- Ehmsen, S.; Pedersen, M.H.; Wang, G.; Terp, M.G.; Arslanagic, A.; Hood, B.L.; Conrads, T.P.; Leth-Larsen, R.; Ditzel, H.J. Increased Cholesterol Biosynthesis Is a Key Characteristic of Breast Cancer Stem Cells Influencing Patient Outcome. Cell Rep. 2019, 27, 3927–3938.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Li, J.; Vickman, R.E.; Li, J.; Liu, R.; Durkes, A.C.; Elzey, B.D.; Yue, S.; Liu, X.; Ratliff, T.L.; et al. Cholesterol Esterification Inhibition Suppresses Prostate Cancer Metastasis by Impairing the Wnt/β-catenin Pathway. Mol. Cancer Res. 2018, 16, 974–985. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Rong, X.; Palladino, E.N.D.; Wang, J.; Fogelman, A.M.; Martín, M.G.; Alrefai, W.A.; Ford, D.A.; Tontonoz, P. Phospholipid Remodeling and Cholesterol Availability Regulate Intestinal Stemness and Tumorigenesis. Cell Stem Cell 2018, 22, 206–220.e4. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.K.; Wai-Hung Ho, D.; Kam, C.S.; Yung-Tuen Chiu, E.; Lai-Oi Lo, I.; Tsz-Wai Yau, D.; Tin-Yan Cheung, E.; Tang, C.N.; Wai-Lun Tang, V.; Kin-Wah Lee, T.; et al. RSK2-inactivating mutations potentiate MAPK signaling and support cholesterol metabolism in hepatocellular carcinoma. J. Hepatol. 2020. [Google Scholar] [CrossRef]
- Huang, B.; Song, B.L.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab 2020, 2, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Khavari, P.A.; Peterson, C.L.; Tamkun, J.W.; Mendel, D.B.; Crabtree, G.R. BRG1 contains a conserved domain of the SWI2/SNF2 family necessary for normal mitotic growth and transcription. Nature 1993, 366, 170–174. [Google Scholar] [CrossRef]
- Kim, J.; Guermah, M.; Roeder, R.G. The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell 2010, 140, 491–503. [Google Scholar] [CrossRef] [Green Version]
- Bell, E.H.; Chakraborty, A.R.; Mo, X.; Liu, Z.; Shilo, K.; Kirste, S.; Stegmaier, P.; McNulty, M.; Karachaliou, N.; Rosell, R.; et al. SMARCA4/BRG1 Is a Novel Prognostic Biomarker Predictive of Cisplatin-Based Chemotherapy Outcomes in Resected Non-Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 2396–2404. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, S.; Dey, P.; Vaz, A.P.; Bhaumik, S.R.; Ponnusamy, M.P.; Batra, S.K. PD2/PAF1 at the Crossroads of the Cancer Network. Cancer Res. 2018, 78, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e14. [Google Scholar] [CrossRef] [Green Version]
- Ebright, R.Y.; Lee, S.; Wittner, B.S.; Niederhoffer, K.L.; Nicholson, B.T.; Bardia, A.; Truesdell, S.; Wiley, D.F.; Wesley, B.; Li, S.; et al. Deregulation of ribosomal protein expression and translation promotes breast cancer metastasis. Science 2020, 367, 1468–1473. [Google Scholar] [CrossRef]
- Schleich, K.; Kase, J.; Dorr, J.R.; Trescher, S.; Bhattacharya, A.; Yu, Y.; Wailes, E.M.; Fan, D.N.Y.; Lohneis, P.; Milanovic, M.; et al. H3K9me3-mediated epigenetic regulation of senescence in mice predicts outcome of lymphoma patients. Nat. Commun. 2020, 11, 3651. [Google Scholar] [CrossRef]
- Sutherland, R.M. Cell and environment interactions in tumor microregions: The multicell spheroid model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef]
- Ojha, H.; Ghosh, P.; Singh Panwar, H.; Shende, R.; Gondane, A.; Mande, S.C.; Sahu, A. Spatially conserved motifs in complement control protein domains determine functionality in regulators of complement activation-family proteins. Commun. Biol. 2019, 2, 290. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.Q.; Luo, S.; Ma, S.A.; Saminathan, P.; Li, H.; Gunnersen, J.; Gelbard, H.A.; Hammond, J.W. The Sez6 family inhibits complement at the level of the C3 convertase. bioRxiv 2020. [Google Scholar] [CrossRef]
- Nash, A.; Aumann, T.D.; Pigoni, M.; Lichtenthaler, S.F.; Takeshima, H.; Munro, K.M.; Gunnersen, J.M. Lack of Sez6 Family Proteins Impairs Motor Functions, Short-Term Memory, and Cognitive Flexibility and Alters Dendritic Spine Properties. Cereb. Cortex 2020, 30, 2167–2184. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-S.; Kim, H.Y.; Won, B.; Kang, S.W.; Kim, Y.-N.; Jang, H. SEZ6L2 Is an Important Regulator of Drug-Resistant Cells and Tumor Spheroid Cells in Lung Adenocarcinoma. Biomedicines 2020, 8, 500. https://doi.org/10.3390/biomedicines8110500
Lee J-S, Kim HY, Won B, Kang SW, Kim Y-N, Jang H. SEZ6L2 Is an Important Regulator of Drug-Resistant Cells and Tumor Spheroid Cells in Lung Adenocarcinoma. Biomedicines. 2020; 8(11):500. https://doi.org/10.3390/biomedicines8110500
Chicago/Turabian StyleLee, Jang-Seok, Hee Yeon Kim, Bomyi Won, Sang Won Kang, Yong-Nyun Kim, and Hyonchol Jang. 2020. "SEZ6L2 Is an Important Regulator of Drug-Resistant Cells and Tumor Spheroid Cells in Lung Adenocarcinoma" Biomedicines 8, no. 11: 500. https://doi.org/10.3390/biomedicines8110500
APA StyleLee, J.-S., Kim, H. Y., Won, B., Kang, S. W., Kim, Y.-N., & Jang, H. (2020). SEZ6L2 Is an Important Regulator of Drug-Resistant Cells and Tumor Spheroid Cells in Lung Adenocarcinoma. Biomedicines, 8(11), 500. https://doi.org/10.3390/biomedicines8110500





