Human Depotentiation following Induction of Spike Timing Dependent Plasticity
Abstract
1. Introduction
1.1. Studying Physiological Mechanisms of Plasticity in Animal Models
1.2. Translation of Animal Model of Plasticity to Humans
1.3. Role of Human Model of Plasticity
1.4. Objectives
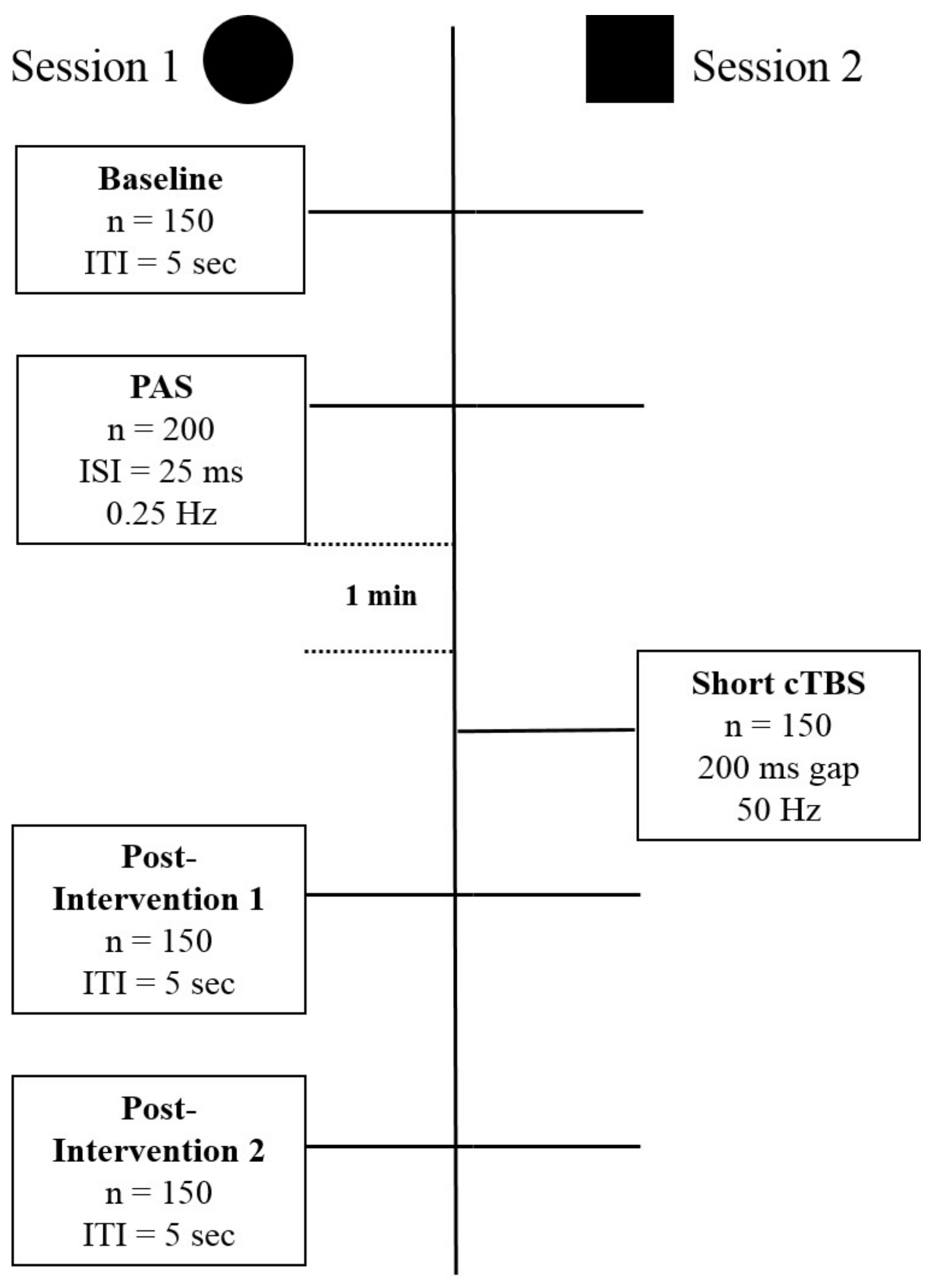
2. Experimental Session
2.1. Participants
2.2. Recording and Stimulation
2.2.1. TMS
2.2.2. Thresholds
2.3. Session Type I: Ltp Induction
2.3.1. Baseline Recording (Pre-Recording)
2.3.2. PAS
2.3.3. P1 and P2 (Post-Recording)
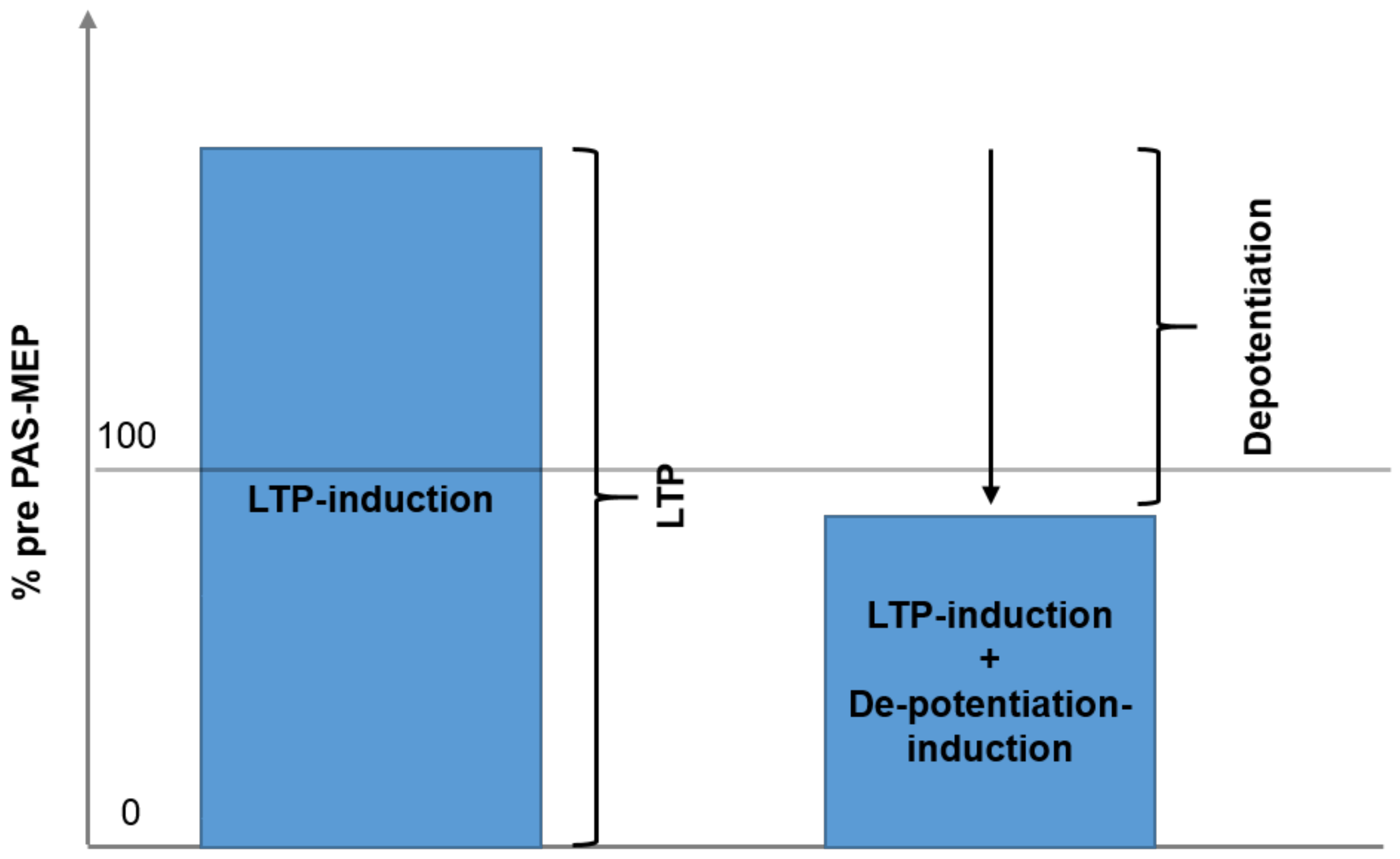
2.3.4. Session Type 2: DP
2.3.5. Coil Orientations
2.3.6. Statistics
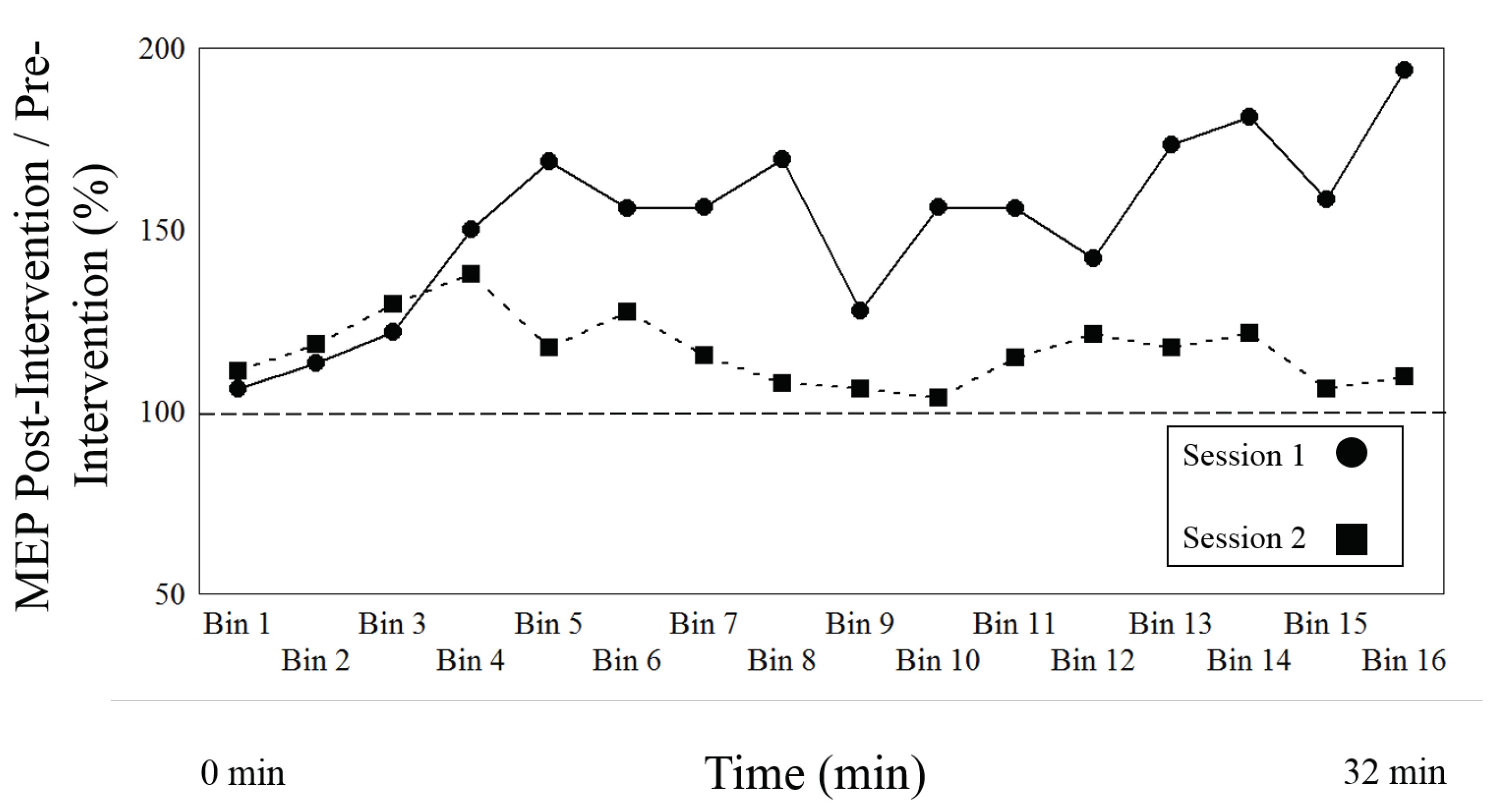
3. Results
3.1. Effect of Session (Type of Intervention, LTP vs. LTP + DP) and Time
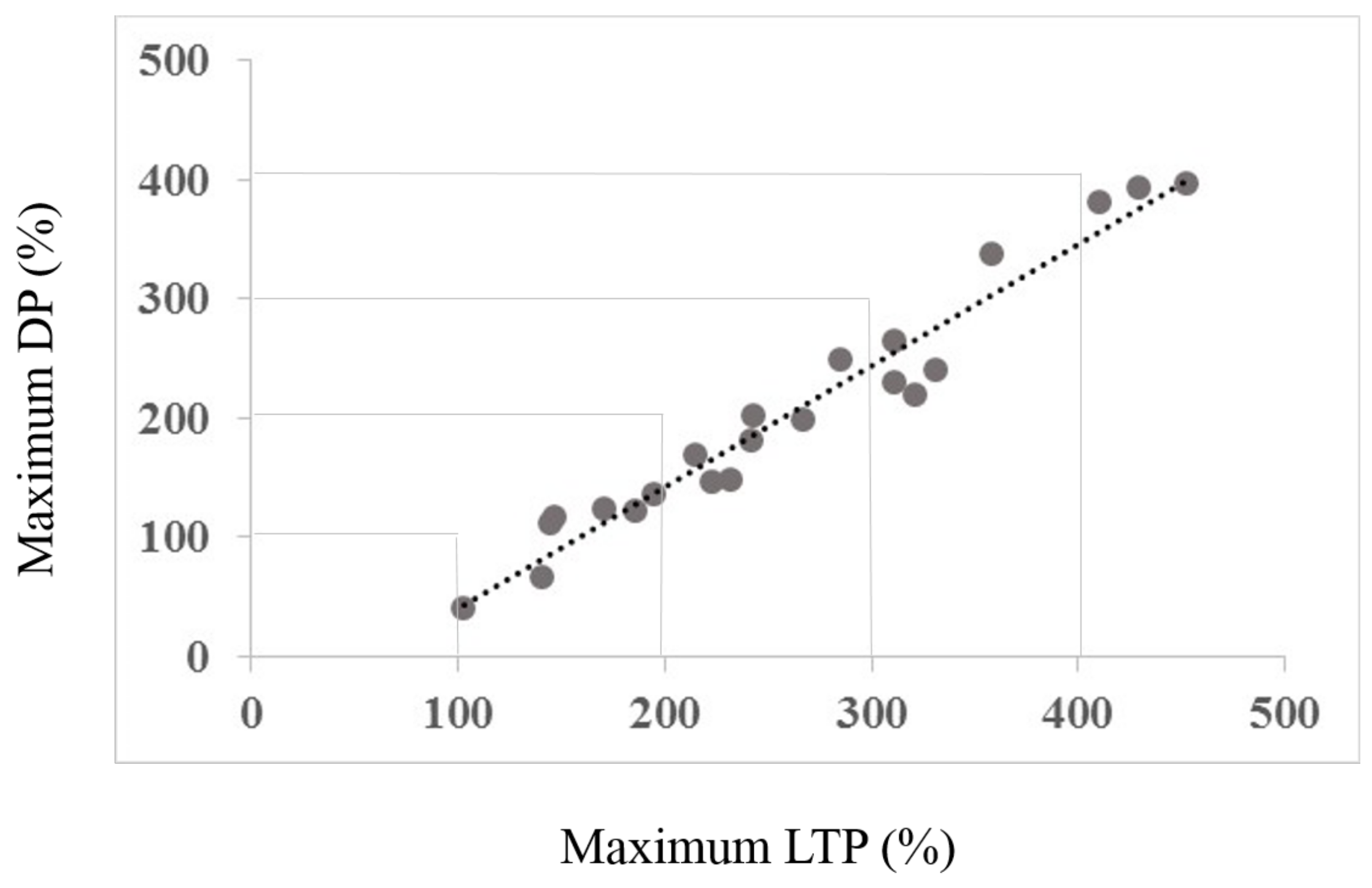
3.2. Interaction between Maximum LTP and Mamixum Change
4. Discussion
4.1. Difference of Effect on Sessions
4.2. Interaction between Maximum LTP (Induced by Session Type 1) and Maximum Change (Induced by Session Type 2)
4.3. Implication
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cohen, L.G.; Ziemann, U.; Chen, R.; Classen, J.; Hallett, M.; Gerloff, C.; Butefisch, C. Studies of neuroplasticity with transcranial magnetic stimulation. J. Clin. Neurophysiol. Off. Pub. Am. Electroencephalogr. Soc. 1998, 15, 305–324. [Google Scholar] [CrossRef]
- Bi, G.Q.; Poo, M.M. Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 10464–10472. [Google Scholar] [CrossRef]
- Gustafsson, B.; Wigström, H.; Abraham, W.C.; Huang, Y.Y. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J. Neurosci. Off. J. Soc. Neurosci. 1987, 7, 774–780. [Google Scholar] [CrossRef]
- Martin, S.J.; Grimwood, P.D.; Morris, R.G. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu. Rev. Neurosci. 2000, 23, 649–711. [Google Scholar] [CrossRef] [PubMed]
- Staubli, U.; Lynch, G. Stable hippocampal long-term potentiation elicited by “theta” pattern stimulation. Brain Res. 1987, 435, 227–234. [Google Scholar] [CrossRef]
- Rose, G.M.; Dunwiddie, T.V. Induction of hippocampal long-term potentiation using physiologically patterned stimulation. Neurosci. Lett. 1986, 69, 244–248. [Google Scholar] [CrossRef]
- Larson, J.; Wong, D.; Lynch, G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986, 368, 347–350. [Google Scholar] [CrossRef]
- Pavlides, C.; Greenstein, Y.J.; Grudman, M.; Winson, J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res. 1988, 439, 383–387. [Google Scholar] [CrossRef]
- Huerta, P.T.; Lisman, J.E. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature 1993, 364, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.W.; Cotman, C.W. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl d-aspartate antagonists. Neurosci. Lett. 1986, 70, 132–137. [Google Scholar] [CrossRef]
- Weisskopf, M.G.; Nicoll, R.A. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature 1995, 376, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Rothwell, J.C.; Lu, C.-S.; Chuang, W.-L.; Lin, W.-Y.; Chen, R.-S. Reversal of plasticity-like effects in the human motor cortex. J. Physiol. 2010, 588 Pt 19, 3683–3693. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Hsu, K.S. Progress in understanding the factors regulating reversibility of long-term potentiation. Rev. Neurosci. 2001, 12, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Kulla, A.; Manahan-Vaughan, D. Depotentiation in the dentate gyrus of freely moving rats is modulated by D1/D5 dopamine receptors. Cereb. Cortex 2000, 10, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.; Xiao, P.; Lynch, G. Reversal of LTP by theta frequency stimulation. Brain Res. 1993, 600, 97–102. [Google Scholar] [CrossRef]
- Barrionuevo, G.; Schottler, F.; Lynch, G. The effects of repetitive low frequency stimulation on control and “potentiated” synaptic responses in the hippocampus. Life Sci. 1980, 27, 2385–2391. [Google Scholar] [CrossRef]
- Bashir, Z.I.; Collingridge, G.L. An investigation of depotentiation of long-term potentiation in the CA1 region of the hippocampus. Exp. Brain Res. 1994, 100, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Staubli, U.; Lynch, G. Stable depression of potentiated synaptic responses in the hippocampus with 1–5 Hz stimulation. Brain Res. 1990, 513, 113–118. [Google Scholar] [CrossRef]
- Stäubli, U.; Chun, D. Factors regulating the reversibility of long-term potentiation. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 853–860. [Google Scholar] [CrossRef]
- Picconi, B.; Centonze, D.; Håkansson, K.; Bernardi, G.; Greengard, P.; Fisone, G.; Calabresi, P. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat. Neurosci. 2003, 6, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Martella, G.; Tassone, A.; Sciamanna, G.; Platania, P.; Cuomo, D.; Viscomi, M.T.; Pisani, A. Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: Role of endogenous acetylcholine. Brain J. Neurol. 2009, 132 Pt 9, 2336–2349. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.P.; Carter, R.J.; Lione, L.A.; Mangiarini, L.; Mahal, A.; Bates, G.P.; Morton, A.J. Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington’s disease mutation. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 5115–5123. [Google Scholar] [CrossRef]
- Beck, H.; Goussakov, I.V.; Lie, A.; Helmstaedter, C.; Elger, C.E. Synaptic Plasticity in the Human Dentate Gyrus. J. Neurosci. 2000, 20, 7080–7086. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Lee, S.; Kato, K.; Spencer, D.D.; Shepherd, G.M.; Williamson, A. Long-term modifications of synaptic efficacy in the human inferior and middle temporal cortex. Proc. Natl. Acad. Sci. USA 1996, 93, 8011–8015. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Kehl, S.J.; McLennan, H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J. Physiol. 1983, 334, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Errington, M.L.; Lynch, M.A.; Bliss, T.V. Long-term potentiation in the dentate gyrus: Induction and increased glutamate release are blocked by D(−)aminophosphonovalerate. Neuroscience 1987, 20, 279–284. [Google Scholar] [CrossRef]
- Morris, R.G.; Anderson, E.; Lynch, G.S.; Baudry, M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 1986, 319, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Stefan, K. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 2000, 123, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta burst stimulation of the human motor cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Pridmore, S.; Fernandes Filho, J.A.; Nahas, Z.; Liberatos, C.; George, M.S. Motor threshold in transcranial magnetic stimulation: A comparison of a neurophysiological method and a visualization of movement method. J. ECT 1998, 14, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Huang, C.C.; Hsu, K.S. Time-dependent reversal of long-term potentiation by low-frequency stimulation at the hippocampal mossy fiber-CA3 synapses. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 3705–3714. [Google Scholar] [CrossRef]
- Stäubli, U.; Scafidi, J. Time-Dependent Reversal of Long-Term Potentiation in Area CA1 of the Freely Moving Rat Induced by Theta Pulse Stimulation. J. Neurosci. 1999, 19, 8712–8719. [Google Scholar] [CrossRef]
- Wassermann, E.M. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, 5–7 June 1996. Electroencephalogr. Clin. Neurophysiol. 1998, 108, 1–16. [Google Scholar] [CrossRef]
- Zhang, L.; Kirschstein, T.; Sommersberg, B.; Merkens, M.; Manahan-Vaughan, D.; Elgersma, Y.; Beck, H. Hippocampal synaptic metaplasticity requires inhibitory autophosphorylation of Ca2+/calmodulin-dependent kinase II. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 7697–7707. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, C.; Anwyl, R.; Rowan, M.J. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can Be depotentiated by stimulation on the negative phase in area CA1 in vivo. J. Neurosci. Off. J. Soc. Neurosci. 1997, 17, 6470–6477. [Google Scholar] [CrossRef]
- Rossini, P.M.; Barker, A.T.; Berardelli, A.; Caramia, M.D.; Caruso, G.; Cracco, R.Q.; Tomberg, C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Saturno, E.; Pilato, F.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Expe. Brain Res. 2001, 138, 268–273. [Google Scholar] [CrossRef]
- O’Dell, T.J.; Kandel, E.R. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn. Mem. 1994, 1, 129–139. [Google Scholar] [PubMed]
- Yamazaki, Y.; Sugihara, T.; Goto, J.-I.; Chida, K.; Fujiwara, H.; Kaneko, K.; Mikoshiba, K. Role of inositol 1, 4, 5-trisphosphate receptors in the postsynaptic expression of guinea pig hippocampal mossy fiber depotentiation. Brain Res. 2011, 1387, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Huerta, P.T.; Lisman, J.E. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron 1995, 15, 1053–1063. [Google Scholar] [CrossRef]
- Huerta, P.T.; Lisman, J.E. Synaptic plasticity during the cholinergic theta-frequency oscillation in vitro. Hippocampus 1996, 6, 58–61. [Google Scholar] [CrossRef]
- Rioult-Pedotti, M.S.; Friedman, D.; Donoghue, J.P. Learning-induced LTP in neocortex. Science 2000, 290, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Rioult-Pedotti, M.S.; Friedman, D.; Hess, G.; Donoghue, J.P. Strengthening of horizontal cortical connections following skill learning. Nat. Neurosci. 1998, 1, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Rioult-Pedotti, M.-S.; Donoghue, J.P.; Dunaevsky, A. Plasticity of the synaptic modification range. J. Neurophysiol. 2007, 98, 3688–3695. [Google Scholar] [CrossRef] [PubMed]
- Malenka, R.C. Postsynaptic factors control the duration of synaptic enhancement in area CA1 of the hippocampus. Neuron 1991, 6, 53–60. [Google Scholar] [CrossRef]
- Goelet, P.; Castellucci, V.F.; Schacher, S.; Kandel, E.R. The long and the short of long-term memory—A molecular framework. Nature 1986, 322, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Krug, M.; Lössner, B.; Ott, T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res. Bull. 1984, 13, 39–42. [Google Scholar] [CrossRef]
- Nguyen, P.V.; Abel, T.; Kandel, E.R. Requirement of a critical period of transcription for induction of a late phase of LTP. Science 1994, 265, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Frey, U.; Morris, R.G. Synaptic tagging and long-term potentiation. Nature 1997, 385, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Liang, Y.-C.; Hsu, K.-S. A Role for Extracellular Adenosine in Time-Dependent Reversal of Long-Term Potentiation by Low-Frequency Stimulation at Hippocampal CA1 Synapses. J. Neurosci. 1999, 19, 9728–9738. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Rothwell, J.C.; Edwards, M.J.; Chen, R.-S. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb. Cortex 2008, 18, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Staubli, U.; Chun, D. Proactive and retrograde effects on LTP produced by theta pulse stimulation: Mechanisms and characteristics of LTP reversal in vitro. Learn. Mem. 1996, 3, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, R.A.; Ji, Z.; Standish, S.; Boyd-Hodgson, T.E.; Henderson, A.K.; Racine, R.J. Training-induced and electrically induced potentiation in the neocortex. Neurobiol. Learn. Mem. 2005, 83, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Monfils, M.-H.; Teskey, G.C. Skilled-learning-induced potentiation in rat sensorimotor cortex: A transient form of behavioural long-term potentiation. Neuroscience 2004, 125, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Cantarero, G.; Lloyd, A.; Celnik, P. Reversal of Long-Term Potentiation-Like Plasticity Processes after Motor Learning Disrupts Skill Retention. J. Neurosci. 2013, 33, 12862–12869. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Murase, N.; Hasan, A.; Balaratnam, M.; Rothwell, J.C. The role of interneuron networks in driving human motor cortical plasticity. Cereb. Cortex 2013, 23, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Saturno, E.; Pilato, F.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr. Clin. Neurophysiol./Electromyogr. Motor Control 1998, 109, 397–401. [Google Scholar] [CrossRef]
- Abraham, W.C.; Christie, B.R.; Logan, B.; Lawlor, P.; Dragunow, M. Immediate early gene expression associated with the persistence of heterosynaptic long-term depression in the hippocampus. Proc. Natl. Acad. Sci. USA 1994, 91, 10049–10053. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.C.; Mason-Parker, S.E.; Williams, J.; Dragunow, M. Analysis of the decremental nature of LTP in the dentate gyrus. Brain Res. Mol. Brain Res. 1995, 30, 367–372. [Google Scholar] [CrossRef]
- Doyère, V.; Burette, F.; Rédini-Del Negro, C.; Laroche, S. Long-term potentiation of hippocampal afferents and efferents to prefrontal cortex: Implications for associative learning. Neuropsychologia 1993, 31, 1031–1053. [Google Scholar] [CrossRef]
- Jeffery, K.J.; Abraham, W.C.; Dragunow, M.; Mason, S.E. Induction of Fos-like immunoreactivity and the maintenance of long-term potentiation in the dentate gyrus of unanesthetized rats. Brain Res. Mol. Brain Res. 1990, 8, 267–274. [Google Scholar] [CrossRef]
- McEachern, J.C.; Shaw, C.A. The plasticity-pathology continuum: Defining a role for the LTP phenomenon. J. Neurosci. Res. 1999, 58, 42–61. [Google Scholar] [CrossRef]
| Subject | RMT (% mso) |
|---|---|
| 1 | 63 |
| 2 | 73 |
| 3 | 50 |
| 4 | 47 |
| 5 | 54 |
| 6 | 60 |
| 7 | 60 |
| 8 | 54 |
| 9 | 58 |
| 10 | 58 |
| 11 | 55 |
| 12 | 43 |
| 13 | 44 |
| 14 | 55 |
| 15 | 44 |
| 16 | 58 |
| 17 | 70 |
| 18 | 47 |
| 19 | 41 |
| 20 | 54 |
| 21 | 53 |
| 22 | 61 |
| Mean | 54.64 |
| Standard Deviation | 8.16 |
| Standard Error | 1.74 |
| Subject | AP Latency (ms) | LM Latency (ms) | Latency (ms) (Difference AP-LM) |
|---|---|---|---|
| 1 | 21.98 | 17.68 | 4.30 |
| 2 | 24.25 | 19.85 | 4.40 |
| 3 | 24.13 | 21.44 | 2.69 |
| 4 | 21.28 | 20.02 | 1.79 |
| 5 | 22.69 | 19.27 | 3.43 |
| 6 | 24.50 | 22.31 | 2.19 |
| 7 | 24.28 | 22.52 | 1.76 |
| 8 | 23.36 | 20.61 | 2.76 |
| 9 | 24.02 | 20.16 | 3.86 |
| 10 | 24.39 | 20.57 | 3.82 |
| 11 | 24.18 | 22.75 | 1.43 |
| 12 | 22.71 | 20.67 | 2.05 |
| 13 | 22.74 | 20.26 | 2.48 |
| 14 | 22.03 | 20.35 | 1.68 |
| 15 | 21.75 | 20.71 | 1.03 |
| 16 | 25.08 | 23.53 | 1.56 |
| 17 | 22.28 | 19.42 | 2.87 |
| 18 | 22.52 | 20.20 | 2.32 |
| 19 | 22.39 | 21.37 | 1.02 |
| 20 | 22.83 | 20.18 | 2.65 |
| 21 | 24.05 | 22.08 | 1.97 |
| 22 | 23.17 | 19.70 | 3.47 |
| Mean | 23.23 | 20.71 | 2.52 |
| Variable (1-Session, 2-Time) | df Effect | MS Effect | df | MS Error | F | p-Level |
|---|---|---|---|---|---|---|
| 1 | 1 | 219684.1094 | 21 | 28121.38672 | 7.811994553 | 0.010850717 |
| 2 | 15 | 6917.164063 | 315 | 2978.043457 | 2.322721004 | 0.003729491 |
| 12 | 15 | 8113.486328 | 315 | 2825.038086 | 2.871991873 | 0.000298259 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroarena-Leal, N.; Heidemeyer, L.; Trenado, C.; Ruge, D. Human Depotentiation following Induction of Spike Timing Dependent Plasticity. Biomedicines 2018, 6, 71. https://doi.org/10.3390/biomedicines6020071
Pedroarena-Leal N, Heidemeyer L, Trenado C, Ruge D. Human Depotentiation following Induction of Spike Timing Dependent Plasticity. Biomedicines. 2018; 6(2):71. https://doi.org/10.3390/biomedicines6020071
Chicago/Turabian StylePedroarena-Leal, Nicole, Larissa Heidemeyer, Carlos Trenado, and Diane Ruge. 2018. "Human Depotentiation following Induction of Spike Timing Dependent Plasticity" Biomedicines 6, no. 2: 71. https://doi.org/10.3390/biomedicines6020071
APA StylePedroarena-Leal, N., Heidemeyer, L., Trenado, C., & Ruge, D. (2018). Human Depotentiation following Induction of Spike Timing Dependent Plasticity. Biomedicines, 6(2), 71. https://doi.org/10.3390/biomedicines6020071




