Muscle–Bone Crosstalk: Emerging Opportunities for Novel Therapeutic Approaches to Treat Musculoskeletal Pathologies
Abstract
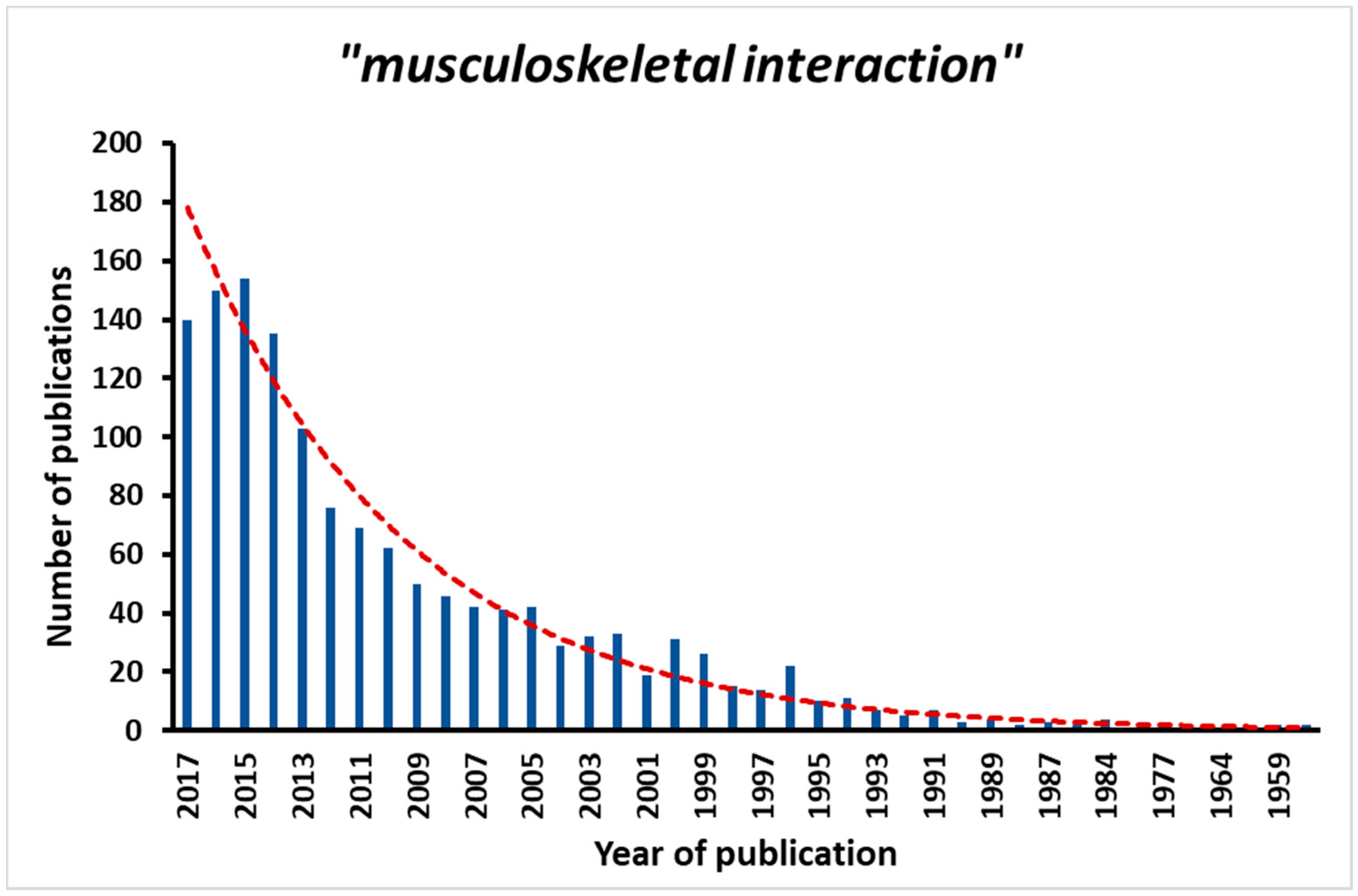
1. Introduction
2. Biomechanical Regulation of Muscle and Bone
3. Biochemical Communication between Muscle and Bone: Muscle and Bone as Endocrine Organs
4. Muscle Secreted Factors Have Effects on Bone Tissue
5. Bone Secreted Factors Have Effects on Muscle Tissue
6. Common Mechanisms Influencing Bone and Muscle Mass
7. Indirect Links
8. Nervous System
9. Fracture Healing
10. Other Factors Affecting the Musculoskeletal Health—The Molecular Clock
11. Pharmacological Interventions
12. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fabbri, E.; Zoli, M.; Gonzalez-Freire, M.; Salive, M.E.; Studenski, S.A.; Ferrucci, L. Aging and Multimorbidity: New Tasks, Priorities, and Frontiers for Integrated Gerontological and Clinical Research. J. Am. Med. Dir. Assoc. 2015, 16, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, Dynapenia, and the Impact of Advancing Age on Human Skeletal Muscle Size and Strength; a Quantitative Review. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Avin, K.G.; Bloomfield, S.A.; Gross, T.S.; Warden, S.J. Biomechanical Aspects of the Muscle-Bone Interaction. Curr. Osteoporos. Rep. 2015, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, H.; Frost, H.M.; Jee, W.S.S. Estrogen and Bone-Muscle Strength and Mass Relationships. Bone 1998, 22, 1–6. [Google Scholar] [CrossRef]
- Ducher, G.; Courteix, D.; Même, S.; Magni, C.; Viala, J.F.; Benhamou, C.L. Bone geometry in response to long-term tennis playing and its relationship with muscle volume: A quantitative magnetic resonance imaging study in tennis players. Bone 2005, 37, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.-Y.; Beaudart, C.; Buckinx, F.; Bruyère, O. Osteoporosis and sarcopenia: Two diseases or one? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 31–36. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lee, S.-J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McPherron, A.C.; Lovejoy, C.O.; Hudson, J. Femoral morphology and cross-sectional geometry of adult myostatin-deficient mice. Bone 2000, 27, 343–349. [Google Scholar] [CrossRef]
- Quinn, L.S.; Anderson, B.G.; Strait-Bodey, L.; Stroud, A.M.; Argilés, J.M. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E191–E202. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W. The skeletal muscle secretome: An emerging player in muscle–bone crosstalk. BoneKEy Rep. 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Harry, L.E.; Sandison, A.; Paleolog, E.M.; Hansen, U.; Pearse, M.F.; Nanchahal, J. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J. Orthop. Res. 2008, 26, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Muscle, bone, and the Utah paradigm: A 1999 overview. Med. Sci. Sports Exerc. 2000, 32, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Sharir, A.; Stern, T.; Rot, C.; Shahar, R.; Zelzer, E. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development 2011, 138, 3247–3259. [Google Scholar] [CrossRef] [PubMed]
- Rot-Nikcevic, I.; Reddy, T.; Downing, K.J.; Belliveau, A.C.; Hallgrímsson, B.; Hall, B.K.; Kablar, B. Myf5−/−:MyoD−/− amyogenic fetuses reveal the importance of early contraction and static loading by striated muscle in mouse skeletogenesis. Dev. Genes Evol. 2006, 216, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gunter, K.B.; Almstedt, H.C.; Janz, K.F. Physical Activity in Childhood May Be the Key to Optimizing Lifespan Skeletal Health. Exerc. Sport Sci. Rev. 2012, 40, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 275A, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- Dudley-Javoroski, S.; Shields, R.K. Muscle and bone plasticity after spinal cord injury: Review of adaptations to disuse and to electrical muscle stimulation. J. Rehabil. Res. Dev. 2008, 45, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F. Regulation of bone remodeling by the central and peripheral nervous system. Arch. Biochem. Biophys. 2008, 473, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Poliachik, S.L.; Bain, S.D.; Threet, D.; Huber, P.; Gross, T.S. Transient muscle paralysis disrupts bone homeostasis by rapid degradation of bone morphology. Bone 2010, 46, 18. [Google Scholar] [CrossRef] [PubMed]
- Manske, S.L.; Boyd, S.K.; Zernicke, R.F. Muscle and bone follow similar temporal patterns of recovery from muscle-induced disuse due to botulinum toxin injection. Bone 2010, 46, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.A.; Lang, C.H.; Zhang, Y.; Paul, E.M.; Laufenberg, L.J.; Lewis, G.S.; Donahue, H.J. Interdependence of Muscle Atrophy and Bone Loss Induced by Mechanical Unloading. J. Bone Miner. Res. 2014, 29, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J.; Galley, M.R.; Richard, J.S.; George, L.A.; Dirks, R.C.; Guildenbecher, E.A.; Judd, A.M.; Robling, A.G.; Fuchs, R.K. Reduced gravitational loading does not account for the skeletal effect of botulinum toxin-induced muscle inhibition suggesting a direct effect of muscle on bone. Bone 2013, 54, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Brotto, M.; Johnson, M.L. Endocrine Crosstalk between Muscle and Bone. Curr. Osteoporos. Rep. 2014, 12, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, N.; Chan, M.H.S.; Bisucci, T.; Darby, I.A.; Febbraio, M.A. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: Evidence of fiber type specificity. FASEB J. 2004, 18, 992–994. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Wallenius, V.; Wallenius, K.; Ahrén, B.; Rudling, M.; Carlsten, H.; Dickson, S.L.; Ohlsson, C.; Jansson, J.-O. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002, 8, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Åkerström, T.C.A.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Cuppini, R.; Sartini, S.; Agostini, D.; Guescini, M.; Ambrogini, P.; Betti, M.; Bertini, L.; Vallasciani, M.; Stocchi, V. Bdnf expression in rat skeletal muscle after acute or repeated exercise. Arch. Ital. Biol. 2007, 145, 99–110. [Google Scholar] [PubMed]
- Yu, T.; Chang, Y.; Gao, X.L.; Li, H.; Zhao, P. Dynamic Expression and the Role of BDNF in Exercise-induced Skeletal Muscle Regeneration. Int. J. Sports Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Aström, M.-B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.L.; Cleary, A.S.; Speaker, K.J.; Lindsay, S.F.; Uyenishi, J.; Reed, J.M.; Madden, M.C.; Mehan, R.S. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E918–E927. [Google Scholar] [CrossRef] [PubMed]
- Langley, B.; Thomas, M.; Bishop, A.; Sharma, M.; Gilmour, S.; Kambadur, R. Myostatin Inhibits Myoblast Differentiation by Down-regulating MyoD Expression. J. Biol. Chem. 2002, 277, 49831–49840. [Google Scholar] [CrossRef] [PubMed]
- Joulia-Ekaza, D.; Cabello, G. The myostatin gene: Physiology and pharmacological relevance. Curr. Opin. Pharmacol. 2007, 7, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, J.; Tang, W.; Jiang, X.; Rowe, D.W.; Quarles, L.D. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E38–E49. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shiizaki, K.; Kuro-o, M.; Moe, O.W. Fibroblast Growth Factor 23 and Klotho: Physiology and Pathophysiology of an Endocrine Network of Mineral Metabolism. Annu. Rev. Physiol. 2013, 75, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Quarles, L.D. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat. Rev. Endocrinol. 2012, 8, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Gattineni, J.; Bates, C.; Twombley, K.; Dwarakanath, V.; Robinson, M.L.; Goetz, R.; Mohammadi, M.; Baum, M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am. J. Physiol. Ren. Physiol. 2009, 297, F282–F291. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.-C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.K.; Price, P.A. Proof that the gamma-carboxyglutamic acid-containing bone protein is synthesized in calf bone. Comparative synthesis rate and effect of coumadin on synthesis. J. Biol. Chem. 1979, 254, 437–441. [Google Scholar] [PubMed]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Balemans, W.; Piters, E.; Cleiren, E.; Ai, M.; Van Wesenbeeck, L.; Warman, M.L.; Van Hul, W. The binding between sclerostin and LRP5 is altered by DKK1 and by high-bone mass LRP5 mutations. Calcif. Tissue Int. 2008, 82, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.L.; Drake, M.T. Clinical utility of serum sclerostin measurements. BoneKEy Rep. 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.-S.M.; Rouzi, A.A.; Al-Sibiani, S.A.; Al-Senani, N.S.; Qari, M.H.; Mousa, S.A. High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: The Center of Excellence for Osteoporosis Research Study. J. Bone Miner. Res. 2012, 27, 2592–2602. [Google Scholar] [CrossRef] [PubMed]
- Toyosawa, S.; Shintani, S.; Fujiwara, T.; Ooshima, T.; Sato, A.; Ijuhin, N.; Komori, T. Dentin Matrix Protein 1 Is Predominantly Expressed in Chicken and Rat Osteocytes but not in Osteoblasts. J. Bone Miner. Res. 2001, 16, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Q.; Ward, L.M.; Liu, S.; Lu, Y.; Xie, Y.; Yuan, B.; Yu, X.; Rauch, F.; Davis, S.I.; Zhang, S.; et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006, 38, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Linkhart, T.A.; Mohan, S.; Baylink, D.J. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone 1996, 19, S1–S12. [Google Scholar] [CrossRef]
- Ishitobi, H.; Matsumoto, K.; Azami, T.; Itoh, F.; Itoh, S.; Takahashi, S.; Ema, M. Flk1-GFP BAC Tg mice: An animal model for the study of blood vessel development. Exp. Anim. 2010, 59, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Juffer, P.; Jaspers, R.T.; Klein-Nulend, J.; Bakker, A.D. Mechanically Loaded Myotubes Affect Osteoclast Formation. Calcif. Tissue Int. 2014, 94, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.C.; McGregor, N.E.; Poulton, I.J.; Pompolo, S.; Allan, E.H.; Quinn, J.M.; Gillespie, M.T.; Martin, T.J.; Sims, N.A. Cardiotrophin-1 Is an Osteoclast-Derived Stimulus of Bone Formation Required for Normal Bone Remodeling. J. Bone Miner. Res. 2008, 23, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A. Cell-specific paracrine actions of IL-6 family cytokines from bone, marrow and muscle that control bone formation and resorption. Int. J. Biochem. Cell Biol. 2016, 79, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Manes-Gravina, E.; Spaziani, L.; Landi, F.; Bernabei, R.; Marzetti, E. Bone-muscle crosstalk: Unraveling new therapeutic targets for osteoporosis. Curr. Pharm. Des. 2017. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Mongelli, T.; Colucci, S.; Cinti, S.; Grano, M. Crosstalk Between Muscle and Bone Via the Muscle-Myokine Irisin. Curr. Osteoporos. Rep. 2016, 14, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin Enhances Osteoblast Differentiation In Vitro. Int. J. Endocrinol. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.; Shi, X.; Zhang, W.; Pennington, C.; Thakore, H.; Haque, M.; Kang, B.; Isales, C.M.; Fulzele, S.; Wenger, K. Loss of Myostatin (GDF8) Function Increases Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells but the Osteogenic Effect is Ablated with Unloading. Bone 2007, 40, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Dankbar, B.; Fennen, M.; Brunert, D.; Hayer, S.; Frank, S.; Wehmeyer, C.; Beckmann, D.; Paruzel, P.; Bertrand, J.; Redlich, K.; et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat. Med. 2015, 21, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Jähn, K.; Lara-Castillo, N.; Brotto, L.; Mo, C.L.; Johnson, M.L.; Brotto, M.; Bonewald, L.F. Skeletal Muscle Secreted Factors Prevent Glucocorticoid-Induced Osteocyte Apoptosis through Activation of B-Catenin. Eur. Cells Mater. 2012, 24, 197–210. [Google Scholar] [CrossRef]
- Mera, P.; Laue, K.; Ferron, M.; Confavreux, C.; Wei, J.; Galán-Díez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016, 23, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Klein-Nulend, J.; Bonewald, L. Principles of Bone Biology; Bilezikian, J.P., Raisz, L.G., Eds.; Academic Press: Amsterdam, The Netherlands, 2008; Volume 1, ISBN 978-0-08-056875-1. [Google Scholar]
- Bonewald, L.F.; Johnson, M.L. Osteocytes, Mechanosensing and Wnt Signaling. Bone 2008, 42, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mo, C.; Bonewald, L.; Brotto, M. Wnt3a potentiates myogenesis in C2C12 myoblasts through changes of signaling pathways including Wnt and NFκB. In Proceedings of the ASBMR 2014 Annual Meeting, Houston, TX, USA, 12–15 September 2014; p. s266. [Google Scholar]
- Mo, C.; Romero-Suarez, S.; Bonewald, L.; Johnson, M.; Brotto, M. Prostaglandin E2: From clinical applications to its potential role in bone-muscle crosstalk and myogenic differentiation. Recent Pat. Biotechnol. 2012, 6, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Levinger, I.; Scott, D.; Nicholson, G.C.; Stuart, A.L.; Duque, G.; McCorquodale, T.; Herrmann, M.; Ebeling, P.R.; Sanders, K.M. Undercarboxylated osteocalcin, muscle strength and indices of bone health in older women. Bone 2014, 64, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Laue, K.; Wei, J.; Berger, J.M.; Karsenty, G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol. Metab. 2016, 5, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Mera, P. Molecular bases of the crosstalk between bone and muscle. Bone 2017. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.K.; Spector, T.D. Genetic Influences on Muscle Strength, Lean Body Mass, and Bone Mineral Density: A Twin Study. J. Bone Miner. Res. 1997, 12, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Prior, S.J.; Roth, S.M.; Wang, X.; Kammerer, C.; Miljkovic-Gacic, I.; Bunker, C.H.; Wheeler, V.W.; Patrick, A.L.; Zmuda, J.M. Genetic and environmental influences on skeletal muscle phenotypes as a function of age and sex in large, multigenerational families of African heritage. J. Appl. Physiol. 2007, 103, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.G.; Lyons, G.E.; Martin, J.F.; Olson, E.N. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development 1994, 120, 1251–1263. [Google Scholar] [PubMed]
- Kramer, I.; Baertschi, S.; Halleux, C.; Keller, H.; Kneissel, M. Mef2c deletion in osteocytes results in increased bone mass. J. Bone Miner. Res. 2012, 27, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F.; Kiel, D.; Clemens, T.; Esser, K.; Orwoll, E.; O’Keefe, R.; Fielding, R. Forum on Bone and Skeletal Muscle Interactions: Summary of the Proceedings of an ASBMR Workshop. J. Bone Miner. Res. 2013, 28, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Meyer, A.; Jacobi, C.; Feige, J.N.; Glass, D.J. TAK-1/p38/nNFκB signaling inhibits myoblast differentiation by increasing levels of Activin A. Skelet. Muscle 2012, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Abdelmagid, S.M.; Belcher, J.Y.; Moussa, F.M.; Lababidi, S.L.; Sondag, G.R.; Novak, K.M.; Sanyurah, A.S.; Frara, N.A.; Razmpour, R.; Del Carpio-Cano, F.E.; et al. Mutation in Osteoactivin Decreases Bone Formation In Vivo and Osteoblast Differentiation In Vitro. Am. J. Pathol. 2014, 184, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.H.-C.; Wergedal, J.E.; Mohan, S.; Amoui, M.; Baylink, D.J.; Lau, K.-H.W. Targeted Overexpression of Osteoactivin in Cells of Osteoclastic Lineage Promotes Osteoclastic Resorption and Bone Loss in Mice. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, T.; Ishidoh, K.; Hirasaka, K.; Ishihara, I.; Ikemoto, M.; Kano, M.; Kominami, E.; Nonaka, I.; Ogawa, T.; Adams, G.R.; et al. Skeletal muscle gene expression in space-flown rats. FASEB J. 2004, 18, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Sondag, G.R.; Salihoglu, S.; Lababidi, S.L.; Crowder, D.C.; Moussa, F.M.; Abdelmagid, S.M.; Safadi, F.F. Osteoactivin induces transdifferentiation of C2C12 myoblasts into osteoblasts. J. Cell. Physiol. 2014, 229, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Venken, K.; Movérare-Skrtic, S.; Kopchick, J.J.; Coschigano, K.T.; Ohlsson, C.; Boonen, S.; Bouillon, R.; Vanderschueren, D. Impact of androgens, growth hormone, and IGF-I on bone and muscle in male mice during puberty. J. Bone Miner. Res. 2007, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Paloyan, E.; Gopalsami, C. Symptoms in patients with primary hyperparathyroidism: Muscle weakness or sleepiness. Endocr. Pract. 2004, 10, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.; Manolagas, S.C. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone 2015, 80, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, R.; Kasperk, C. Glucocorticoid-induced osteoporosis: Prevention and treatment. Steroids 1998, 63, 344–348. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Jilka, R.L.; Parfitt, A.M.; Manolagas, S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Investig. 1998, 102, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.S.; Feeback, D.L. Mechanical load induces sarcoplasmic wounding and FGF release in differentiated human skeletal muscle cultures. FASEB J. 1996, 10, 502–509. [Google Scholar] [PubMed]
- Liang, H.; Pun, S.; Wronski, T.J. Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology 1999, 140, 5780–5788. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.F.; Chang, H.; Knothe Tate, M.L. Elucidating Multiscale Periosteal Mechanobiology: A Key to Unlocking the Smart Properties and Regenerative Capacity of the Periosteum? Tissue Eng. Part B Rev. 2013, 19, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McNeil, P.L.; Patterson, S.L. Role of muscle-derived growth factors in bone formation. J. Musculoskelet. Neuronal Interact. 2010, 10, 64–70. [Google Scholar] [PubMed]
- Lai, X.; Price, C.; Lu, X. (Lucas); Wang, L. Imaging and Quantifying Solute Transport across Periosteum: Implications for Muscle-Bone Crosstalk. Bone 2014, 66, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin Regulates Bone Formation via the Sympathetic Nervous System. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Baldock, P.A.; Sainsbury, A.; Couzens, M.; Enriquez, R.F.; Thomas, G.P.; Gardiner, E.M.; Herzog, H. Hypothalamic Y2 receptors regulate bone formation. J. Clin. Investig. 2002, 109, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Baldock, P.A.; Allison, S.J.; Lundberg, P.; Lee, N.J.; Slack, K.; Lin, E.-J.D.; Enriquez, R.F.; McDonald, M.M.; Zhang, L.; During, M.J.; et al. Novel Role of Y1 Receptors in the Coordinated Regulation of Bone and Energy Homeostasis. J. Biol. Chem. 2007, 282, 19092–19102. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.J.; Nguyen, A.D.; Enriquez, R.F.; Doyle, K.L.; Sainsbury, A.; Baldock, P.A.; Herzog, H. Osteoblast specific Y1 receptor deletion enhances bone mass. Bone 2011, 48, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Houweling, P.; Kulkarni, R.N.; Baldock, P.A. Neuronal control of bone and muscle. Bone 2015, 80, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, R.T.; Hodge, K.M.B.; Cody, D.B.; Sheldon, R.J.; Kobilka, B.K.; Isfort, R.J. Skeletal muscle hypertrophy and anti-atrophy effects of clenbuterol are mediated by the beta2-adrenergic receptor. Muscle Nerve 2002, 25, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Joassard, O.R.; Durieux, A.-C.; Freyssenet, D.G. β2-Adrenergic agonists and the treatment of skeletal muscle wasting disorders. Int. J. Biochem. Cell Biol. 2013, 45, 2309–2321. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.S.; Ryall, J.G. Role of β-Adrenoceptor Signaling in Skeletal Muscle: Implications for Muscle Wasting and Disease. Physiol. Rev. 2008, 88, 729–767. [Google Scholar] [CrossRef] [PubMed]
- Downie, D.; Delday, M.I.; Maltin, C.A.; Sneddon, A.A. Clenbuterol increases muscle fiber size and GATA-2 protein in rat skeletal muscle in utero. Mol. Reprod. Dev. 2008, 75, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Beitzel, F.; Sillence, M.N.; Lynch, G.S. β-Adrenoceptor signaling in regenerating skeletal muscle after β-agonist administration. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E932–E940. [Google Scholar] [CrossRef] [PubMed]
- Beitzel, F.; Gregorevic, P.; Ryall, J.G.; Plant, D.R.; Sillence, M.N.; Lynch, G.S. β2-Adrenoceptor agonist fenoterol enhances functional repair of regenerating rat skeletal muscle after injury. J. Appl. Physiol. 2004, 96, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Armamento-Villareal, R.; Parimi, N.; Chode, S.; Sinacore, D.R.; Hilton, T.N.; Napoli, N.; Qualls, C.; Villareal, D.T. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip BMD induced by weight loss despite decline in bone-active hormones. J. Bone Miner. Res. 2011, 26, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Bermeo, S.; Gunaratnam, K.; Duque, G. Fat and bone interactions. Curr. Osteoporos. Rep. 2014, 12, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Takahashi, N.; Akatsu, T.; Tanaka, H.; Sasaki, T.; Nishihara, T.; Koga, T.; Martin, T.J.; Suda, T. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7260–7264. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.K.; Raggatt, L.-J.; Alexander, K.A.; Kuliwaba, J.S.; Fazzalari, N.L.; Schroder, K.; Maylin, E.R.; Ripoll, V.M.; Hume, D.A.; Pettit, A.R. Osteal Tissue Macrophages Are Intercalated throughout Human and Mouse Bone Lining Tissues and Regulate Osteoblast Function In Vitro and In Vivo. J. Immunol. 2008, 181, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Yamashita, Y.; Abe, T.; Hirasaka, K.; Oarada, M.; Ohno, A.; Teshima-Kondo, S.; Higashibata, A.; Choi, I.; Mills, E.M.; et al. Unloading stress disturbs muscle regeneration through perturbed recruitment and function of macrophages. J. Appl. Physiol. 2012, 112, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Utvåg, S.E.; Iversen, K.B.; Grundnes, O.; Reikerås, O. Poor muscle coverage delays fracture healing in rats. Acta Orthop. Scand. 2002, 73, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.; Perren, S.M.; Cordey, J.; Kenwright, J.; Mosheiff, R.; Francis, M.J.O. The muscle bed—A crucial factor for fracture healing: A physiological concept. Orthopedics 2002, 25, 1379–1383. [Google Scholar] [PubMed]
- Hao, Y.; Ma, Y.; Wang, X.; Jin, F.; Ge, S. Short-term muscle atrophy caused by botulinum toxin-A local injection impairs fracture healing in the rat femur. J. Orthop. Res. 2012, 30, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Schindeler, A.; Liu, R.; Little, D.G. The contribution of different cell lineages to bone repair: Exploring a role for muscle stem cells. Differ. Res. Biol. Divers. 2009, 77, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Schindeler, A.; Little, D.G. The potential role of muscle in bone repair. J. Musculoskelet. Neuronal Interact. 2010, 10, 71–76. [Google Scholar] [PubMed]
- Prasadam, I.; Zhou, Y.; Du, Z.; Chen, J.; Crawford, R.; Xiao, Y. Osteocyte-induced angiogenesis via VEGF-MAPK-dependent pathways in endothelial cells. Mol. Cell. Biochem. 2014, 386, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.A.; Esser, K.A. The Role of the Molecular Clock in Skeletal Muscle and What It Is Teaching Us about Muscle-Bone Crosstalk. Curr. Osteoporos. Rep. 2017, 15, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Schroder, E.A.; Harfmann, B.D.; Zhang, X.; Srikuea, R.; England, J.H.; Hodge, B.A.; Wen, Y.; Riley, L.A.; Yu, Q.; Christie, A.; et al. Intrinsic muscle clock is necessary for musculoskeletal health. J. Physiol. 2015, 593, 5387–5404. [Google Scholar] [CrossRef] [PubMed]
- Hodge, B.A.; Wen, Y.; Riley, L.A.; Zhang, X.; England, J.H.; Harfmann, B.D.; Schroder, E.A.; Esser, K.A. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet. Muscle 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.P.; Huffman, N.T.; Vallejo, J.; Brotto, L.; Chittur, S.V.; Breggia, A.; Stern, A.; Huang, J.; Mo, C.; Seidah, N.G.; et al. Deletion of Mbtps1 (Pcsk8, S1p, Ski-1) Gene in Osteocytes Stimulates Soleus Muscle Regeneration and Increased Size and Contractile Force with Age. J. Biol. Chem. 2016, 291, 4308–4322. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.P.; Price, J.L. Bone muscle crosstalk targets muscle regeneration pathway regulated by core circadian transcriptional repressors DEC1 and DEC2. BoneKEy Rep. 2016, 5, 850. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, C.P.; Graham, Z.A. Muscle-bone interactions: Movement in the field of mechano-humoral coupling of muscle and bone. Ann. N. Y. Acad. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bernabei, R.; Martone, A.M.; Ortolani, E.; Landi, F.; Marzetti, E. Screening, diagnosis and treatment of osteoporosis: A brief review. Clin. Cases Miner. Bone Metab. 2014, 11, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, R.C.; Mohan, S. Skeletal effects of growth hormone and insulin-like growth factor-I therapy. Mol. Cell. Endocrinol. 2016, 432, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Mödder, U.I.; Khosla, S.; Rosen, C.J. Emerging Therapeutic Opportunities for Skeletal Restoration. Nat. Rev. Drug Discov. 2011, 10, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Brotto, M.; Bonewald, L. Bone and Muscle: Interactions beyond Mechanical. Bone 2015, 80, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Abou-Khalil, R.; Yang, F.; Lieu, S.; Julien, A.; Perry, J.; Pereira, C.; Relaix, F.; Miclau, T.; Marcucio, R.; Colnot, C. Role of muscle stem cells during skeletal regeneration. Stem Cells (Dayt. Ohio) 2015, 33, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
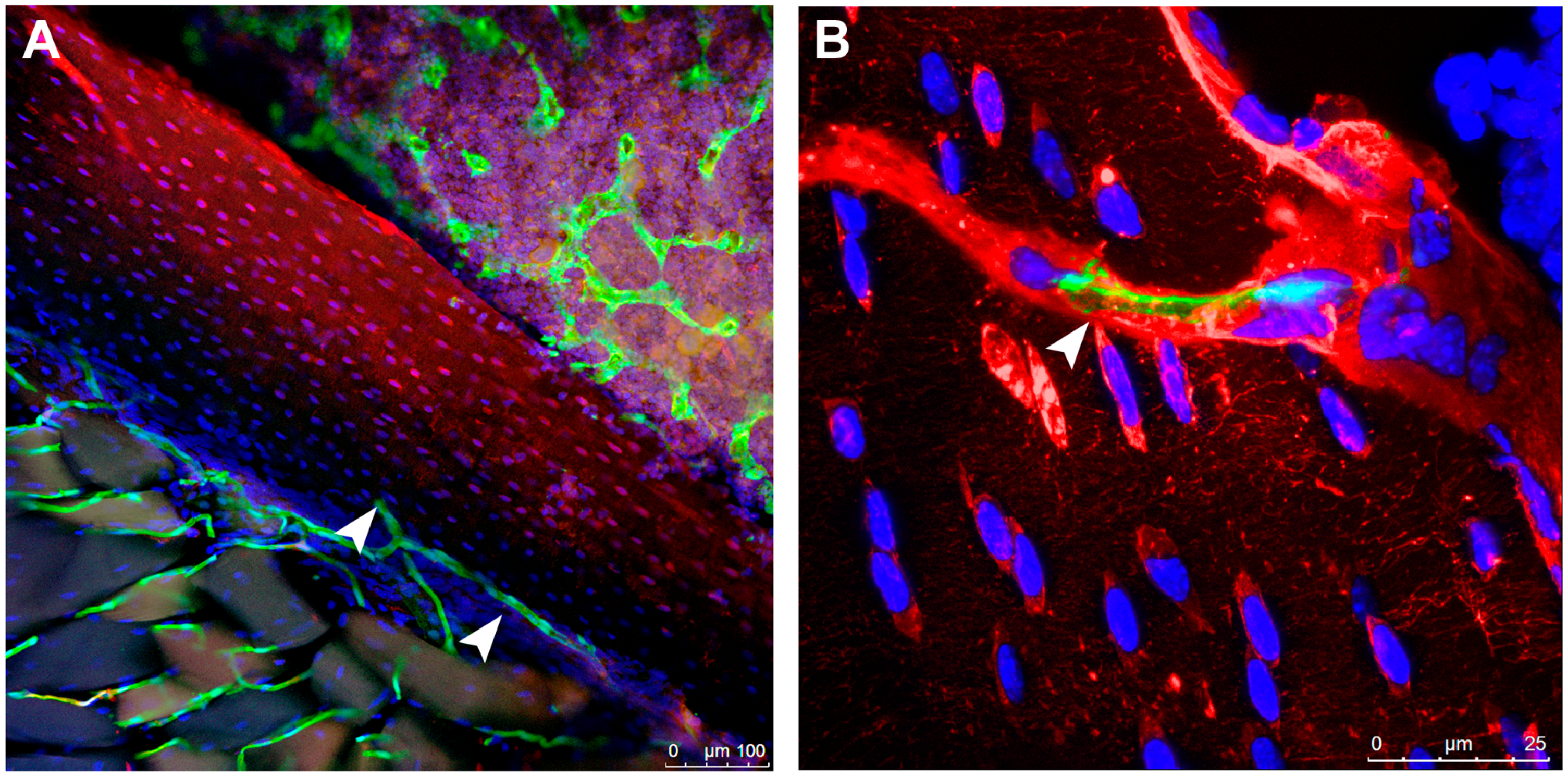
| Molecule | Effect on Bone |
|---|---|
| Myostatin | Promotes osteoclastogenesis |
| Irisin | Promotes osteoblast differentiation |
| Insulin-like Growth Factor (IGF-1) | Increases ability of osteoblast to deposit bone |
| Basic Fibroblast Growth Factor-2 (FGF-2) | Promotes osteoblastogenesis |
| Interleukin-6 (IL-6) | Increases osteoclastogenesis by promoting RANKL secretion by osteoblasts |
| Interleukin-15 (IL-15) | Promotes osteoblast capacity to deposit mineral matrix |
| Interleukin-5 (IL-5) | Not determined |
| Interleukin-7 (IL-7) | Inhibitor of osteoclastogenesis in bone marrow cultures |
| Interleukin-8 (IL-8) | Not determined |
| Brain-Derived Neurotrophic Factor (BDNF) | Regulates expression and secretion of VEGF from osteoblasts |
| Ciliary Neurotrophic Factor (CNTF) | Suppresses osteoblast differentiation in vitro |
| Follistatin-like protein 1 | Not determined |
| Decorin | Promotes bone matrix formation and calcium deposition |
| Osteoglycin (OGN) | Increases alkaline phosphatase, type I collagen and osteocalcin |
| Molecule | Effect on Muscle |
|---|---|
| Osteocalcin or Bone Gamma-Carboxyglutamate Protein (BGLAP) | Increases insulin sensitivity, promotes protein synthesis in myotubes |
| Fibroblast Growth Factor (FGF23) | Not determined |
| Sclerostin | Not determined |
| Dentin Matrix Protein-1 (DMP-1) | Not determined |
| Matrix Extracellular Phosphoglycoprotein (MEPE) | Not determined |
| Phosphate-regulating gene with Homologies to Endopeptidases on the X chromosome (PHEX) | Not determined |
| Receptor Activator of Nuclear Factor-kappa B Ligand (RANKL) | Not determined |
| Prostaglandin E2 (PEG2) | Promotes proliferation of myoblasts |
| WNT-3a | Enhances muscle ability to contract |
| Generic Name | Commercial Name | Approved by FDA | Route of Administration | Effect on Bone | Mechanism of Action | Major Side Effect |
|---|---|---|---|---|---|---|
| Alendronate | Fosamax, Binosto | Yes | Oral (daily or weekly) | Anti-resorptive | Inhibits osteoclast formation and activity | Atypical subtrochanteric and diaphyseal femoral fractures |
| Risedronate | Actonel Atelvia | Yes | Oral, long-lasting tablet (one tablet weekly or on tablet monthly or one tablet per day for 2 consecutive days each month) | Anti-resorptive | Inhibits osteoclast activity | Atypical subtrochanteric and diaphyseal femoral fractures |
| Ibandronate | Boniva | Yes | Intravenous injection once every three months | Anti-resorptive | Inhibits osteoclast activity | Atypical subtrochanteric and diaphyseal femoral fractures |
| Zoledronic acid | Reclast | Yes | Intravenous injection once a year | Anti-resorptive | Inhibits release of acid by osteoclasts | Atypical subtrochanteric and diaphyseal femoral fractures |
| SERM (Raloxifene) | Evista, Keoxifene | Yes | Oral (daily) | Anabolic | Binds to estrogen receptors (Estrogen agonist) | Might develop blood clot in lung or lungs |
| Denosumab | Prolia, Xgeva | Yes | Subcutaneous injection (once every 6 months for osteoporosis treatment) | Anti-resorptive | Binds to RANKL | Femoral bone fracture |
| Estrogens | Amnestrogen, Cenestin, Enjuvia, Estrace, Estratab, Evex, Femogen, Menest, Ogen Tablets, Ortho-est, Premarin | Yes | Oral (daily) | Anabolic/Anti-resorptive | Binds to DNA activating targeted genes. Promotes osteoclast apoptosis | Increase risk to develop endometrial cancer |
| Hormone replacement therapy | Activella, Angeliq, FemHRT, Jinteli, Mimvey, Prefest, Premphase, Prempro | Yes | Oral (daily) | Anabolic | Binds to DNA activating targeted genes. Promotes osteoclast apoptosis | May increase the risk of heart attack, stroke, breast cancer, and blood clots in the lungs and legs |
| PTH (Teriparatide) | Forteo | Yes | Subcutaneous injection daily for up to 2 years | Anabolic | Increases osteoblast activity and recruitment | Osteosarcoma |
| Strontium ranelate | Protelos, Osseor | Alternative use only | Oral (daily) | Anabolic/Anti-resorptive | May induce osteoblast proliferation and osteoclast apoptosis | Heart problems, blood clots |
| Blosozumab | No (Phase III Clinical trials) | Subcutaneous injection | Anabolic | Inhibits Sclerostin (activates Wnt/b-catenin pathway | Increase cardiovascular events | |
| Romosozumab | Evenity | No (Phase III of Clinical trials) | Subcutaneous injection | Anabolic | Inhibits Sclerostin (activates Wnt/b-catenin pathway | Increase cardiovascular events |
| Abaloparatide | Tymlos | Yes | subcutaneous injection once daily | Anabolic | Parathyroid hormone-related peptide analogue | Increase incidence of osteosarcoma (in mice) |
| Odonacatib | No | Anti-resorptive | Cathepsin-K antagonist | Elevated incidence of stroke |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurel, D.B.; Jähn, K.; Lara-Castillo, N. Muscle–Bone Crosstalk: Emerging Opportunities for Novel Therapeutic Approaches to Treat Musculoskeletal Pathologies. Biomedicines 2017, 5, 62. https://doi.org/10.3390/biomedicines5040062
Maurel DB, Jähn K, Lara-Castillo N. Muscle–Bone Crosstalk: Emerging Opportunities for Novel Therapeutic Approaches to Treat Musculoskeletal Pathologies. Biomedicines. 2017; 5(4):62. https://doi.org/10.3390/biomedicines5040062
Chicago/Turabian StyleMaurel, Delphine B., Katharina Jähn, and Nuria Lara-Castillo. 2017. "Muscle–Bone Crosstalk: Emerging Opportunities for Novel Therapeutic Approaches to Treat Musculoskeletal Pathologies" Biomedicines 5, no. 4: 62. https://doi.org/10.3390/biomedicines5040062
APA StyleMaurel, D. B., Jähn, K., & Lara-Castillo, N. (2017). Muscle–Bone Crosstalk: Emerging Opportunities for Novel Therapeutic Approaches to Treat Musculoskeletal Pathologies. Biomedicines, 5(4), 62. https://doi.org/10.3390/biomedicines5040062





