Comparative Efficacy of Platelet-Rich Plasma, Autologous Serum, and Artificial Tears in Dry Eye Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Eligibility Criteria
2.3. Data Extraction and Outcomes
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
3.1. Overview of Studies
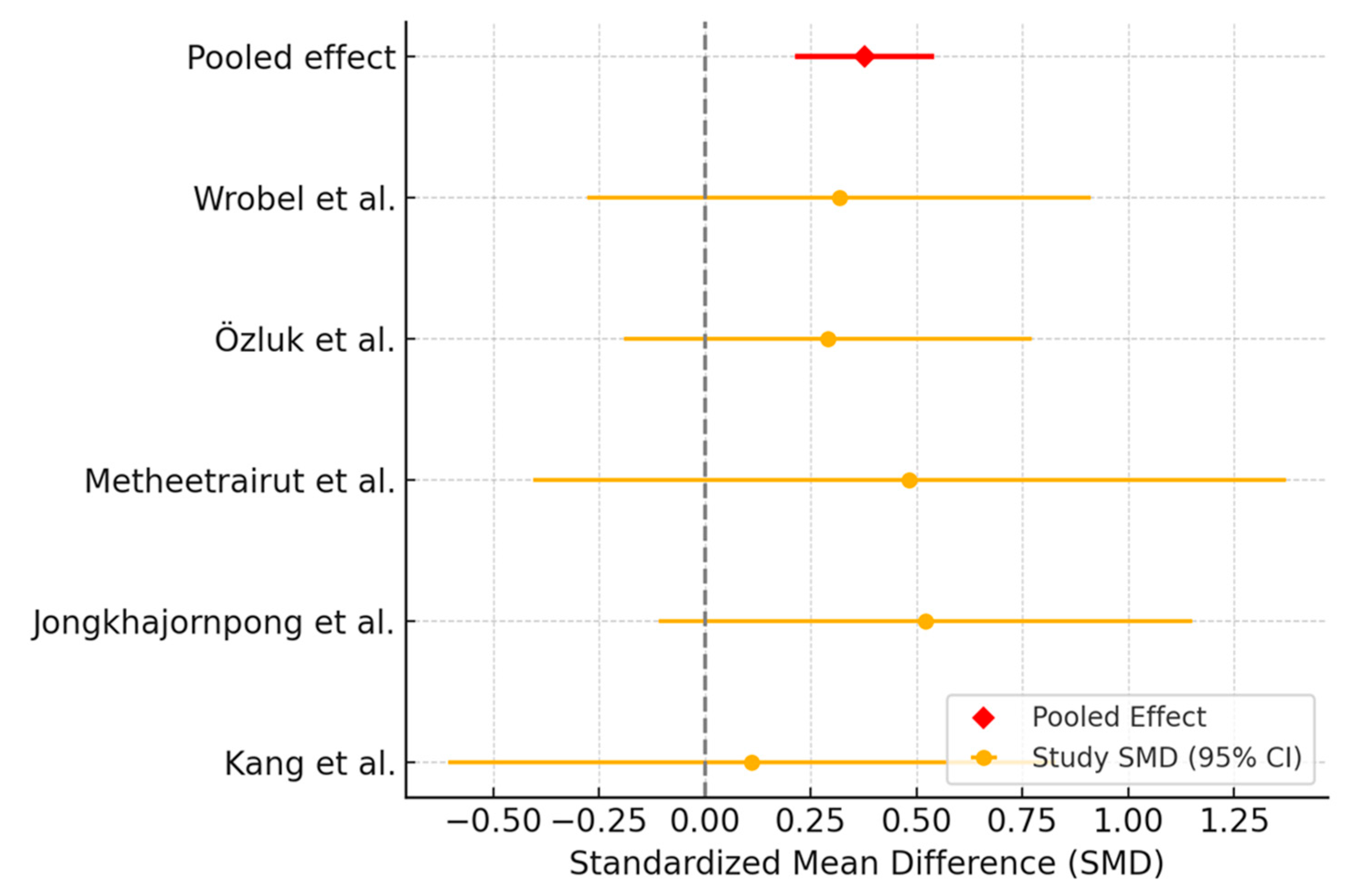
3.2. Platelet-Rich Plasma Versus Autologous Serum
3.2.1. Schirmer Test Assessment
3.2.2. Tear Break-Up Time Assessment
3.2.3. Ocular Surface Disease Index Assessment
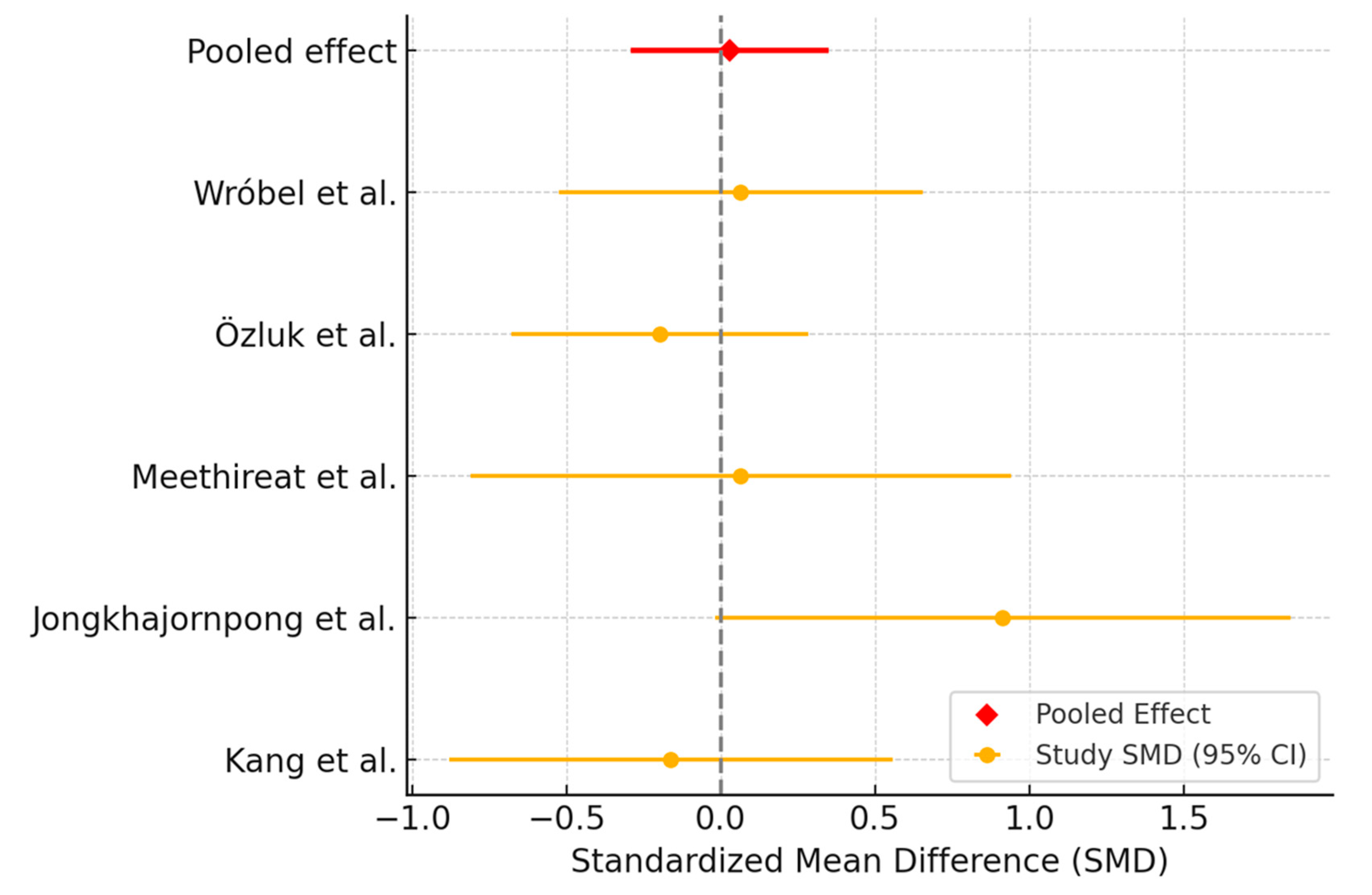
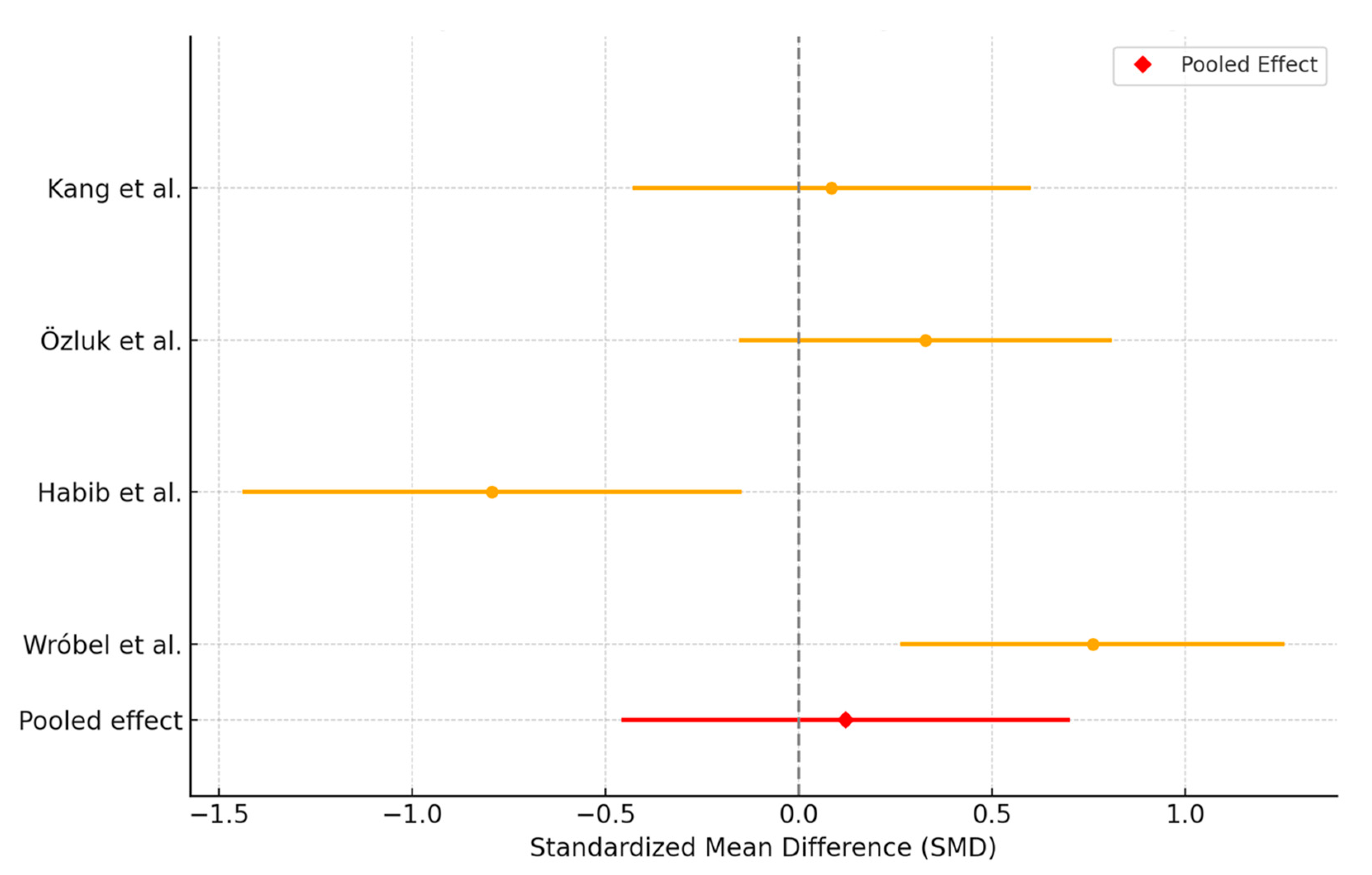
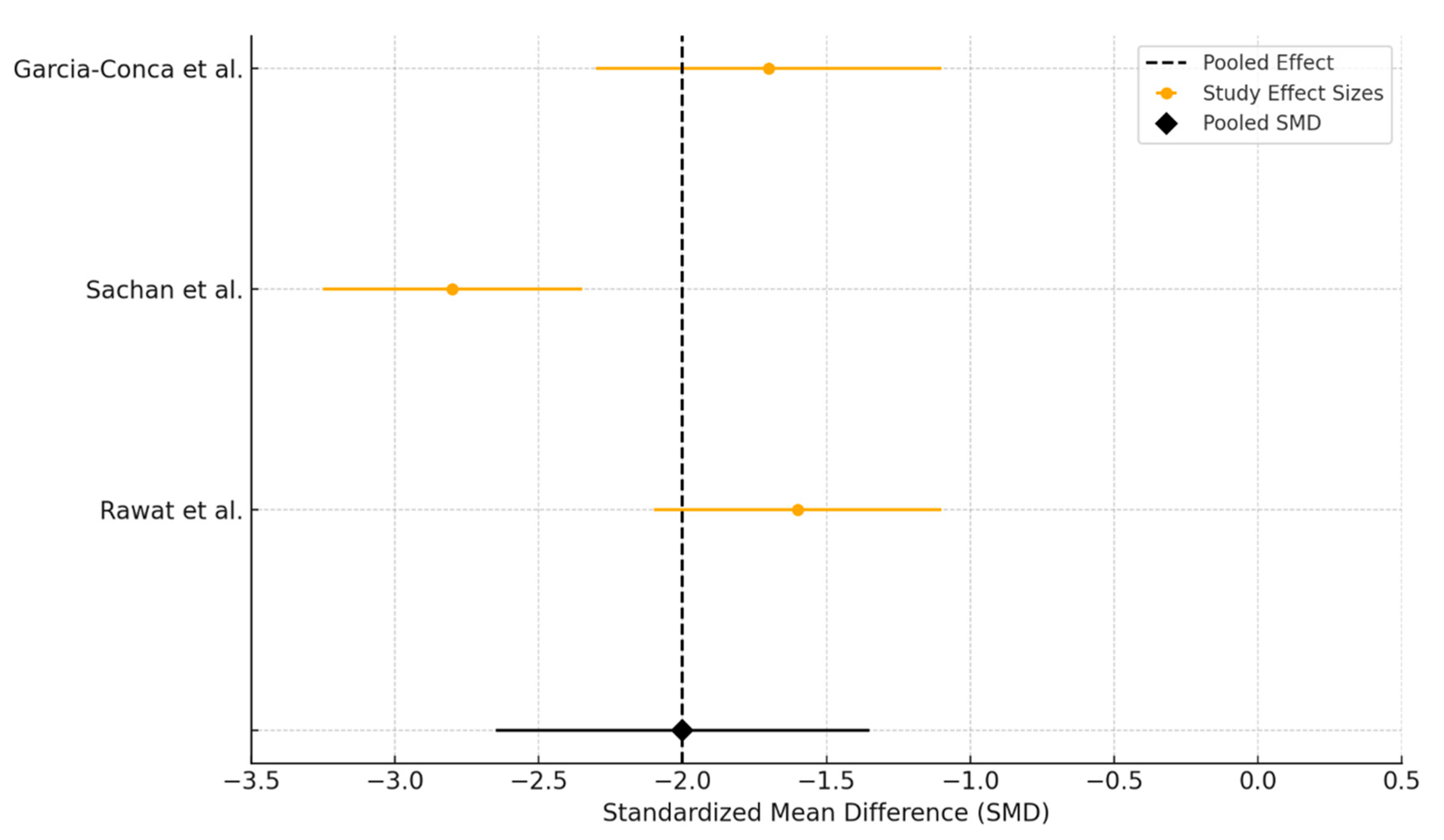
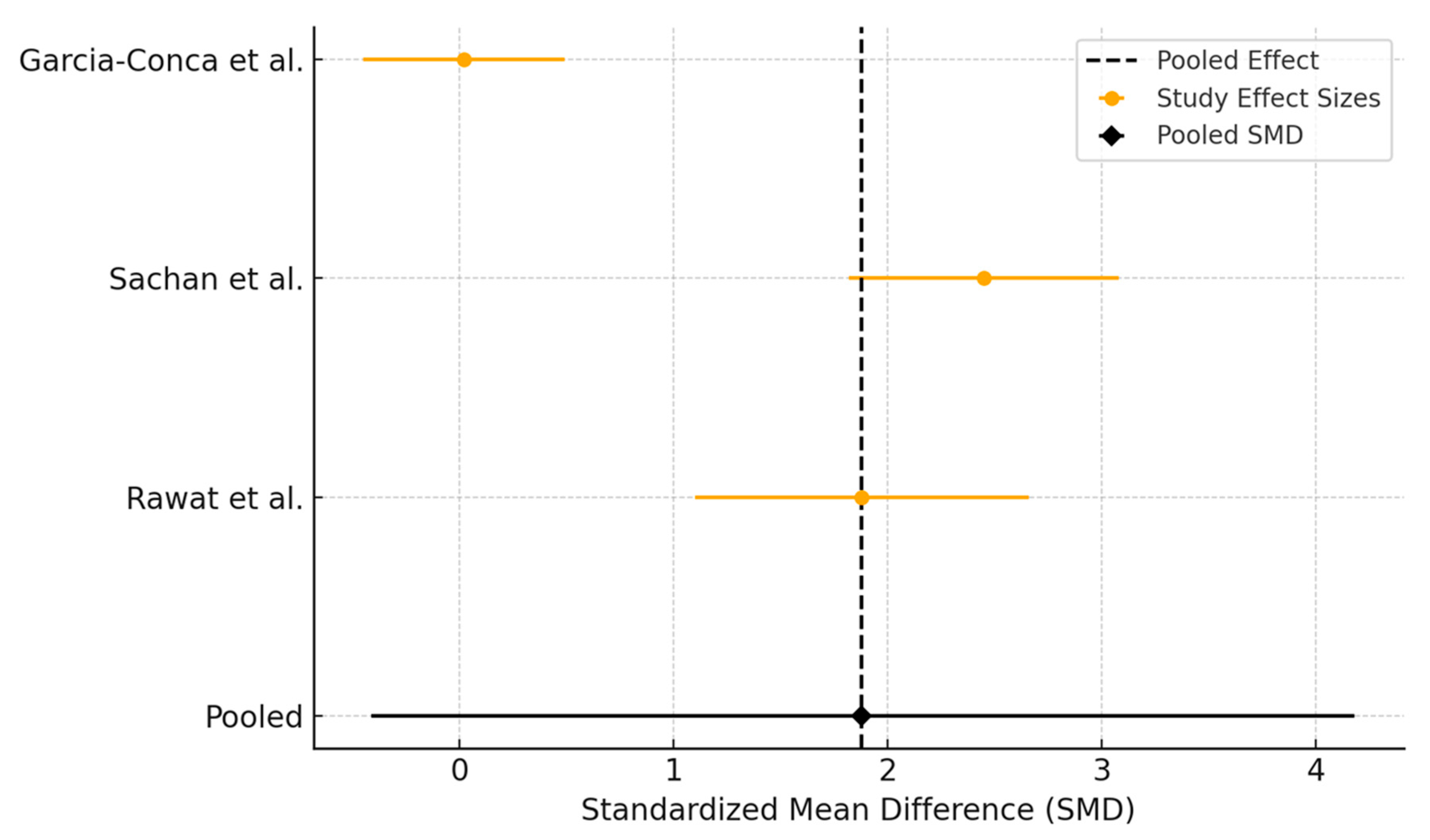
3.3. Platelet-Rich Plasma Versus Artificial Tears
3.3.1. Ocular Surface Disease Index Assessment
3.3.2. Tear Break-Up Time Assessment
3.3.3. Schirmer Test Assessment
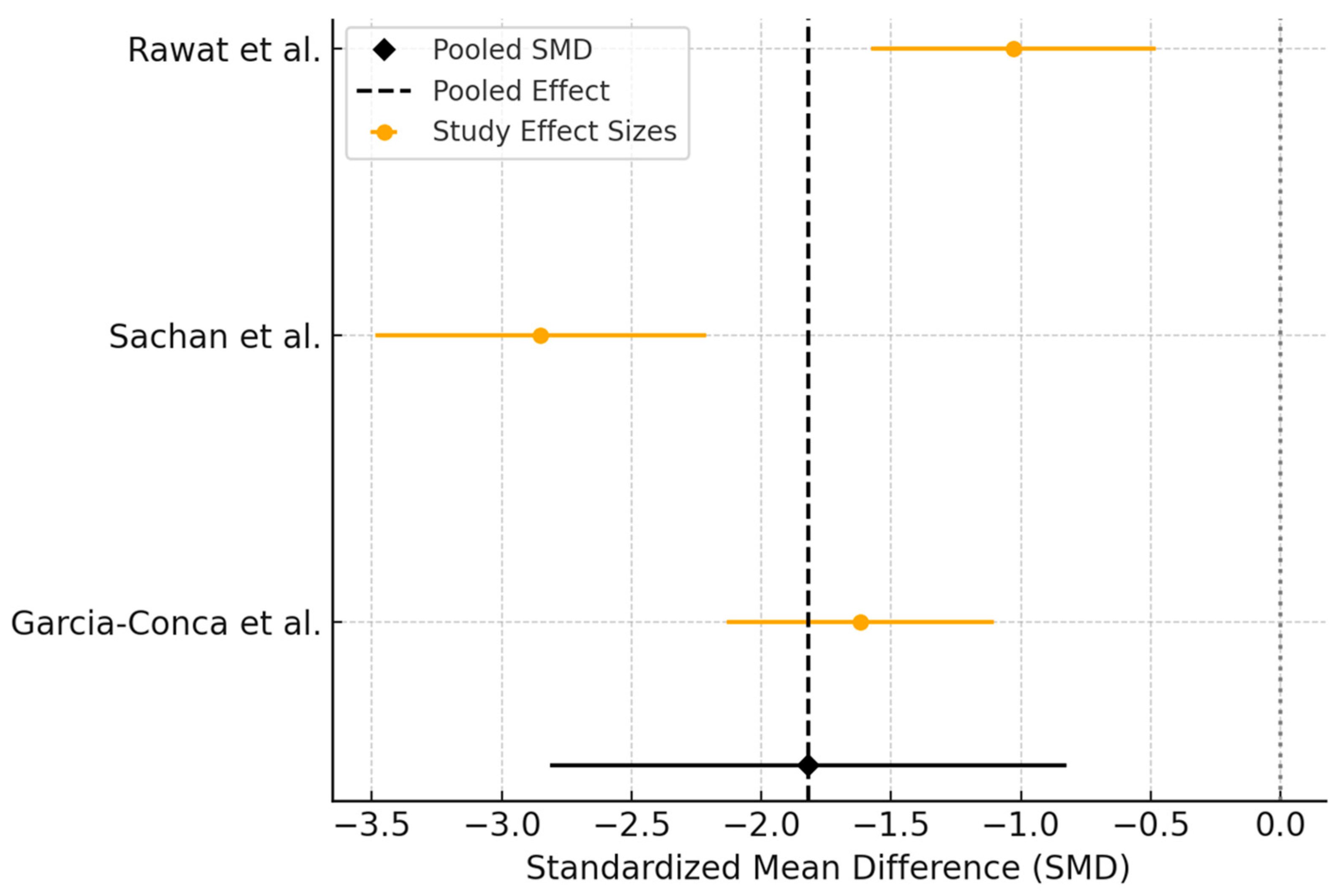
3.4. Autologous Serum Versus Artificial Tears
3.5. Subgroup Analysis
3.5.1. Outcome Analysis Depending on Route of Administration in Platelet-Rich Plasma Versus Artificial Tears
3.5.2. Outcome Analysis Depending on Route of Administration in Platelet-Rich Plasma Versus Autologous Serum
3.6. Risk of Bias
4. Discussion
Advantages and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golden, M.I.; Meyer, J.J.; Zeppieri, M.; Patel, B.C. Dry Eye Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hu, J.-W.; Zhu, X.-P.; Pan, S.-Y.; Yang, H.; Xiao, X.-H. Prevalence and Risk Factors of Dry Eye Disease in Young and Middle-Aged Office Employee: A Xi’an Study. Int. J. Ophthalmol. 2021, 14, 567–573. [Google Scholar] [CrossRef]
- Aragona, P.; Barabino, S.; Di Zazzo, A.; Giannaccare, G.; Villani, E.; Aiello, F.; Antoniazzi, E.; Bonini, S.; Cantera, E.; Carlini, G.; et al. Dry Eye Disease: From Causes to Patient Care and Clinical Collaboration—A Narrative Review. Ophthalmol. Ther. 2025, 14, 1411–1428. [Google Scholar] [CrossRef]
- Guo, L.W.; Akpek, E.K. The Negative Effects of Dry Eye Disease on Quality of Life and Visual Function. Turk. J. Med. Sci. 2020, 50, 1611–1615. [Google Scholar] [CrossRef]
- Semp, D.A.; Beeson, D.; Sheppard, A.L.; Dutta, D.; Wolffsohn, J.S. Artificial Tears: A Systematic Review. Clin. Optom. 2023, 15, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.B.; Abd El-Hamid, B.N.; Fathalla, D.; Fouad, E.A. Current Trends in Pharmaceutical Treatment of Dry Eye Disease: A Review. Eur. J. Pharm. Sci. 2022, 175, 106206. [Google Scholar] [CrossRef] [PubMed]
- Sjö, A.D.; Rovelt, J.; Ahrenberg, S.; Kolko, M. Preservatives in Artificial Tears: Assessing Impact on Dry Eye Symptoms: Findings from 67 951 Questionnaire Responses—Project FOREVER. Acta Ophthalmol. 2025, 103, aos.17500. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.H.; Silva, F.Q.; Blender, N.; Tran, T.; Vantipalli, S. Ocular Benzalkonium Chloride Exposure: Problems and Solutions. Eye 2022, 36, 361–368. [Google Scholar] [CrossRef]
- Ribeiro, M.V.M.R.; Barbosa, F.T.; Ribeiro, L.E.F.; Sousa-Rodrigues, C.F.D.; Ribeiro, E.A.N. Effectiveness of Using Preservative-Free Artificial Tears versus Preserved Lubricants for the Treatment of Dry Eyes: A Systematic Review. Arq. Bras. Oftalmol. 2019, 82, 436–445. [Google Scholar] [CrossRef]
- Nasser, L.; Rozycka, M.; Gomez Rendon, G.; Navas, A. Real-Life Results of Switching from Preserved to Preservative-Free Artificial Tears Containing Hyaluronate in Patients with Dry Eye Disease. Clin. Ophthalmol. 2018, 12, 1519–1525. [Google Scholar] [CrossRef]
- O’Neil, E.C.; Henderson, M.; Massaro-Giordano, M.; Bunya, V.Y. Advances in Dry Eye Disease Treatment. Curr. Opin. Ophthalmol. 2019, 30, 166–178. [Google Scholar] [CrossRef]
- Kong, L.; Sun, J.; Abedi-Firouzjah, R. Emerging Treatment Strategies in Dry Eye Disease: Potential of Blood-Derived Approaches and Natural Plant-Based Products. Exp. Eye Res. 2025, 251, 110217. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; Tayebba, A.; Riestra, A.; Perez, V.L.; Merayo-Lloves, J.; Orive, G. Autologous Serum and Plasma Rich in Growth Factors in Ophthalmology: Preclinical and Clinical Studies. Acta Ophthalmol. 2015, 93, e605–e614. [Google Scholar] [CrossRef]
- Soni, N.G.; Jeng, B.H. Blood-Derived Topical Therapy for Ocular Surface Diseases. Br. J. Ophthalmol. 2016, 100, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, A.M.; Soleimani, M.; Eleiwa, T.K.; ElSheikh, R.H.; Frank, C.R.; Naderan, M.; Yazdanpanah, G.; Rosenblatt, M.I.; Djalilian, A.R. Current and Emerging Therapies for Limbal Stem Cell Deficiency. Stem Cells Transl. Med. 2022, 11, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; De La Fuente, M.; Sánchez-Ávila, R.M.; De La Sen-Corcuera, B.; Merayo-Lloves, J.; Muruzábal, F. Beneficial Effects of Plasma Rich in Growth Factors (PRGF) Versus Autologous Serum and Topical Insulin in Ocular Surface Cells. Curr. Eye Res. 2023, 48, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Ma, I.-H.; Chen, L.; Tu, W.-H.; Lu, C.-J.; Huang, C.-J. Serum Components and Clinical Efficacies of Autologous Serum Eye Drops in Dry Eye Patients with Active and Inactive Sjogren Syndrome. Taiwan J. Ophthalmol. 2017, 7, 213. [Google Scholar] [CrossRef]
- Anitua, E.; De La Fuente, M.; Merayo-Lloves, J.; Muruzabal, F.; Orive, G. Allogeneic Blood-Based Therapies: Hype or Hope? Eye 2017, 31, 509–510. [Google Scholar] [CrossRef][Green Version]
- Vermeulen, C.; van der Burg, L.L.J.; van Geloven, N.; Eggink, C.A.; Cheng, Y.Y.Y.; Nuijts, R.M.M.A.; Wisse, R.P.L.; van Luijk, C.M.; Nieuwendaal, C.; Remeijer, L.; et al. Allogeneic Serum Eye Drops: A Randomized Clinical Trial to Evaluate the Clinical Effectiveness of Two Drop Sizes. Ophthalmol. Ther. 2023, 12, 3347–3359. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Chandler, J.; McKenzie, J.; Boutron, I.; Welch, V. Cochrane Methods 2016; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, J.; Zheng, L.; Zhao, X.; Chen, S. Risk of bias in non-randomized studies on ophthalmic interventions: Application of ROBINS-I in a systematic review. BMC Ophthalmol. 2022, 22, 327. [Google Scholar] [CrossRef]
- Allam, I.Y. Autologous Serum Eye Drops versus Lacrimal Gland Injection of Platelet-Rich Plasma for Severe Dry Eye. Delta J. Ophthalmol. 2021, 22, 251–258. [Google Scholar] [CrossRef]
- Metheetrairut, C.; Ngowyutagon, P.; Tunganuntarat, A.; Khowawisetsut, L.; Kittisares, K.; Prabhasawat, P. Comparison of Epitheliotrophic Factors in Platelet-Rich Plasma versus Autologous Serum and Their Treatment Efficacy in Dry Eye Disease. Sci. Rep. 2022, 12, 8906. [Google Scholar] [CrossRef] [PubMed]
- Wróbel-Dudzińska, D.; Przekora, A.; Kazimierczak, P.; Ćwiklińska-Haszcz, A.; Kosior-Jarecka, E.; Żarnowski, T. The Comparison between the Composition of 100% Autologous Serum and 100% Platelet-Rich Plasma Eye Drops and Their Impact on the Treatment Effectiveness of Dry Eye Disease in Primary Sjogren Syndrome. J. Clin. Med. 2023, 12, 3126. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-J.; Lee, J.H.; Hwang, J.; Chung, S.-H. Efficacy and Safety of Platelet-Rich Plasma and Autologous-Serum Eye Drops for Dry Eye in Primary Sjögren’s Syndrome: A Randomized Trial. Sci. Rep. 2023, 13, 19279. [Google Scholar] [CrossRef]
- Habib, A.; Allam, I.; El-Mansy, M.; Abd El-Raouf, A. Comparative Study Between Platelet-Rich-Plasma and Autologous Serum Eye Drops in Severe Dry Eye Disease. ALEXMED ePosters 2023, 5, 28–29. [Google Scholar] [CrossRef]
- Jongkhajornpong, P.; Numthavaj, P.; Anothaisintawee, T.; Lekhanont, K.; McKay, G.; Attia, J.; Thakkinstian, A. Comparison of Treatment Efficacy between 100% Platelet-Rich Plasma and 100% Serum Eye Drops in Moderate-to-Severe Dry Eye Disease: A Randomised Controlled Trial Protocol. BMJ Open 2021, 11, e048479. [Google Scholar] [CrossRef]
- Özlük, İ.; Yüksel, B.; Küsbeci, T. Comparison of Autologous Serum and Platelet-Rich Plasma in the Treatment of Severe Dry Eye and Persistent Epithelial Defects. Contact Lens Anterior Eye 2024, 47, 102247. [Google Scholar] [CrossRef]
- García-Conca, V.; Abad-Collado, M.; Hueso-Abancens, J.R.; Mengual-Verdú, E.; Piñero, D.P.; Aguirre-Balsalobre, F.; Molina, J.C. Efficacy and Safety of Treatment of Hyposecretory Dry Eye with Platelet-rich Plasma. Acta Ophthalmol. 2019, 97, E170–E178. [Google Scholar] [CrossRef]
- Elessawy, K.B.; Araissi, A.B.; Nasr, H.E.; Abdelbaky, S.H. Assessment of the Efficacy of Platelet-Rich Plasma Injections in the Management of Severe Dry Eye. J. Egypt. Ophthalmol. Soc. 2021, 114, 63–68. [Google Scholar] [CrossRef]
- Rawat, P.; Agrawal, R.; Bhaisare, V.; Walia, S.; Kori, N.; Gupta, R. Autologous Platelet-Rich Plasma Eye Drop versus Artificial Tear Eye Drop for Symptomatic Dry Eye Disease: A Prospective Comparative Interventional Study. Indian J. Ophthalmol. 2022, 70, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Allam, I.Y.; Shaheen, M.S.; Lazreg, S.; Doheim, M.F. Lacrimal Gland Injection of Platelet Rich Plasma for Treatment of Severe Dry Eye: A Comparative Clinical Study. BMC Ophthalmol. 2022, 22, 343. [Google Scholar] [CrossRef] [PubMed]
- Sachan, S.; Dwivedi, K.; Singh, S.P.; Kumar, S.; Singh, V.K. Comparison of 20% Autologous Platelet-Rich Plasma Versus Conventional Treatment in Moderate to Severe Dry Eye Patients. Turk. J. Ophthalmol. 2025, 55, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Ishida, R.; Dogru, M.; Goto, E.; Matsumoto, Y.; Kaido, M.; Tsubota, K. The Effect of Autologous Serum Eyedrops in the Treatment of Severe Dry Eye Disease: A Prospective Randomized Case-Control Study. Am. J. Ophthalmol. 2005, 139, 242–246. [Google Scholar] [CrossRef]
- Urzua, C.A.; Vasquez, D.H.; Huidobro, A.; Hernandez, H.; Alfaro, J. Randomized Double-Blind Clinical Trial of Autologous Serum Versus Artificial Tears in Dry Eye Syndrome. Curr. Eye Res. 2012, 37, 684–688. [Google Scholar] [CrossRef]
- Celebi, A.R.C.; Ulusoy, C.; Mirza, G.E. The Efficacy of Autologous Serum Eye Drops for Severe Dry Eye Syndrome: A Randomized Double-Blind Crossover Study. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 619–626. [Google Scholar] [CrossRef]
- Yılmaz, U.; Küçük, E.; Koç, Ç.; Gökler, E. Comparison of Autologous Serum Versus Preservative Free Artificial Tear in Patients with Dry Eyes Due to Systemic Isotretinoin Therapy. Curr. Eye Res. 2017, 42, 827–831. [Google Scholar] [CrossRef]
- Zheng, N.; Zhu, S.-Q. Randomized Controlled Trial on the Efficacy and Safety of Autologous Serum Eye Drops in Dry Eye Syndrome. World J. Clin. Cases 2023, 11, 6774–6781. [Google Scholar] [CrossRef]
- Alahmadawy, Y.; Youssef, A.; Elmekkawy, H.; Abdelrahman, A. Schirmer’s Test and Tear Breakup Time in an Egyptian Population Sample: A Hospital-Based Study. Delta J. Ophthalmol. 2020, 21, 6. [Google Scholar] [CrossRef]
- Vidas Pauk, S. Noninvasive Tear Film Break-Up Time Assessment Using Handheld Lipid Layer Examination Instrument. Acta Clin. Croat. 2019, 58, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gialelis, T.K.; Mouzaka, A.E.; Chouzouris, M. Comparison of the OSDI Questionnaire, the Tear Film Break-up Time and Schirmer Tests for the Evaluation of Tear Film in Computer Users and Contact Lenses without Dry Eye Symptoms. Ophthalmol. Res. Int. J. 2022, 17, 18–24. [Google Scholar] [CrossRef]
- Nichols, K.K.; Mousavi, M. Clinical Assessments of Dry Eye Disease: Tear Film and Ocular Surface Health. In Dry Eye Disease; Elsevier: Amsterdam, The Netherlands, 2023; pp. 15–23. ISBN 978-0-323-82753-9. [Google Scholar]
- King-Smith, P.E.; Begley, C.G.; Braun, R.J. Mechanisms, Imaging and Structure of Tear Film Breakup. Ocul. Surf. 2018, 16, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Sánchez-González, J.-M.; Silva-Viguera, C.; Sánchez-González, M.C.; Capote-Puente, R.; De-Hita-Cantalejo, C.; Ballesteros-Sánchez, A.; Ballesteros-Durán, L.; García-Romera, M.-C.; Gutiérrez-Sánchez, E. Tear Film Stabilization and Symptom Improvement in Dry Eye Disease: The Role of Hyaluronic Acid and Trehalose Eyedrops versus Carmellose Sodium. J. Clin. Med. 2023, 12, 6647. [Google Scholar] [CrossRef]
- Sweeney, D.F.; Millar, T.J.; Raju, S.R. Tear Film Stability: A Review. Exp. Eye Res. 2013, 117, 28–38. [Google Scholar] [CrossRef]
- Kaštelan, S.; Gabrić, K.; Mikuličić, M.; Mrazovac Zimak, D.; Karabatić, M.; Gverović Antunica, A. The Influence of Tear Film Quality on Visual Function. Vision 2024, 8, 8. [Google Scholar] [CrossRef]
- Alio, J.L.; Rodriguez, A.E.; Ferreira-Oliveira, R.; Wróbel-Dudzińska, D.; Abdelghany, A.A. Treatment of Dry Eye Disease with Autologous Platelet-Rich Plasma: A Prospective, Interventional, Non-Randomized Study. Ophthalmol. Ther. 2017, 6, 285–293. [Google Scholar] [CrossRef]
- Mason, L.; Jafri, S.; Dortonne, I.; Sheppard, J.D. Emerging Therapies for Dry Eye Disease. Expert Opin. Emerg. Drugs 2021, 26, 401–413. [Google Scholar] [CrossRef]
- Vazirani, J.; Sridhar, U.; Gokhale, N.; Doddigarla, V.R.; Sharma, S.; Basu, S. Autologous Serum Eye Drops in Dry Eye Disease: Preferred Practice Pattern Guidelines. Indian J. Ophthalmol. 2023, 71, 1357–1363. [Google Scholar] [CrossRef]
- Kandel, H.; Stapleton, F.; Downie, L.E.; Chidi-Egboka, N.C.; MIngo-Botin, D.; Arnalich-Montiel, F.; Rauz, S.; Recchioni, A.; Sitaula, S.; Markoulli, M.; et al. The Impact of Dry Eye Disease on Patient-Reported Quality of Life: A Save Sight Dry Eye Registry Study. Ocul. Surf. 2025, 37, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Gabbriellini, G.; Baldini, C.; Varanini, V.; Ferro, F.; Pepe, P.; Luciano, N.; Fanucci, F.; Mosca, M.; Nardi, M.; Bombardieri, S. Ocular Surface Disease Index (OSDI): A Potential Useful Instrument for the Assessment of Vision-Targeted Health-Related Quality of Life (VT-HRQ) in Primary Sjögren’s Syndrome (pSS) Clinical Trials? Clin. Exp. Rheumatol. 2012, 30, 812–813. [Google Scholar] [PubMed]
- Aljarousha, M.; Alghamdi, W.M.; Attaallah, S.; Alhoot, M.A. Ocular Surface Disease Index Questionnaire in Different Languages. Med. Hypothesis Discov. Innov. Ophthalmol. 2025, 13, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Martin, R. EMO Research Group Comparison of the Ocular Surface Disease Index and the Symptom Assessment in Dry Eye Questionnaires for Dry Eye Symptom Assessment. Life 2023, 13, 1941. [Google Scholar] [CrossRef]
- Lyu, Y.-R.; Kwon, O.-J.; Park, B.; Jung, H.-A.; Lee, G.-Y.; Kim, C.-S. Efficacy and Safety of Useul for Dry Eye Disease: Protocol for a Randomized, Double-Blind, Placebo-Controlled, Parallel, Phase 2 Clinical Trial. Healthcare 2024, 12, 2383. [Google Scholar] [CrossRef]
- Akowuah, P.K.; Obinwanne, C.J.; Owusu, E.; Kyeremeh, S.; Bonsu, K.; Karikari, L.A.A.; Akomeah, F.A.; Nkansah, E.K.; Kobia-Acquah, E. Platelet-Rich Plasma for Treating Dry Eye Disease—A Systematic Review and Meta-Analysis. Cont. Lens Anterior Eye 2024, 47, 102091. [Google Scholar] [CrossRef]
- Ribeiro, M.V.M.R.; Ribeiro, E.A.N.; Ribeiro, L.F. The Use of Platelet-Rich Plasma in Dry Eye Disease. In Plasma Medicine—Concepts and Clinical Applications; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Feng, Y.; Li, X.; Lu, Z.; Gu, H.; Li, W.; Hill, L.J.; Ou, S. Evolution of Therapeutic Strategy Based on Oxidant–Antioxidant Balance for Fuchs Endothelial Corneal Dystrophy. Ocul. Surf. 2024, 34, 247–261. [Google Scholar] [CrossRef]
- Higuchi, A. Autologous Serum and Serum Components. Invest. Ophthalmol. Vis. Sci. 2018, 59, DES121–DES129. [Google Scholar] [CrossRef]
- Labetoulle, M.; Benitez-Del-Castillo, J.M.; Barabino, S.; Herrero-Vanrell, R.; Daull, P.; Garrigue, J.-S. Artificial Tears: Biological Role of Their Ingredients in the Management of Dry Eye Disease. Int. J. Mol. Sci. 2022, 23, 2434. [Google Scholar] [CrossRef]
- Song, J.S.; Hyon, J.Y.; Lee, D.; Kim, T.H.; Chung, S.-H.; Kim, J.H.; Choi, S.H.; Yoon, K.C. Current Practice Pattern for Dry Eye Patients in South Korea: A Multicenter Study. Korean J. Ophthalmol. 2014, 28, 115–121. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.L.; Walt, J.G.; Mink, D.R.; Satram-Hoang, S.; Wilson, S.E.; Perry, H.D.; Asbell, P.A.; Pflugfelder, S.C. Minimal clinically important difference for the ocular surface disease index. Arch. Ophthalmol. 2010, 128, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, K.; Wei, Z.; Baudouin, C.; Labbé, A.; Liang, Q. Autologous Serum Eye Drops versus Artificial Tear Drops for Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ophthalmic Res. 2020, 63, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.G.; Leslie, L.; Li, T. Autologous Serum Eye Drops for Dry Eye: Systematic Review. Optom. Vis. Sci. 2023, 100, 564–571. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Ge, Z.; Li, F. Blood Component Therapy for Dry Eye Disease: A Systematic Review and Network Meta-Analysis. Front. Med. 2024, 11, 1500160. [Google Scholar] [CrossRef]

| Study | Year | Country | Comparison | Sample Size | Age | Outcome | Included in Meta-Analysis |
|---|---|---|---|---|---|---|---|
| Allam et al. [25] | 2021 | Egypt | PRP vs. AS | 20/20 | 56.0 ± 8.2 | OSDI score, Schirmer test, CFS, TMH, NIBUT, LLT | No |
| Metheetrairut et al. [26] | 2022 | Thailand | PRP vs. AS | 10/10 | 65.6 ± 14.35 | OSDI score, CFS, TBUT, Schirmer test, BCVA, IOP | Yes |
| Wróbel-Dudzińska et al. [27] | 2023 | Poland | PRP vs. AS | 22/22 | 62.2 ± 8.5/62.9 ± 10.9 | OSDI score, BCVA, Schirmer test, TBUT, CFS, meibomian gland parameters | Yes |
| Kang et al. [28] | 2023 | South Korea | PRP vs. AS | 14/16 | 54.07 ± 11.28/54.56 ± 11.93 | OSDI score, CFS, CDVA, Schirmer test, TBUT | Yes |
| Habib et al. [29] | 2023 | Egypt | PRP vs. AS | 20/20 | N/A | OSDI score, CFS, CDVA, Schirmer test, NIBUT | Yes |
| Jongkhajornpong et al. [30] | 2024 | Thailand | PRP vs. AS | 48/48 | N/A | OSDI score, Oxford scale, TBUT Schirmer test, meibum quality, adverse effects | Yes |
| Ozluk et al. [31] | 2024 | Turkey | PRP vs. AS | 34/33 | 55.1 ± 19.7/65.7 ± 12.3 | BCVA, Schirmer test, OSDI score, TBUT, Oxford scale | Yes |
| Garcia Conca et al. [32] | 2018 | Spain | PRP vs. AT | 44/39 | 64 ± 11.2 | OSDI score, osmolarity, Schirmer test, TBUT, CFS | Yes |
| Elessawy et al. [33] | 2021 | Egypt | PRP vs. AT | 20/20 | 54 ± 10/51 ± 6 | Schirmer test, TBUT, OSDI score, CFS, CIC | No |
| Rawat et al. [34] | 2022 | India | PRP vs. AT | 31/30 | 52.8 ± 12.8/55.5 ± 13.4 | Schirmer test, TBUT, OSDI score, CFS | Yes |
| Mohammed et al. [35] | 2022 | Egypt | PRP vs. AT | 14/14 | 43.4 ± 7.85 | TMH, NITBUT, LLT, Schirmer test, CFS, meiboscore | No |
| Sachan et al. [36] | 2025 | India | PRP vs. AT | 20/20 | 51 ± 14/50 ± 17 | OSDI score, BCVA, TMH, TBUT, Schirmer test, CFS, CIC | Yes |
| Kojima et al. [37] | 2005 | Japan | AS vs. AT | 10/10 | 62.3 ± 12.5/65.4 ± 9.7 | Schirmer test, TBUT, CFS | No |
| Urzua et al. [38] | 2012 | Chile | AS vs. AT | 12/12 | 52 ± 6.3 | OSDI score, CFS, TBUT | No |
| Celebi et al. [39] | 2014 | Turkey | AS vs. AT | 20/20 | 56.05 ± 8.07 | OSDI score, TBUT, Schirmer test, Oxford scale | Yes |
| Yilmaz et al. [40] | 2017 | Turkey | AS vs. AT | 24/24 | 25 ± 4.02 | BCVA, IOP, TBUT, Schirmer test, OSDI score | No |
| Zheng et al. [41] | 2023 | China | AS vs. AT | 116/116 | 54.6 ± 12.4/55.2 ± 12.4 | OSDI score, TBUT, Schirmer test, CFS, CIC | Yes |
| Study | Sample Size | Treatment Method | Preparation/Administration | Therapeutic Dose | Follow-Up | Side Effects |
|---|---|---|---|---|---|---|
| Allam et al. [25] | 20/20 | AS: eye drops PRP: injection into lacrimal gland | AS: 20 mL whole blood → clot → centrifugation → diluted 50% with 0.9% saline PRP: 10 mL blood with sodium citrate → centrifugation → activated with CaCl, injected transcutaneously into lacrimal gland area | AS: 5×/day for 12 weeks PRP: 1 mL (days 0, 30, 60, 90) | 1 month/2 months/3 months | mild ocular irritation in 4 eyes (AS group) |
| Metheetrairut et al. [26] | 10/10 | AS/PRP: eye drops | AS: 35 mL blood incubated 2 h at 31 °C; centrifuged at 3000× g for 30 min; diluted to 20% with BSS PRP: 35 mL blood with sodium citrate; centrifuged at 2200× g for 10 min; diluted to 20% with balanced salt solution | AS/PRP: instilled hourly for 1 month | 1 month | None reported |
| Wróbel-Dudzińska et al. [27] | 22/22 | AS/PRP: eye drops | AS: 5 mL blood without anticoagulant; clotting 1 h; centrifuged twice at 3000 rpm ×10 min; filtered; dispensed undiluted PRP: 9 mL blood with sodium citrate; centrifuged 1400 rpm ×10 min (no platelet activation); platelet-rich top layer collected (avoiding buffy coat); dispensed undiluted | AS/PRP: instilled 1 drop ×5/day for 3 months | 1 month/3 months | None reported |
| Kang et al. [28] | 14/16 | AS/PRP: eye drops | AS: 24 mL blood → clot 2 h → centrifuged 3500× g for 15 min → diluted to 20% with sodium hyaluronate PRP: 22 mL blood + sodium citrate → processed with PRS Bio Kit; centrifuged 3000× g for 3 min → buffy coat + plasma mixed → diluted to 20% with sodium hyaluronate | AS/PRP: instilled 1 drop, 6× daily for 12 weeks | 1 month/3 months | None reported |
| Habib et al. [29] | 20/20 | AS/PRP: eye drops | Not reported | AS/PRP: instilled regularly over 3 months | 1 month/3 months | None reported |
| Jongkhajornpong et al. [30] | 48/48 | AS/PRP: eye drops | AS: blood allowed to clot 1–2 h → centrifuged at 3000× g for 15 min → serum filtered → dispensed undiluted (100%) PRP:Blood anticoagulated with sodium citrate (9:1 ratio), centrifuged at 350× g for 10 min → plasma fraction collected as APRP → dispensed undiluted (100%) | AS: 1 drop/eye every 2 h between 08:00–22:00 (≈8×/day) for 4 weeks. | 2 weeks/4 weeks | None reported |
| Ozluk et al. [31] | 34/33 | AS/PRP: eye drops | Not reported | AS/PRP: instilled regularly over one month | 1 month | None reported |
| Study | Sample Size | Treatment Method | Components/Composition | Preparation/Administration | Therapeutic Dose | Follow-Up | Side Effects |
|---|---|---|---|---|---|---|---|
| Garcia Conca et al. [32] | 44/39 | PRP/AT: eye drops | AT: Hypotonic aqueous solution of SH 0.18% + ions (Vismed®, TRB Chemedica, Newcastle-under-Lyme, UK) | PRP: Blood (40 mL) collected → centrifuged; pellet resuspended → ~4.5 mL PRP/tube. | PRP: 1 drop every 3 h, 6×/day, for 30 days. AT: instilled regularly | 2 weeks/4 weeks | None reported |
| Elessawy et al. [33] | 20/20 | AT: eye drops PRP: injection into lacrimal gland | AT: Preservative-free (Refresh Plus®, Allergan, Dublin, Ireland; carboxymethylcellulose-based) | PRP: 10 mL whole blood → 1st spin 160 g ×10 min → plasma + buffy coat transferred → 2nd spin 160 g ×15 min → PRP layer collected; 0.1 mL CaCl2 added | AT: instillation 3× daily for 3 months PRP: injection repeated at day 0, 30, 60 | 3 months | PRP: 1 ocular infection, 2 vasovagal episodes, 2 ecchymoses, 5 transient periorbital edemas |
| Rawat et al. [34] | 31/30 | PRP/AT: eye drops | AT:0.5% Carboxymethylcellulose (CMC) solution | PRP: Blood collected in citrated tubes → centrifuged per Alió 2012 protocol → platelet-rich plasma fraction aspirated → diluted 1:4 with BSS | AT/PRP: 4–6×/day, for 3 months | 1 week/2 weeks/1 month/3 months | None reported |
| Mohammed et al. [35] | 14/14 | AT: eye drops PRP: injection into lacrimal gland | AT: preservative-free lubricants | PRP: 10 mL blood in sodium citrate → centrifuged → platelet-rich fraction collected → activated with 10% CaCl2. | AT: instilled regularly PRP: 1 mL PRP injected transcutaneously into lacrimal gland | 1 month/2 months/3 months | None reported |
| Sachan et al. [36] | 20/20 | PRP/AT: eye drops | AT: Systane Complete (propylene glycol 0.6%, Alcon, Geneva, Switzerland) | PRP: 10 mL blood in sodium citrate → soft spin → supernatant spun→ 3–5 mL PRP extracted → diluted to 20% with BSS. | AT/PRP: 1 drop, 4–6×/day, for 3 months | 1 week/1 month/3 months | None reported |
| Study | Sample Size | Treatment Method | Components/Composition | Preparation/Administration | Therapeutic Dose | Follow-Up | Side Effects |
|---|---|---|---|---|---|---|---|
| Kojima et al. [37] | 10/10 | AS/AT: eye drops | AT: Preservative-free lubricant | AS: 40 mL venous blood → centrifuged 1500 rpm, 5 min → serum separated → diluted to 20% saline. | AS/AT: 1 drop, 6×/day, both eyes, for 2 weeks | 2 weeks | None reported |
| Urzua et al. [38] | 12/12 | AS/AT: eye drops | AT: Systane (propylene glycol 0.6%, Alcon) | AS: 20 mL blood → clotting 2 h → centrifugation → supernatant serum collected → diluted 20% | AS/AT: 1 drop 4×/day for 14 days. | 1 month/2 months | None reported |
| Celebi et al. [39] | 20/20 | AS/AT: eye drops | AT: Refresh® Preservative-Free AT (Allergan, carboxymethylcellulose and lubricants) | AS: 20 mL venous blood → clot 2 h RT → centrifugation → ~5 mL serum collected → diluted 20% | AS/AT: 1 drop, 4×/day, for 1 month | 1 month/2 months | None reported |
| Yilmaz et al. [40] | 24/24 | AS/AT: eye drops | AT: Preservative-free lubricant | AS: 20 mL venous blood → clot 2 h → centrifuge → supernatant serum collected → diluted 40% saline | AS/AT: 1 drop, 4×/day, for 1 month. | 1 month/2 months | None reported |
| Zheng et al. [41] | 116/116 | AS/AT: eye drops | AT: Preservative-free 0.1% sodium hyaluronate (Hylo-Comod®, Saarbrücken, Germany). | AS: 40 mL venous blood → centrifugation → serum separated, diluted to 20% with saline | AS/AT: 1 drop/eye, 4× daily, for 12 weeks. | 3 months | AS/AT: Mild, transient ocular irritation/redness/itching/discharge |
| Study | D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall |
|---|---|---|---|---|---|---|---|---|
| Rawat et al. [34] | Moderate | |||||||
| Sachan et al. [36] | Moderate | |||||||
| Mohammed et al. [35] | Low/Moderate | |||||||
| Ozluk et al. [31] | Moderate | |||||||
| Wróbel-Dudzińska et al. [27] | Low/Moderate |
| Study | D1 | D2 | D3 | D4 | D5 | D6 | Overall |
|---|---|---|---|---|---|---|---|
| Garcia-Conca et al. [32] | Low/Moderate | ||||||
| Allam et al. [25] | Low/Moderate | ||||||
| Metheetrairut et al. [26] | Low | ||||||
| Yilmaz et al. [40] | Low | ||||||
| Celebi et al. [39] | Low | ||||||
| Urzua et al. [38] | Low | ||||||
| Kojima et al. [37] | Moderate | ||||||
| Zheng et al. [41] | Low | ||||||
| Kang et al. [28] | Low | ||||||
| Jongkhajornpong et al. [30] | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mederle, A.L.; Andrei, D.; Ghenciu, L.A.; Stoicescu, E.R.; Iacob, R.; Haţegan, O.A. Comparative Efficacy of Platelet-Rich Plasma, Autologous Serum, and Artificial Tears in Dry Eye Disease: A Systematic Review and Meta-Analysis. Biomedicines 2025, 13, 2316. https://doi.org/10.3390/biomedicines13092316
Mederle AL, Andrei D, Ghenciu LA, Stoicescu ER, Iacob R, Haţegan OA. Comparative Efficacy of Platelet-Rich Plasma, Autologous Serum, and Artificial Tears in Dry Eye Disease: A Systematic Review and Meta-Analysis. Biomedicines. 2025; 13(9):2316. https://doi.org/10.3390/biomedicines13092316
Chicago/Turabian StyleMederle, Alexandra Laura, Diana Andrei, Laura Andreea Ghenciu, Emil Robert Stoicescu, Roxana Iacob, and Ovidiu Alin Haţegan. 2025. "Comparative Efficacy of Platelet-Rich Plasma, Autologous Serum, and Artificial Tears in Dry Eye Disease: A Systematic Review and Meta-Analysis" Biomedicines 13, no. 9: 2316. https://doi.org/10.3390/biomedicines13092316
APA StyleMederle, A. L., Andrei, D., Ghenciu, L. A., Stoicescu, E. R., Iacob, R., & Haţegan, O. A. (2025). Comparative Efficacy of Platelet-Rich Plasma, Autologous Serum, and Artificial Tears in Dry Eye Disease: A Systematic Review and Meta-Analysis. Biomedicines, 13(9), 2316. https://doi.org/10.3390/biomedicines13092316







