Mammographic Calcifications in Lung Transplant Recipients: Prevalence and Evolution
Abstract
1. Introduction
2. Materials and Methods
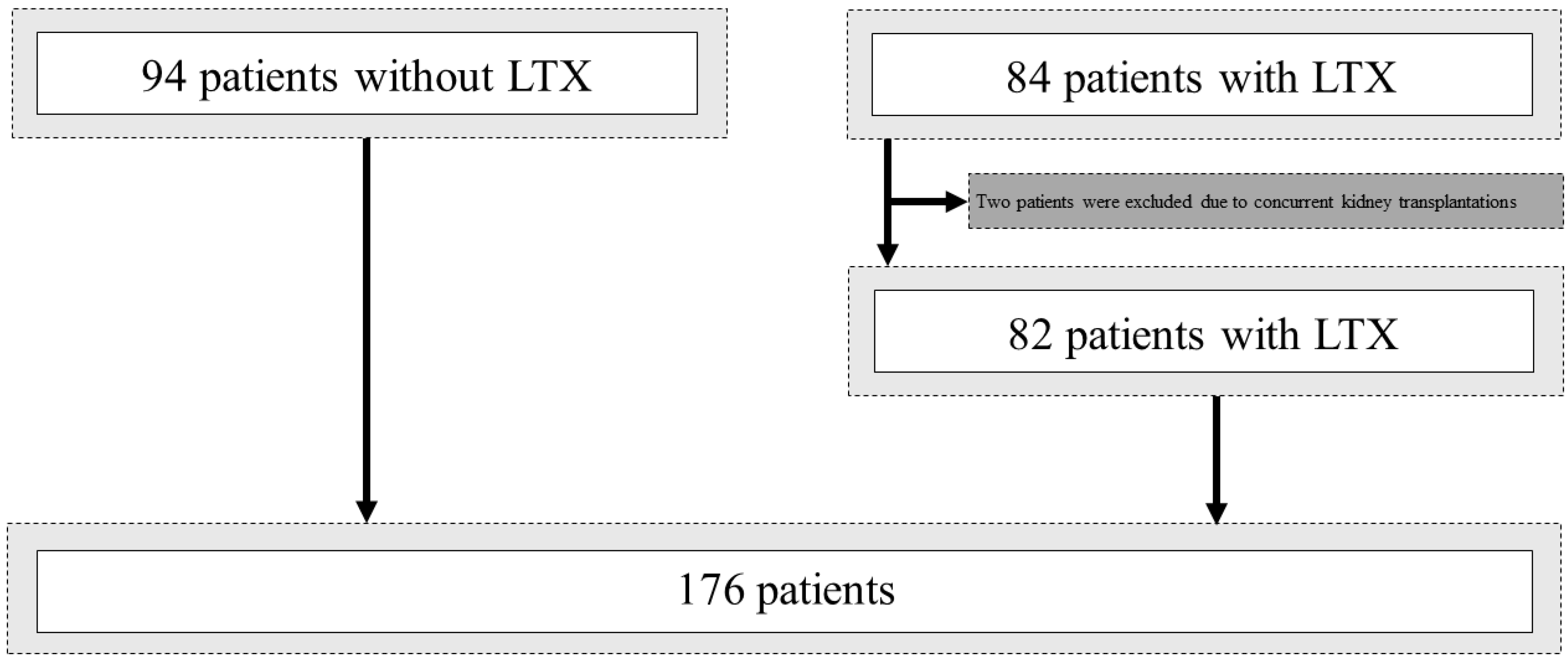
2.1. Patient Selection
2.2. Mammography Protocol
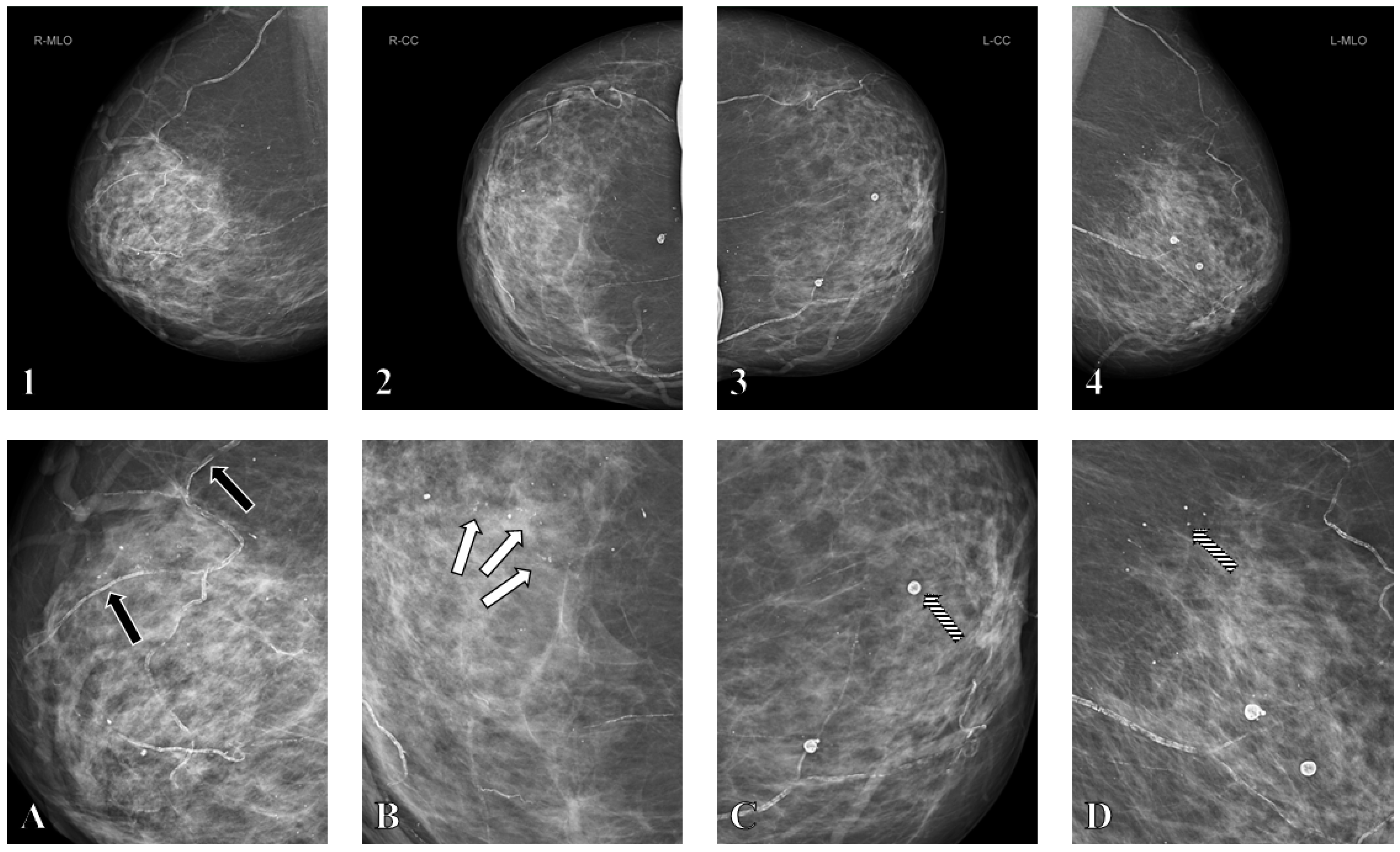
2.3. Mammographic Assessment
2.4. Reader Protocol
2.5. Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. Imaging Characteristics and Follow-Up
3.3. Mammographic Assessment and BAC Scoring
3.4. Multivariable Analysis
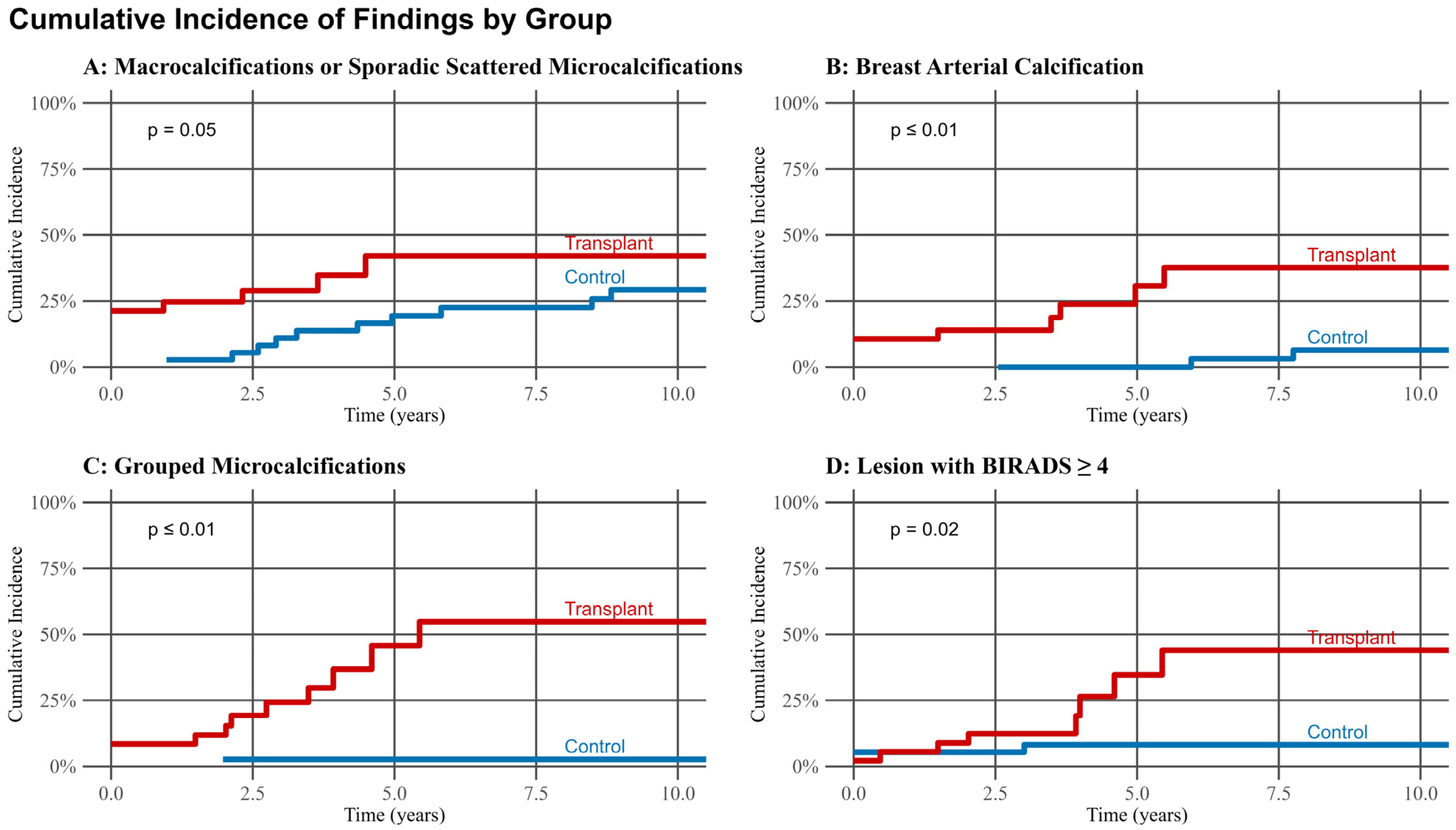
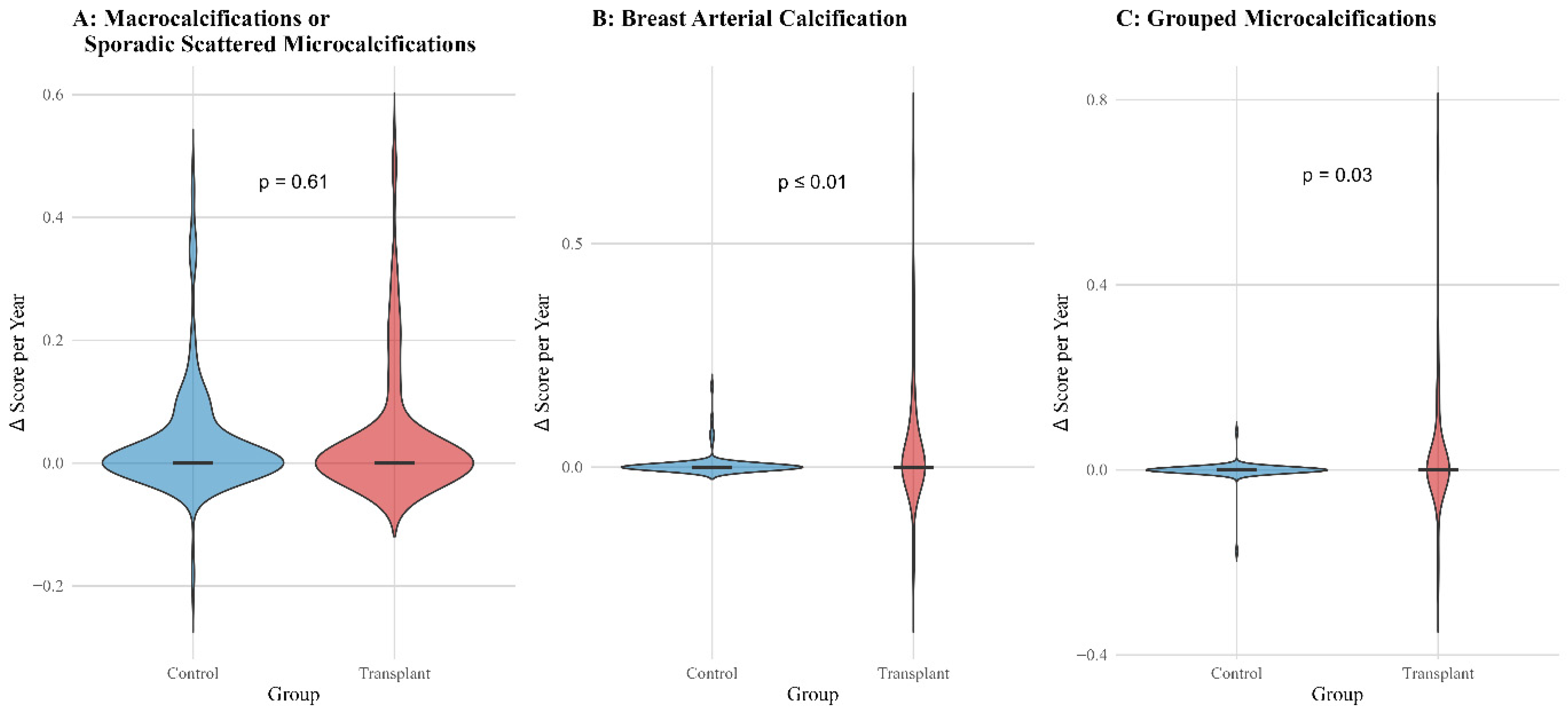
3.5. Progression Analysis and Cumulative Incidence Curves
3.6. Interobserver Agreement
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAC | breast arterial calcifications |
| BI-RADS | Breast Imaging Reporting and Data System |
| DCIS | ductal carcinoma in situ |
| LTX | Lung transplant recipients |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bleyer, A.; Welch, H.G. Effect of Three Decades of Screening Mammography on Breast-Cancer Incidence. N. Engl. J. Med. 2012, 367, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Fu, R.; Cantor, A.; Pappas, M.; Daeges, M.; Humphrey, L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2016, 164, 244–255. [Google Scholar] [CrossRef]
- Capiro, N.; Chalfant, J.S. Breast Cancer Screening and Solid Organ Transplantation. J. Breast Imaging 2025, wbaf016. [Google Scholar] [CrossRef]
- Kato, T.; Kakuta, Y.; Yamanaka, K.; Okumi, M.; Abe, T.; Imamura, R.; Ichimaru, N.; Takahara, S.; Nonomura, N. Early diagnosis and treatment of breast cancer in Japanese kidney transplant recipients: A single center experience. SpringerPlus 2015, 4, 196. [Google Scholar] [CrossRef]
- D’Arcy, M.E.; Coghill, A.E.; Lynch, C.F.; Koch, L.A.; Li, J.; Pawlish, K.S.; Morris, C.R.; Rao, C.; Engels, E.A. Survival after a cancer diagnosis among solid organ transplant recipients in the United States. Cancer 2019, 125, 933–942. [Google Scholar] [CrossRef]
- Wong, G.; Au, E.; Badve, S.V.; Lim, W.H. Breast Cancer and Transplantation. Am. J. Transplant. 2017, 17, 2243–2253. [Google Scholar] [CrossRef]
- N’da Marcelin, H.; Dasse, R.S.; Yeboah, R.O.; Tariam, A.D.; Kagambega, A.G.Z.; Oseni, A.M.; Kouassi, Y.; Bilé, M.A.; Toure, M.; Thakar, M.; et al. Circulating natural killer cells and their association with breast cancer and its clinico-pathological characteristics. Ecancermedicalscience 2023, 17, 1567. [Google Scholar] [CrossRef]
- Thacker, G.; Henry, S.; Nandi, A.; Debnath, R.; Singh, S.; Nayak, A.; Susnik, B.; Boone, M.M.; Zhang, Q.; Kesmodel, S.B.; et al. Immature natural killer cells promote progression of triple-negative breast cancer. Sci. Transl. Med. 2023, 15, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Murray, J.; Hilton, J.F.; Karamchandani, J.; Muradali, D.; Faragalla, H.; Polenz, C.; Han, D.; Bell, D.C.; Brezden-Masley, C. Mammographic microcalcifications and breast cancer tumorigenesis: A radiologic-pathologic analysis. BMC Cancer 2015, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Strax, P.; Venet, L.; Shapiro, S.; Gross, S. Mammography and clinical examination in mass screening for cancer of the breast. Cancer 1967, 20, 2184–2188. [Google Scholar] [CrossRef] [PubMed]
- Gosling, S.B.; Arnold, E.L.; Davies, S.K.; Cross, H.; Bouybayoune, I.; Calabrese, D.; Nallala, J.; Pinder, S.E.; Fu, L.; Lips, E.H.; et al. Microcalcification crystallography as a potential marker of DCIS recurrence. Sci. Rep. 2023, 13, 9331. [Google Scholar] [CrossRef]
- Wilkinson, L.; Thomas, V.; Sharma, N. Microcalcification on mammography: Approaches to interpretation and biopsy. Br. J. Radiol. 2017, 90, 20160594. [Google Scholar] [CrossRef] [PubMed]
- Janzen, K.; Janzen, J. Arterial Microcalcifications in the Breast Mimicking Malignancy. Case Rep. Radiol. 2012, 2012, 946317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bae, M.J.; Lee, S.Y.; Kim, Y.J.; Lee, J.G.; Jeong, D.W.; Yi, Y.H.; Cho, Y.H.; Choi, E.J.; Choo, K.S. Association of Breast Arterial Calcifications, Metabolic Syndrome, and the 10-Year Coronary Heart Disease Risk: A Cross-Sectional Case-Control Study. J. Women’s Health 2013, 22, 625–630. [Google Scholar] [CrossRef]
- Burnside, E.S.; Ochsner, J.E.; Fowler, K.J.; Fine, J.P.; Salkowski, L.R.; Rubin, D.L.; Sisney, G.A. Use of Microcalcification Descriptors in BI-RADS 4th Edition to Stratify Risk of Malignancy. Radiology 2007, 242, 388–395. [Google Scholar] [CrossRef]
- Osman, M.; Regner, S.; Osman, K.; Shahan, C.; Kheiri, B.; Kadiyala, M.; Sokos, G.; Sengupta, P.P.; Shapiro, M.D.; Michos, E.D.; et al. Association Between Breast Arterial Calcification on Mammography and Coronary Artery Disease: A Systematic Review and Meta-Analysis. J. Women’s Health 2022, 31, 1719–1726. [Google Scholar] [CrossRef]
- D’Orsi, C.J.; Sickler, E.A.; Mendelson, E.B.; Morris, E.S.; Creech, W.E.; Butler, P.F.; Wiegmann, P.G.; Chatfield, M.B.; Meyer, L.W.; Wilcox, P.A. ACR BI-RADS®-Atlas der Mammadiagnostik; Springer Berlin Heidelberg: Berlin, Heidelberg, 2016; ISBN 978-3-662-48817-1. [Google Scholar]
- Kim, H.; Greenberg, J.S.; Javitt, M.C. Residents’ Teaching Files: Breast Calcifications due to Mönckeberg Medial Calcific Sclerosis. RadioGraphics 1999, 19, 1401–1403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saenger, J.A.; Uenal, E.; Mann, E.; Winnik, S.; Eriksson, U.; Boss, A. Mammographic Vascular Microcalcifications as a Surrogate Parameter for Coronary Heart Disease: Correlation to Cardiac Computer Tomography and Proposal of a Classification Score. Diagnostics 2024, 14, 2803. [Google Scholar] [CrossRef]
- Ge, F.; Wang, R.; Huo, Z.; Wen, Y.; Liang, H.; Jiang, Y.; Su, Z.; Liang, W.; He, J. Cancer Risk in Heart or Lung Transplant Recipients: A Meta-Analysis. Transplantation 2020, 104, S582–S583. [Google Scholar] [CrossRef]
- Houn, F.; Brown, M.L. Current practice of screening mammography in the United States: Data from the National Survey of Mammography Facilities. Radiology 1994, 190, 209–215. [Google Scholar] [CrossRef]
- Lee, S.C.; Phillips, M.; Bellinge, J.; Stone, J.; Wylie, E.; Schultz, C. Is breast arterial calcification associated with coronary artery disease?—A systematic review and meta-analysis. PLoS ONE 2020, 15, e0236598. [Google Scholar] [CrossRef]
- Van Berkel, B.; Van Ongeval, C.; Van Craenenbroeck, A.H.; Pottel, H.; De Vusser, K.; Evenepoel, P. Prevalence, progression and implications of breast artery calcification in patients with chronic kidney disease. Clin. Kidney J. 2022, 15, 295–302. [Google Scholar] [CrossRef]
- Duhn, V.; D’Orsi, E.T.; Johnson, S.; D’Orsi, C.J.; Adams, A.L.; O’Neill, W.C. Breast Arterial Calcification. Clin. J. Am. Soc. Nephrol. 2011, 6, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Palit, S.; Kendrick, J. Vascular Calcification in Chronic Kidney Disease: Role of Disordered Mineral Metabolism. Curr. Pharm. Des. 2014, 20, 5829–5833. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, E.J.E.; de Jong, P.A.; van der Graaf, Y.; Mali, W.P.T.M.; van der Schouw, Y.T.; Beulens, J.W.J. Breast arterial calcifications: A systematic review and meta-analysis of their determinants and their association with cardiovascular events. Atherosclerosis 2015, 239, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Opałka, B.; Żołnierczuk, M.; Grabowska, M. Immunosuppressive Agents—Effects on the Cardiovascular System and Selected Metabolic Aspects: A Review. J. Clin. Med. 2023, 12, 6935. [Google Scholar] [CrossRef]
- Oudijk, E.D.; Lammers, J.J.; Koenderman, L. Systemic inflammation in chronic obstructive pulmonary disease. Eur. Respir. J. 2003, 22, 5s–13s. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef]
- George, J.T.; Green, L. Calciphylaxis of the breast, mimicking advanced breast cancer with skin involvement. Radiol. Case Rep. 2021, 16, 1211–1215. [Google Scholar] [CrossRef]
- Rzepka, A.M.; Tile, L.; Chow, C.-W.; McDonald-Blumer, H.; Chaparro, C.; Ridout, R.; Cheung, A.M. Lung transplantation and bone health: A narrative review. J. Heart Lung Transplant. 2025, 44, 849–857. [Google Scholar] [CrossRef]
- Spak, D.A.; Plaxco, J.S.; Santiago, L.; Dryden, M.J.; Dogan, B.E. BI-RADS ® fifth edition: A summary of changes. Diagn. Interv. Imaging 2017, 98, 179–190. [Google Scholar] [CrossRef]
- Maxwell, A.J.; Ridley, N.T.; Rubin, G.; Wallis, M.G.; Gilbert, F.J.; Michell, M.J. The Royal College of Radiologists Breast Group breast imaging classification. Clin. Radiol. 2009, 64, 624–627. [Google Scholar] [CrossRef]
- Cohen, Y. Digital Breast Tomosynthesis Assists in Differentiating Vascular Calcification From Malignant Calcification. Am. J. Roentgenol. 2017, 209, W115. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, H.; Bidgoli, F.A.; Zhou, B.; Iribarren, C.; Molloi, S.; Baldi, P. Detecting Cardiovascular Disease from Mammograms With Deep Learning. IEEE Trans. Med. Imaging 2017, 36, 1172–1181. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of Cancer Risk Among US Solid Organ Transplant Recipients. JAMA 2011, 306, 1891. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Edwards, L.B.; Kucheryavaya, A.Y.; Aurora, P.; Dobbels, F.; Kirk, R.; Rahmel, A.O.; Stehlik, J.; Hertz, M.I. The Registry of the International Society for Heart and Lung Transplantation: Twenty-seventh official adult lung and heart-lung transplant report—2010. J. Heart Lung Transplant. 2010, 29, 1104–1118. [Google Scholar] [CrossRef]
- Van Raemdonck, D.; Vos, R.; Yserbyt, J.; Decaluwe, H.; De Leyn, P.; Verleden, G.M. Lung cancer: A rare indication for, but frequent complication after lung transplantation. J. Thorac. Dis. 2016, 8, S915–S924. [Google Scholar] [CrossRef] [PubMed]
- Acuna, S.A.; Fernandes, K.A.; Daly, C.; Hicks, L.K.; Sutradhar, R.; Kim, S.J.; Baxter, N.N. Cancer Mortality Among Recipients of Solid-Organ Transplantation in Ontario, Canada. JAMA Oncol. 2016, 2, 463. [Google Scholar] [CrossRef]
- Stenman, C.; Wallinder, A.; Holmberg, E.; Karason, K.; Magnusson, J.; Dellgren, G. Malignancies After Lung Transplantation. Transpl. Int. 2024, 37, 12127. [Google Scholar] [CrossRef]
- Tseng, S.-C.; Gagne, S.M.; Hatabu, H.; Lin, G.; Sholl, L.M.; Nishino, M. Lung Cancer in Lung Transplant Recipients: Clinical, Radiologic, and Pathologic Characteristics and Treatment Outcome. J. Comput. Assist. Tomogr. 2023, 47, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Collett, D.; Mumford, L.; Banner, N.; Neuberger, J.; Watson, C. Comparison of the Incidence of Malignancy in Recipients of Different Types of Organ: A UK Registry Audit. Am. J. Transplant. 2010, 10, 1889–1896. [Google Scholar] [CrossRef]
- Adami, J.; Gäbel, H.; Lindelöf, B.; Ekström, K.; Rydh, B.; Glimelius, B.; Ekbom, A.; Adami, H.-O.; Granath, F. Cancer risk following organ transplantation: A nationwide cohort study in Sweden. Br. J. Cancer 2003, 89, 1221–1227. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, G.B.; Peng, F.H.; Xie, X.B. Cancer risks in recipients of renal transplants: A meta-analysis of cohort studies. Oncotarget 2018, 9, 15375–15385. [Google Scholar] [CrossRef]
- Yeoh, H.-L.; Shingles, H.; Paul, E.; Levvey, B.J.; Schwarz, M.; Voskoboynik, M.; Haydon, A.M.; Shackleton, M.; Snell, G.I.; Andrews, M.C. Malignancy risk and mortality after lung transplantation: A single-institution experience over 31 years. JHLT Open 2024, 4, 100094. [Google Scholar] [CrossRef]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Jiskra, J.; Límanová, Z.; Barkmanová, J.; Friedmannová, Z. Autoimunne thyroid diseases in women with breast cancer and colorectal cancer. Physiol. Res. 2006, 53, 693–702. [Google Scholar] [CrossRef]
- Han, J.Y.; Kim, H.; Jung, S.Y.; Jang, E.J.; Cho, S.K.; Sung, Y.K. Increased risk of malignancy in patients with systemic lupus erythematosus: Population-based cohort study in Korea. Arthritis Res. Ther. 2021, 23, 270. [Google Scholar] [CrossRef]
- Miao, Y.; Everly, J.J.; Gross, T.G.; Tevar, A.D.; First, M.R.; Alloway, R.R.; Woodle, E.S. De Novo Cancers Arising in Organ Transplant Recipients are Associated With Adverse Outcomes Compared With the General Population. Transplantation 2009, 87, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- AlBugami, M.; Kiberd, B. Malignancies: Pre and post transplantation strategies. Transplant. Rev. 2014, 28, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Chapman, J.R.; Craig, J.C. Cancer screening in renal transplant recipients: What is the evidence? Clin. J. Am. Soc. Nephrol. 2008, 3, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Cantor, A.; Humphrey, L.; Fu, R.; Pappas, M.; Daeges, M.; Griffin, J. Evidence Synthesis Number 124 Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2016; Available online: http://europepmc.org/abstract/med/26889531 (accessed on 20 June 2025).
- Duffy, S.W. Reduction in Breast Cancer Mortality from Organized Service Screening with Mammography: 1. Further Confirmation with Extended Data. Cancer Epidemiol. Biomark. Prev. 2006, 15, 45–51. [Google Scholar] [CrossRef]
- Kiberd, B.A.; Keough-Ryan, T.; Clase, C.M. Screening for Prostate, Breast and Colorectal Cancer in Renal Transplant Recipients. Am. J. Transplant. 2003, 3, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Fenichel, M. American Cancer Society Changes Breast Cancer Screening Guidelines To Reflect Analysis of Benefits and Harms. J. Natl. Cancer Inst. 2016, 108, djw022. [Google Scholar] [CrossRef][Green Version]
| Characteristic | Overall, N = 176 | Control, N = 94 | LTX, N = 82 | p-Value |
|---|---|---|---|---|
| Age at first mammography | 56 (50, 61) | 55 (51, 61) | 56 (50, 62) | 0.7 1 |
| Current or former smoker | 40 (23) | 9 (9.6) | 31 (38) | <0.001 2 |
| Pack years, Mean (SD) | 7.5 (15.4) | 2.4 (8.0) | 13.4 (19.4) | <0.001 3 |
| Chronic Kidney Disease | 56 (32) | 8 (8.5) | 48 (59) | <0.001 2 |
| Diabetes mellitus | 21 (12) | 14 (15) | 7 (8.5) | 0.2 2 |
| Arterial Hypertension | 56 (32) | 25 (27) | 31 (38) | 0.14 2 |
| Pulmonary Arterial Hypertension | 6 (3.4) | 0 (0) | 6 (7.3) | 0.009 2 |
| Characteristic | Overall, N = 176 | Control, N = 94 | LTX, N = 82 | p-Value 1 |
|---|---|---|---|---|
| Number of available mammographies | 3.00 (2.00, 5.00) | 4.00 (3.00, 6.00) | 2.00 (1.00, 4.00) | <0.001 |
| Interval between first and last mammography | 6.85 (3.37, 10.47) | 9.01 (5.05, 11.69) | 4.68 (2.17, 8.02) | <0.001 |
| Patients with ≥3 mammograms | 114 (64) | 77 (82) | 37 (45) | <0.001 |
| Follow-up, years | 6.95 (3.37, 10.47) | 9.01 (5.05, 11.69) | 4.68 (2.17, 8.02) | <0.001 |
| Characteristic | First Available Mammography | Last Available Mammography | ||||
|---|---|---|---|---|---|---|
| No LTX, N = 94 | LTX, N = 82 | p-Value | No LTX, N = 94 | LTX, N = 82 | p-Value | |
| ACR | 0.10 1 | 0.008 1 | ||||
| a | 13 (14) | 8 (9.8) | 17 (18) | 6 (7.3) | ||
| b | 32 (34) | 20 (24) | 39 (41) | 25 (30) | ||
| c | 35 (37) | 30 (37) | 34 (36) | 39 (48) | ||
| d | 14 (15) | 24 (29) | 4 (4.3) | 12 (15) | ||
| BIRADs, n (%) | 0.3 1 | 0.2 1 | ||||
| 0 | 0 (0) | 1 (1.2) | 0 (0) | 0 (0) | ||
| 1 | 8 (8.5) | 2 (2.4) | 4 (4.3) | 0 (0) | ||
| 2 | 80 (85) | 71 (87) | 84 (89) | 73 (89) | ||
| 3 | 3 (3.2) | 4 (4.9) | 4 (4.3) | 6 (7.3) | ||
| 4 | 3 (3.2) | 4 (4.9) | 1 (1.1) | 3 (3.7) | ||
| 5 | 0 (0) | 0 (0) | 1 (1.1) | 0 (0) | ||
| 6 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Grouped microcalcifications | 3 (3.2) | 5 (6.1) | 0.5 2 | 3 (3.2) | 17 (21) | <0.001 2 |
| Macrocalcifications or sporadic, scattered microcalcifications | 45 (48) | 44 (54) | 0.4 2 | 66 (70) | 66 (80) | 0.12 2 |
| BAC score | 0.003 1 | <0.001 1 | ||||
| 1 | 89 (95) | 67 (82) | 84 (89) | 49 (60) | ||
| 2 | 4 (4.3) | 3 (3.7) | 4 (4.3) | 4 (4.9) | ||
| 3 | 1 (1.1) | 9 (11) | 6 (6.4) | 14 (17) | ||
| 4 | 0 (0) | 3 (3.7) | 0 (0) | 15 (18) | ||
| Characteristic | Macrocalcifications or Sporadic Scattered Microcalcifications | Grouped Microcalcifications | BAC |
|---|---|---|---|
| Lung transplantation | 1.37 (0.55–3.52), 0.50 | 9.26 (2.29–50.4), 0.004 | 8.15 (2.77–27.0), <0.001 |
| Age (years) | 1.00 (0.96–1.05), >0.90 | 0.93 (0.87–1.00), 0.051 | 0.99 (0.94–1.04), 0.70 |
| Chronic kidney disease | 1.49 (0.56–4.15), 0.40 | 0.48 (0.15–1.49), 0.20 | 1.41 (0.57–3.48), 0.50 |
| Diabetes mellitus | 2.97 (0.76–19.7), 0.20 | 5.33 (1.15–24.7), 0.029 | 2.33 (0.68–7.97), 0.20 |
| Arterial hypertension | 2.04 (0.88–5.20), 0.11 | 1.65 (0.53–5.04), 0.40 | 3.05 (1.31–7.25), 0.010 |
| Smoking status | 1.18 (0.47–3.20), 0.70 | 3.12 (0.94–10.8), 0.064 | 0.68 (0.25–1.77), 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saenger, J.; Happe, J.; Maier, C.; Kerber, B.; Uenal, E.; Bos, D.; Frauenfelder, T.; Boss, A. Mammographic Calcifications in Lung Transplant Recipients: Prevalence and Evolution. Biomedicines 2025, 13, 2318. https://doi.org/10.3390/biomedicines13092318
Saenger J, Happe J, Maier C, Kerber B, Uenal E, Bos D, Frauenfelder T, Boss A. Mammographic Calcifications in Lung Transplant Recipients: Prevalence and Evolution. Biomedicines. 2025; 13(9):2318. https://doi.org/10.3390/biomedicines13092318
Chicago/Turabian StyleSaenger, Jonathan, Jasmin Happe, Caroline Maier, Bjarne Kerber, Ela Uenal, Denise Bos, Thomas Frauenfelder, and Andreas Boss. 2025. "Mammographic Calcifications in Lung Transplant Recipients: Prevalence and Evolution" Biomedicines 13, no. 9: 2318. https://doi.org/10.3390/biomedicines13092318
APA StyleSaenger, J., Happe, J., Maier, C., Kerber, B., Uenal, E., Bos, D., Frauenfelder, T., & Boss, A. (2025). Mammographic Calcifications in Lung Transplant Recipients: Prevalence and Evolution. Biomedicines, 13(9), 2318. https://doi.org/10.3390/biomedicines13092318







