Heparin Binding Protein in Sepsis—A Comprehensive Overview of Pathophysiology, Clinical Usage and Utility as Biomarker
Abstract
1. Introduction
2. Methods
3. Key Points
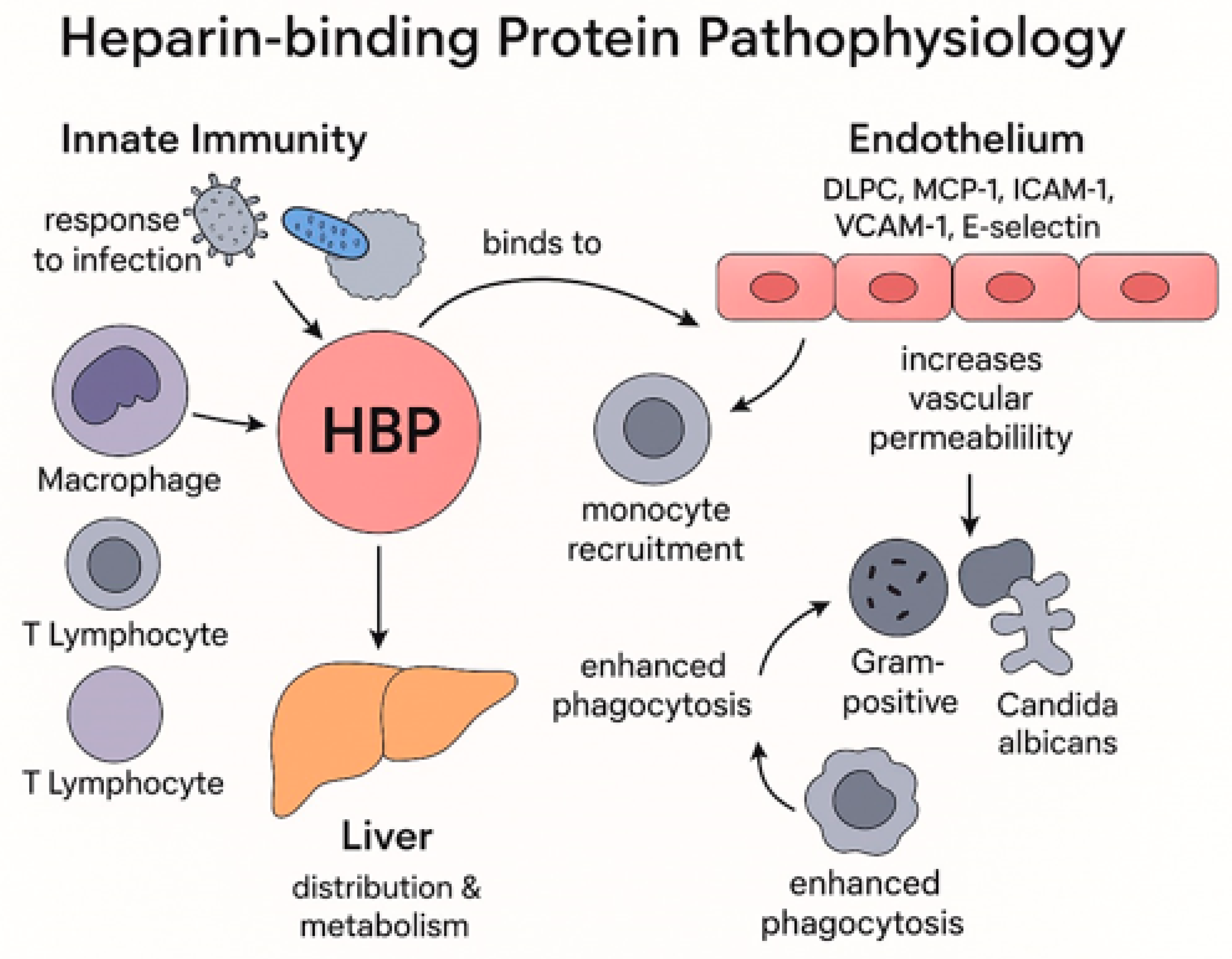
3.1. Heparin Binding Protein Pathophysiology
- HBP is an enzymatically inactive serine protease, mainly stored in azurophilic granules and rapidly released during infection or stress, often preceding the rise of other inflammatory biomarkers.
- HBP actively contributes to sepsis pathophysiology by recruiting monocytes, increasing endothelial permeability, promoting vascular leakage, and enhancing pathogen clearance.
3.2. Biomarkers in Sepsis
- Established biomarkers, including lactate, CRP, PCT, WBC, NLR, and PSP, are valuable diagnostic and prognostic tools for sepsis, but are limited by specificity and response timing.
- HBP emerges as a promising biomarker with potential to enhance early diagnosis and risk stratification in sepsis.
3.3. HBP as Biomarker
- HBP demonstrates high diagnostic and prognostic accuracy across multiple bacterial infections—including central nervous system, urinary tract, respiratory, and postoperative infections—often outperforming or complementing traditional biomarkers.
- Beyond bacterial infections, elevated HBP levels are associated with viral infections such as COVID-19, as well as pancreatitis, cardiothoracic surgery, and other inflammatory conditions, reflecting its broader clinical utility.
3.4. HBP and Sepsis
- HBP demonstrates superior diagnostic and prognostic accuracy for sepsis, septic shock, and organ dysfunction compared to traditional biomarkers, with elevated levels detectable up to 72 h before clinical deterioration, supporting timely initiation of sepsis bundle therapies.
- Dynamic changes, integration of HBP with clinical scores, and cutoff thresholds of HBP significantly enhance mortality prediction, severe sepsis progression, and differentiate conditions such as COVID-19-related sepsis and autoinflammatory diseases.
4. Discussion
4.1. Heparin Binding Protein Pathophysiology
4.2. Biomarkers in Sepsis
4.3. HBP as Biomarker
4.4. HBP and Sepsis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pohl, J.; Pereira, H.A.; Martin, N.M.; Spitznagel, J.K. Amino acid sequence of CAP37, a human neutrophil granule-derived antibacterial and monocyte-specific chemotactic glycoprotein structurally similar to neutrophil elastase. FEBS Lett. 1990, 272, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, S.; Wang, J.; Gao, Y.; Xie, F.; Gong, J.; Bi, S.; Yao, Z.; Li, Y.; Liu, W.; et al. The performance of a combination of heparin-binding protein with other biomarkers for sepsis diagnosis: An observational cohort study. BMC Infect. Dis. 2024, 24, 755. [Google Scholar] [CrossRef]
- Wu, Y.L.; Yo, C.H.; Hsu, W.T.; Qian, F.; Wu, B.S.; Dou, Q.L.; Lee, C.C. Accuracy of heparin-binding protein in diagnosing sepsis: A systematic review and meta-analysis. Crit. Care Med. 2021, 49, e80–e90. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yuan, H.; Wu, Y.L.; Fu, S.; Pan, X.Y. The predictive value of heparin-binding protein and D-dimer in patients with sepsis. Int. J. Gen. Med. 2023, 16, 2295–2303. [Google Scholar] [CrossRef]
- Han, X.; Dou, Q.; Zhu, Y.; Ling, P.; Shen, Y.H.; Liu, J.; Zhang, Z.; Zhou, Y.; Fan, M.; Huang, S.S.; et al. Heparin-binding protein-enhanced quick SOFA score improves mortality prediction in sepsis patients. Front. Med. 2022, 9, 926798. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Patel, A. Editorial: Understanding molecular mechanisms to facilitate the development of biomarkers for therapeutic intervention in gastrointestinal diseases and sepsis. Front. Genet. 2025, 16, 1581299. [Google Scholar] [CrossRef]
- He, R.R.; Yue, G.L.; Dong, M.L.; Wang, J.Q.; Cheng, C. Sepsis biomarkers: Advancements and clinical applications—A narrative review. Int. J. Mol. Sci. 2024, 25, 9010. [Google Scholar] [CrossRef] [PubMed]
- Flodgaard, H.; Østergaard, E.; Bayne, S.; Svendsen, A.; Thomsen, J.; Engels, M.; Wollmer, A. Covalent structure of two novel neutrophil leucocyte-derived proteins of porcine and human origin: Neutrophil elastase homologues with strong monocyte and fibroblast chemotactic activities. Eur. J. Biochem. 1991, 197, 535–547. [Google Scholar] [CrossRef]
- Zuo, L.; Li, X.; Wang, L.; Yuan, H.; Liao, Z.; Zhou, S.; Wu, J.; Guan, X.; Liu, Y. Heparin-binding protein as a biomarker for the diagnosis of sepsis in the intensive care unit: A retrospective cross-sectional study in China. BMJ Open 2024, 14, e078687. [Google Scholar] [CrossRef]
- Halldorsdottir, H.; Lindbom, L.; Ebberyd, A.; Oldner, A.; Weitzberg, E. The effect of heparins on plasma concentration of heparin-binding protein: A pilot study. BJA Open 2024, 9, 100256. [Google Scholar] [CrossRef]
- Johannesson, E.; Erixon, C.; Sterner, N.; Thelaus, L.; Dardashti, A.; Nilsson, J.; Ragnarsson, S.; Linder, A.; Zindovic, I. Utility of heparin-binding protein following cardiothoracic surgery using cardiopulmonary bypass. Sci. Rep. 2023, 13, 21566. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; He, Q.; Shen, J.; Xu, L.; Zhu, P.; Zhao, M. A promising candidate: Heparin-binding protein steps onto the stage of sepsis prediction. J. Immunol. Res. 2019, 2019, 7515346. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.L.; Liu, J.; Zhang, W.; Wang, C.W.; Gu, Y.; Li, N.; Hu, R.; Hsu, W.T.; Huang, A.H.; Tong, H.S.; et al. Dynamic changes in heparin-binding protein as a prognostic biomarker for 30-day mortality in sepsis patients in the intensive care unit. Sci. Rep. 2022, 12, 10751. [Google Scholar] [CrossRef]
- Russell, J.A.; Rush, B.; Boyd, J. Pathophysiology of septic shock. Crit. Care Clin. 2018, 34, 43–61. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, M.; Sun, Y.; Li, X.; Cao, L.; Ma, X. Transforming growth factor-β receptor type 2 is required for heparin-binding protein-induced acute lung injury and vascular leakage for transforming growth factor-β/Smad/Rho signaling pathway activation. FASEB J. 2022, 36, e22580. [Google Scholar] [CrossRef]
- Olinder, J.; Börjesson, A.; Norrman, J.; West, T.; Carlström, J.; Gustafsson, A.; Annborn, M.; Herwald, H.; Rydén, C. Hepcidin discriminates sepsis from other critical illness at admission to intensive care. Sci. Rep. 2022, 12, 14857. [Google Scholar] [CrossRef]
- Neumann, A. Rapid release of sepsis markers heparin-binding protein and calprotectin triggered by anaerobic cocci poses an underestimated threat. Anaerobe 2022, 75, 102584. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Yu, F. Changes in heparin-binding protein, procalcitonin, and C-reactive protein within the first 72 hours predict 28-day mortality in patients admitted to the intensive care unit with septic shock. Med. Sci. Monit. 2023, 29, e938538. [Google Scholar] [CrossRef] [PubMed]
- Katsaros, K.; Renieris, G.; Safarika, A.; Adami, E.M.; Gkavogianni, T.; Giannikopoulos, G.; Solomonidi, N.; Halvatzis, S.; Koutelidakis, I.M.; Tsokos, N.; et al. Heparin binding protein for the early diagnosis and prognosis of sepsis in the emergency department: The PROMPT multicenter study. Shock 2022, 57, 518–525. [Google Scholar] [CrossRef]
- Fisher, J.; Kahn, F.; Wiebe, E.; Gustafsson, P.; Kander, T.; Mellhammar, L.; Bentzer, P.; Linder, A. The dynamics of circulating heparin-binding protein: Implications for its use as a biomarker. J. Innate Immun. 2022, 14, 447–460. [Google Scholar] [CrossRef]
- Soehnlein, O.; Zernecke, A.; Eriksson, E.E.; Rothfuchs, A.G.; Pham, C.T.; Herwald, H.; Bidzhekov, K.; Rottenberg, M.E.; Weber, C.; Lindbom, L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008, 112, 1461–1471. [Google Scholar] [CrossRef]
- Lee, T.D.; Gonzalez, M.L.; Kumar, P.; Grammas, P.; Pereira, H.A. CAP37, a neutrophil-derived inflammatory mediator, augments leukocyte adhesion to endothelial monolayers. Microvasc. Res. 2003, 66, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Olofsson, A.M.; Herwald, H.; Iversen, L.F.; Lundgren-Åkerlund, E.; Hedqvist, P.; Arfors, K.-E.; Flodgaard, H.; Lindbom, L. Heparin-binding protein (HBP/CAP37): A missing link in neutrophil-evoked alteration of vascular permeability. Nat. Med. 2001, 7, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Bentzer, P.; Fisher, J.; Kong, H.J.; Mörgelin, M.; Boyd, J.H.; Walley, K.R.; Russell, J.A.; Linder, A. Heparin-binding protein is important for vascular leak in sepsis. Intensive Care Med. Exp. 2016, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Tapper, H.; Karlsson, A.; Mörgelin, M.; Flodgaard, H.; Herwald, H. Secretion of heparin-binding protein from human neutrophils is determined by its localization in azurophilic granules and secretory vesicles. Blood 2002, 99, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Lever, A.; Mackenzie, I. Sepsis: Definition, epidemiology, and diagnosis. BMJ 2007, 335, 879–883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zambon, M.; Ceola, M.; Almeida-de-Castro, R.; Gullo, A.; Vincent, J.L. Implementation of the Surviving Sepsis Campaign guidelines for severe sepsis and septic shock: We could go faster. J. Crit. Care. 2008, 23, 455–460. [Google Scholar] [CrossRef]
- Marshall, J.C.; Reinhart, K. Biomarkers of sepsis. Crit. Care Med. 2009, 37, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kolb-Bachofen, V. A review on the biological properties of C-reactive protein. Immunobiology 1991, 183, 133–145. [Google Scholar] [CrossRef]
- Clyne, B.; Olshaker, J.S. The C-reactive protein. J. Emerg. Med. 1999, 17, 1019–1025. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Pepys, M.B.; Hawkins, P.N. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J. Clin. Investig. 1993, 91, 351–357. [Google Scholar] [CrossRef]
- Young, B.; Gleeson, M.; Cripps, A.W. C-reactive protein: A critical review. Pathology 1991, 23, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Almeida, E.; Moreira, P.; Fernandes, A.; Mealha, R.; Aragão, A.; Sabino, H. C-reactive protein as an indicator of sepsis. Intensive Care Med. 1998, 24, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S.M.; Lobo, F.R.; Bota, D.P.; Lopes-Ferreira, F.; Soliman, H.M.; Mélot, C.; Vincent, J.L. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest 2003, 123, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Petel, D.; Winters, N.; Gore, G.C.; Papenburg, J.; Beltempo, M.; Lacroix, J.; Fontela, P.S. Use of C-reactive protein to tailor antibiotic use: A systematic review and meta-analysis. BMJ Open 2018, 8, e022133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nargis, W.; Ibrahim, M.; Ahamed, B.U. Procalcitonin versus C-reactive protein: Usefulness as biomarker of sepsis in ICU patient. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 195–199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Briel, M.; Schuetz, P.; Mueller, B.; Young, J.; Schild, U.; Nusbaumer, C.; Périat, P.; Bucher, H.C.; Christ-Crain, M. Procalcitonin-guided antibiotic use versus a standard approach for acute respiratory tract infections in primary care. Arch. Intern. Med. 2008, 168, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.F.; Sibbald, W.J. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 1992, 20, 864–874. [Google Scholar] [PubMed]
- Farkas, J.D. The complete blood count to diagnose septic shock. J. Thorac. Dis. 2020, 12 (Suppl. 1), S16–S21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seigel, T.A.; Cocchi, M.N.; Salciccioli, J.; Shapiro, N.I.; Howell, M.; Tang, A.; Donnino, M.W. Inadequacy of temperature and white blood cell count in predicting bacteremia in patients with suspected infection. J. Emerg. Med. 2012, 42, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts—Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar] [PubMed]
- Lee, J.S.; Kim, N.Y.; Na, S.H.; Youn, Y.H.; Shin, C.S. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 2018, 97, e11138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forget, P.; Khalifa, C.; Defour, J.P.; Latinne, D.; Van Pel, M.C.; De Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hwang, S.Y.; Shin, T.G.; Jo, I.J.; Jeon, K.; Suh, G.Y.; Lee, T.R.; Yoon, H.; Cha, W.C.; Sim, M.S. Neutrophil-to-lymphocyte ratio as a prognostic marker in critically-ill septic patients. Am. J. Emerg. Med. 2017, 35, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.; Zhou, L.; Wang, Q.; Ding, B.; Zhan, Y.; Qin, S.; Zhu, N.; Li, Z.; Lei, Z. Diagnostic accuracy of pancreatic stone protein in patients with sepsis: A systematic review and meta-analysis. BMC Infect. Dis. 2024, 24, 472. [Google Scholar] [CrossRef]
- Michailides, C.; Lagadinou, M.; Paraskevas, T.; Papantoniou, K.; Kavvousanos, M.; Vasileiou, A.; Thomopoulos, K.; Velissaris, D.; Marangos, M. The role of the pancreatic stone protein in predicting intra-abdominal infection-related complications: A prospective observational single-center cohort study. Microorganisms 2023, 11, 2579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lagadinou, M.; Paraskevas, T.; Velissaris, D.; Michailides, C.; Eleftherakis, G.; Sampsonas, F.; Siakallis, G.; Assimakopoulos, S.F.; Marangos, M. The role of pancreatic stone protein as a prognostic factor for COVID-19 patients. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6391–6395. [Google Scholar] [CrossRef] [PubMed]
- Filippidis, P.; Hovius, L.; Tissot, F.; Orasch, C.; Flückiger, U.; Siegemund, M.; Pagani, J.L.; Eggimann, P.; Marchetti, O.; Lamoth, F.; et al. Serial monitoring of pancreatic stone protein for the detection of sepsis in intensive care unit patients with complicated abdominal surgery: A prospective, longitudinal cohort study. J. Crit. Care 2024, 82, 154772. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.M.; Abouelmagd, K.; Omar, M.M.; Najah, Q.; Ali, M.; Hasan, M.T.; Allam, S.A.; Arian, R.; Rageh, O.E.S.; Abd-ElGawad, M. The diagnostic utility of heparin-binding protein among patients with bacterial infections: A systematic review and meta-analysis. BMC Infect. Dis. 2024, 24, 150. [Google Scholar] [CrossRef]
- Li, L.; Tian, X.-X.; Feng, G.-L.; Chen, B. The predictive value of heparin-binding protein for bacterial infections in patients with severe polytrauma. PLoS ONE 2024, 19, e0300692. [Google Scholar] [CrossRef]
- Kandil, M.; Khalil, G.; El-Attar, E.; Shehata, G.; Hassan, S. Accuracy of heparin-binding protein as a new marker in prediction of acute bacterial meningitis. Braz. J. Microbiol. 2018, 49 (Suppl. 1), 213–219. [Google Scholar] [CrossRef]
- Namiduru, E.S.; Namiduru, M.; Karaoğlan, İ.; Erbağci, E. Heparin binding protein in early differential diagnosis of bacterial meningitis. Indian J. Clin. Biochem. 2024, 39, 118–123. [Google Scholar] [CrossRef]
- Schade, R.P.; Schinkel, J.; Roelandse, F.W.C.; Geskus, R.B.; Visser, L.G.; van Dijk, M.C.; Voormolen, J.H.C.; van Pelt, H.; Kuijper, E.J. Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage-related bacterial meningitis. J. Neurosurg. 2006, 104, 101–108. [Google Scholar] [CrossRef]
- Linder, A.; Åkesson, P.; Brink, M.; Studahl, M.; Björck, L.; Christensson, B. Heparin-binding protein: A diagnostic marker of acute bacterial meningitis. Crit. Care Med. 2011, 39, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Chen, J.; Xu, J.; Luo, Q.; Fu, P.; Zhao, F.; Huang, Z. The diagnostic and prognostic value of heparin-binding protein in cerebrospinal fluid for patients with intracranial infections. Eur. J. Med. Res. 2024, 29, 579. [Google Scholar] [CrossRef]
- Guan, L.; Wang, F.; Chen, J.; Xu, Y.; Zhang, W.; Zhu, J. Clinical value of heparin-binding protein in adult bacterial intracranial infection. Front. Cell. Infect. Microbiol. 2024, 14, 1439143. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Ye, Y.; Ma, J.; Shi, G. Accuracy of heparin-binding protein for the diagnosis of nosocomial meningitis and ventriculitis. Crit. Care 2022, 26, 56. [Google Scholar] [CrossRef] [PubMed]
- Kjölvmark, C.; Påhlman, L.I.; Åkesson, P.; Linder, A. Heparin-binding protein: A diagnostic biomarker of urinary tract infection in adults. Open Forum Infect. Dis. 2014, 1, ofu004. [Google Scholar] [CrossRef]
- Kjölvmark, C.; Tschernij, E.; Öberg, J.; Påhlman, L.I.; Linder, A.; Åkesson, P. Distinguishing asymptomatic bacteriuria from urinary tract infection in the elderly—The use of urine levels of heparin-binding protein and interleukin-6. Diagn. Microbiol. Infect. Dis. 2016, 85, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Lei, Z.; Wang, Z.; Yu, M.; Li, J.; Chai, W.; Zhao, X. A novel HCP (heparin-binding protein-C reactive protein-procalcitonin) inflammatory composite model can predict severe acute pancreatitis. Sci. Rep. 2023, 13, 9440. [Google Scholar] [CrossRef]
- Sjöbeck, M.; Sternby, H.; Herwald, H.; Thorlacius, H.; Regnér, S. Heparin-binding protein is significantly increased in acute pancreatitis. BMC Gastroenterol. 2021, 21, 337. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, L.; Huang, M.; Sun, G. Blood heparin-binding protein and neutrophil-to-lymphocyte ratio as indicators of the severity and prognosis of community-acquired pneumonia. Respir. Med. 2023, 208, 107144. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, M.; Thelaus, L.; Riesbeck, K.; Qvarfordt, I.; Smith, M.E.; Lindén, A.; Linder, A. Heparin-binding protein in lower airway samples as a biomarker for pneumonia. Respir. Res. 2021, 22, 174. [Google Scholar] [CrossRef]
- Mellhammar, L.; Thelaus, L.; Elén, S.; Fisher, J.; Linder, A. Heparin binding protein in severe COVID-19—A prospective observational cohort study. PLoS ONE 2021, 16, e0249570. [Google Scholar] [CrossRef]
- Barnes, G.D.; Burnett, A.; Allen, A.; Blumenstein, M.; Clark, N.P.; Cuker, A.; Dager, W.E.; Deitelzweig, S.B.; Ellsworth, S.; Garcia, D.; et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: Interim clinical guidance from the Anticoagulation Forum. J. Thromb. Thrombolysis 2020, 50, 72–81. [Google Scholar] [CrossRef]
- Saridaki, M.; Metallidis, S.; Grigoropoulou, S.; Vrentzos, E.; Lada, M.; Argyraki, K.; Tsachouridou, O.; Georgiadou, A.; Vasishta, A.; Giamarellos-Bourboulis, E.J. Integration of heparin-binding protein and interleukin-6 in the early prediction of respiratory failure and mortality in pneumonia by SARS-CoV-2 (COVID-19). Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1405–1412. [Google Scholar] [CrossRef]
- Stjärne Aspelund, A.; Hammarström, H.; Inghammar, M.; Larsson, H.; Hansson, L.; Christensson, B.; Påhlman, L.I. Heparin-binding protein, lysozyme, and inflammatory cytokines in bronchoalveolar lavage fluid as diagnostic tools for pulmonary infection in lung transplanted patients. Am. J. Transplant. 2018, 18, 444–452. [Google Scholar] [CrossRef]
- Xue, M.; Zhang, T.; Lin, R.; Zeng, Y.; Cheng, Z.J.; Li, N.; Zheng, P.; Huang, H.; Zhang, X.D.; Wang, H.; et al. Clinical utility of heparin-binding protein as an acute-phase inflammatory marker in interstitial lung disease. J. Leukoc. Biol. 2022, 112, 861–873. [Google Scholar] [CrossRef]
- Kaukonen, K.-M.; Linko, R.; Herwald, H.; Lindbom, L.; Ruokonen, E.; Ala-Kokko, T.; Pettilä, V. Heparin-binding protein (HBP) in critically ill patients with influenza A (H1N1) infection. Clin. Microbiol. Infect. 2013, 19, 1122–1128. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Linder, A.; Annborn, M.; Rundgren, M.; Friberg, H. Heparin-binding protein: An early indicator of critical illness and predictor of outcome in cardiac arrest. Resuscitation 2013, 84, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, E.; Passov, A.; Salminen, U.S.; Ilmakunnas, M.; Vento, A.; Aittomäki, J.; Andersson, S.; Schramko, A. Heparin-binding protein in adult heart surgery. Ann. Thorac. Surg. 2019, 107, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Sterner, N.; Fisher, J.; Thelaus, L.; Ketteler, C.; Lemež, Š.; Dardashti, A.; Nilsson, J.; Linder, A.; Zindovic, I. The dynamics of heparin-binding protein in cardiothoracic surgery—A pilot study. J. Cardiothorac. Vasc. Anesth. 2021, 35, 2640–2650. [Google Scholar] [CrossRef]
- Ristagno, G.; Masson, S.; Tiainen, M.; Bendel, S.; Bernasconi, R.; Varpula, T.; Milani, V.; Vaahersalo, J.; Magnoli, M.; Spanuth, E.; et al. Elevated plasma heparin-binding protein is associated with early death after resuscitation from cardiac arrest. Crit. Care 2016, 20, 251. [Google Scholar] [CrossRef]
- Busse, L.W.; Barker, N.; Petersen, C. Vasoplegic syndrome following cardiothoracic surgery—Review of pathophysiology and update of treatment options. Crit. Care 2020, 24, 36. [Google Scholar] [CrossRef]
- Pan, T.; Long, G.F.; Chen, C.; Zhang, H.T.; Wang, J.X.; Ahaskar, A.; Chen, H.B.; Wang, D.J. Heparin-binding protein measurement improves the prediction of myocardial injury-related cardiogenic shock. BMC Cardiovasc. Disord. 2020, 20, 124. [Google Scholar] [CrossRef]
- Tverring, J.; Nielsen, N.; Dankiewicz, J.; Linder, A.; Kahn, F.; Åkesson, P. Repeated measures of heparin-binding protein (HBP) and procalcitonin during septic shock: Biomarker kinetics and association with cardiovascular organ dysfunction. Intensive Care Med. Exp. 2020, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Al Farai, A.; Al Moundhri, M.; Panaro, F. Launching of the Sultan Qaboos Comprehensive Cancer Care and Research Center (SQCCCRC) in the Sultanate of Oman to manage HBP type of cancers. Hepatobiliary Surg. Nutr. 2022, 11, 338–339. [Google Scholar] [CrossRef]
- Yang, Z.; Xue, Z.; Tao, Y.; Shi, X.; Li, J. Systemic inflammatory status of patients with obstructive sleep apnea syndrome and the predictive value of heparin-binding protein. Ear Nose Throat J. 2023, 1455613231202490. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.L.; Persson, S.; Lopes-Ferreira, M.; Romero, E.C.; Kirchgatter, K.; Nascimento, A.L.T.; Herwald, H. Heparin-binding protein release is strongly induced by Leptospira species and is a candidate for an early diagnostic marker of human leptospirosis. J. Infect. Dis. 2019, 219, 996–1006. [Google Scholar] [CrossRef]
- Linder, A.; Johansson, L.; Thulin, P.; Hertzén, E.; Mörgelin, M.; Christensson, B.; Björck, L.; Norrby-Teglund, A.; Åkesson, P. Erysipelas caused by group A streptococcus activates the contact system and induces the release of heparin-binding protein. J. Investig. Dermatol. 2010, 130, 1365–1372. [Google Scholar] [CrossRef]
- Herwald, H.; Cramer, H.; Mörgelin, M.; Russell, W.; Sollenberg, U.; Norrby-Teglund, A.; Flodgaard, H.; Lindbom, L.; Björck, L. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell 2004, 116, 367–379. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.-C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of clinical criteria for sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Jekarl, D.W.; Lee, S.; Kim, M.; Kim, Y.; Woo, S.H.; Lee, W.J. Procalcitonin as a prognostic marker for sepsis based on SEPSIS-3. J. Clin. Lab. Anal. 2019, 33, e22996. [Google Scholar] [CrossRef]
- Rudd, K.E.; Seymour, C.W.; Aluisio, A.R.; Augustin, M.E.; Bagenda, D.S.; Beane, A.; Byiringiro, J.C.; Chang, C.-C.H.; Colas, L.N.; Day, N.P.J.; et al. Association of the quick Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA) score with excess hospital mortality in adults with suspected infection in low- and middle-income countries. JAMA 2018, 319, 2202–2211. [Google Scholar] [CrossRef]
- Taha, A.M.; Najah, Q.; Omar, M.M.; Abouelmagd, K.; Ali, M.; Hasan, M.T.; Allam, S.A.; Hamam, Y.A.; Arian, R.; Abd-ElGawad, M. Diagnostic and prognostic value of heparin-binding protein in sepsis: A systematic review and meta-analysis. Medicine 2024, 103, e38525. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, C.; Hong, G.; Wang, Q.; Lian, M. Meta-analysis of the diagnostic efficacy of heparin-binding protein in adult sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 31, 1330–1334. [Google Scholar] [CrossRef]
- Chen, M.; Yuan, J.; Yang, Z.; Cai, G. Value of heparin-binding protein in diagnosis of sepsis in adult patients: A meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 31, 1224–1230. [Google Scholar] [CrossRef]
- Fisher, J.; Linder, A. Heparin-binding protein: A key player in the pathophysiology of organ dysfunction in sepsis. J. Intern. Med. 2017, 281, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Linder, A.; Christensson, B.; Herwald, H.; Björck, L.; Akesson, P. Heparin-binding protein: An early marker of circulatory failure in sepsis. Clin. Infect. Dis. 2009, 49, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Linder, A.; Arnold, R.; Boyd, J.H.; Zindovic, M.; Zindovic, I.; Lange, A.; Paulsson, M.; Nyberg, P.; Russell, J.A.; Pritchard, D.; et al. Heparin-binding protein measurement improves the prediction of severe infection with organ dysfunction in the emergency department. Crit. Care Med. 2015, 43, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Kahn, F.; Tverring, J.; Mellhammar, L.; Wetterberg, N.; Bläckberg, A.; Studahl, E.; Hadorn, N.; Kahn, R.; Nueesch, S.; Jent, P.; et al. Heparin-binding protein as a prognostic biomarker of sepsis and disease severity at the emergency department. Shock 2019, 52, e135–e145. [Google Scholar] [CrossRef]
- Fisher, J.; Russell, J.A.; Bentzer, P.; Parsons, D.; Secchia, S.; Mörgelin, M.; Walley, K.R.; Boyd, J.H.; Linder, A. Heparin-binding protein (HBP): A causative marker and potential target for heparin treatment of human sepsis-induced acute kidney injury. Shock 2017, 48, 313–320. [Google Scholar] [CrossRef]
- Abdou, K.M.; Ali, O.M.H.; Abdelfatah, M.I.; Abdelhamid, H.S.; Soliman, D.A.; Zaki, H.V. Heparin binding protein as a reliable prognostic biomarker for severity of sepsis in the intensive care unit. Egypt. J. Anaesth. 2024, 42, 300–307. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Cao, Y.; Fan, M.; Zhou, Y.; Li, X.; Cao, C.; Han, X. Predictive value of heparin-binding protein for sepsis. China J. Emerg. Resusc. Disaster Med. 2021, 33, 654–658. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J. Value of D-dimer combined with SOFA score in predicting prognosis for patients with sepsis. China J. Emerg. Resusc. Disaster Med. 2021, 16, 50–53. [Google Scholar]
- Xue, M.; Zeng, Y.; Qu, H.Q.; Zhang, T.; Li, N.; Huang, H.; Zheng, P.; Hu, H.; Zhou, L.; Duan, Z.; et al. Heparin-binding protein levels correlate with aggravation and multiorgan damage in severe COVID-19. ERJ Open Res. 2021, 7, 00741-2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Chen, X.; Yang, C.; Teng, J.; Qu, H.; Liu, H.L. Serum heparin-binding protein as a potential biomarker to distinguish adult-onset Still’s disease from sepsis. Front. Immunol. 2021, 12, 654811. [Google Scholar] [CrossRef] [PubMed]
| Diagnostic Role of HBP in Sepsis | ||||
|---|---|---|---|---|
| First Author | Publication Year | Study Type | Major Findings | Sample Size |
| Taha, A.M. [89] | 2024 | Systematic review and meta-analysis | HBP presented moderate diagnostic value in sepsis validating its use as a complementary tool alongside established clinical assessments and other well-studied biomarkers. Sensitivity of 0.71 and a specificity of 0.68. | The meta-analysis enrolled 28 studies including 5508 patients. |
| Wu, Y.L. [3] | 2021 | Systematic review and meta-analysis | HBP demonstrated high diagnostic accuracy in detecting sepsis among critically ill patients with a pooled sensitivity of 0.85 and specificity of 0.91 outperforming PCT (sensitivity = 0.75, specificity = 0.85) CRP (sensitivity = 0.75, specificity = 0.71) and lactate (sensitivity = 0.64, specificity = 0.80) | The meta-analysis enrolled 26 studies including 3868 patients |
| Tian, R. [103] | 2021 | Prospective observational cohort study | Serum HBP differentiates active adult-onset Still’s disease from sepsis at a cutoff of 65.1 ng/mL (sensitivity = 0.759, specificity = 0.552) and from inactive disease at a cutoff of 35.59 ng/mL (sensitivity = 0.650, specificity = 0.889), supporting its potential use as a diagnostic biomarker | In the study were enrolled 30 patients with AOSD, 29 patients with sepsis, and 30 healthy individuals. Of those with AOSD, 20 had active and 10 had inactive disease |
| Chen, S. [90] | 2019 | Meta-analysis | HBP demonstrated good diagnostic accuracy for adult sepsis with a pooled sensitivity of 0.74 and specificity of 0.73 and can be used as an auxiliary biomarker in clinical diagnostic protocols. | This meta-analysis included 14 studies with a total of 2023 subjects: 1120 in the sepsis group and 903 in the non-sepsis control group (795 patients with non-sepsis conditions and 108 healthy volunteers). |
| Chen, M. [91] | 2019 | Systematic review and meta-analysis | HPB presented strong diagnostic performance in adult sepsis, with both pooled sensitivity and specificity of 0.80 | In this meta-analysis 10 studies with 1884 patients were included. |
| Prognostic Role of HBP in Sepsis | ||||
|---|---|---|---|---|
| First Author | Publication Year | Study Type | Major Findings | Sample Size |
| Taha, A.M. [89] | 2024 | Systematic review and meta-analysis | HBP demonstrated moderate prognostic accuracy for mortality at a cutoff value of 161.415 ng/mL, with a sensitivity and specificity of 0.72 and for severe sepsis outcomes at a cutoff value of 58.907 ng/mL, with a sensitivity and specificity of 0.71. | This meta-analysis included 28 studies with 5508 patients. |
| Abdou, K.M. [98] | 2024 | Retrospective observational study | Sustained HBP elevation during the first 3 days strongly correlated with sepsis severity and mortality, confirming its reliability as a prognostic biomarker. The AUC for HBP at day 3 was 0.831 (p = 0.000). and at a cut-off value of > 9.5 ng/mL, sensitivity was 0.83 and specificity was 0.77 | This study enrolled 55 adult patients who have been proven to have sepsis, and were hospitalized into the intensive care unit. |
| Tang, J. [4] | 2023 | Prospective observational cohort study | HBP and D-dimer exhibited significant prognostic value in septic patients, and their combined use—particularly alongside SOFA scores—enhanced the accuracy of 28-day mortality prediction. The AUC of their combination was 0.824, and the sensitivity and specificity were 0.68 and 0.92, respectively. | The study involved 51 ICU patients diagnosed with sepsis, who were monitored over a 28-day period to assess the prognostic value of serial HBP, D-dimer levels, along with the SOFA score. |
| Xue, H. [18] | 2023 | Prospective observational cohort study | Dynamic changes in HBP, PCT, and CRP within 72 h of ICU admission significantly predicted 28-day mortality in septic shock patients, with HBP and PCT levels being significantly higher in non-survivor during this period (p < 0.001) | In the study, 146 patients with septic shock admitted to ICU, of whom 82 and 64 were survivors and non-survivors, respectively. |
| Dou, Q.L. [13] | 2022 | Prospective observational cohort study | 48-h HBP change (HBPc-48 h) was an independent prognostic biomarker for 30-day mortality in ICU patients with sepsis (AUC = 0.82), outperforming baseline HBP (0.79) as well as PCT (0.72), lactate (0.71) and CRP (0.65) | The study enrolled 206 patients with sepsis or septic shock, including 21 patients who died within 48 h of admission |
| Han, X. [5] | 2022 | Prospective observational cohort study | The incorporation of HBP into the quick SOFA score significantly improved 30-day mortality prediction in septic patients (AUC = 0.80) compared with qSOFA model alone (AUC = 0.70) | The study included 794 adult patients who presented to ED with presumed sepsis, classified into three severity groups: survivors, critically-ill, and non-survivors. |
| Mellhammar, L. [66] | 2021 | Prospective observational cohort study | Elevated HBP levels measured by a new point- of- care test within 72 h of admission preceded the onset of organ dysfunction in patients with severe COVID-19 supporting its use as an early prognostic biomarker in COVID-19, with an AUC of 0.88 (95% CI: 0.70–1.00, p < 0.01) | The study totally enrolled 35 patients with PCR-confirmed COVID-19. For analysis of organ dysfunction risk, 29 patients had blood samples collected within 72 h, among them, 23 developed organ dysfunction and 6 did not |
| Saridaki, M. [68] | 2021 | Prospective observational cohort study | Measurement of HBP and IL-6 at hospital admission enhanced early prediction of severe respiratory failure and 28-day mortality in COVID-19 pneumonia patients. For SRF: sensitivity = 0.59, specificity = 0.96. For 28-day mortality: sensitivity = 0.69, specificity = 0.93 | The study included 178 patients with SARS-CoV-2–induced pneumonia, who were evaluated using Sepsis-3 criteria and classified into non-sepsis and sepsis group |
| Xue, M. [101] | 2021 | Prospective cohort study | Elevated HBP levels were significantly associated with disease aggravation and multiorgan injury in severe COVID-19, highlighting its prognostic value in severe COVID-19. | The study investigated 18 critically ill COVID-19 patients who suffered from respiratory failure and sepsis, compared with 15 age- and sex- matched COVID-19-negative patients with respiratory failure. |
| Zhang, Z. [99] | 2021 | Prospective observational cohort study | HBP was positively correlated with SOFA score and organ dysfunction severity (r value was 0.60, p < 0.01) | The study enrolled 188 patients admitted to the department of emergency of Hunan Provincial People’s Hospital were enrolled. The patients were divided into non-sepsis group (87 patients) and sepsis group (101 patients) according to Sepsis-3 criteria. |
| Kahn, F. [96] | 2019 | Prospective, multicenter observational study | HBP predicted impending organ dysfunction within 72 h. AUC for HBP was 0.73 (95% CI 0.68–0.78) among all patients. | The study included a total of 524 emergency department patients, of whom 236 were eventually adjudicated to have a noninfectious disease. 374 had or developed organ dysfunction within 72 h, 54 patients were admitted to an intensive care unit, and 23 patients died within 72 h. |
| Fisher, J. [97] | 2017 | Randomized controlled trial | Elevated plasma HBP is associated with development of sepsis-induced AKI, with an AUC of 0.80, suggesting a potential role in its pathophysiology | The randomized multicenter Vasopressin and Septic Shock Trial (VASST) included 296 septic shock patients. |
| Linder, A. [95] | 2015 | Prospective international multicenter cohort study | HBP predicted progression to organ dysfunction within 72 h at a cutoff value of 30 ng/mL with a sensitivity of 78.0% and a specificity of 76.3%, outperforming other biomarkers (PCT, WBC, CRP, lactate. | The study included 759 ED patients with a suspected infection. 674 patients were infected, of whom 328 had signs of organ dysfunction within the 72-h study period, including 29 patients with septic shock. |
| Linder, A. [93] | 2009 | Prospective observational cohort study | HBP is an early biomarker of circulatory failure in sepsis, at a cutoff of 15 ng/mL with sensitivity = 0.87 and specificity = 0.95 enabling timely identification of patients at risk | In the study were included 233 febrile adult patients with a suspected infection and were classified into 5 groups. 26 patients were diagnosed with severe sepsis and septic shock, 44 patients had severe sepsis without septic shock, 100 patients had sepsis, 43 patients had an infection without sepsis, and 20 patients had an inflammatory response caused by a noninfectious disease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasouli, F.; Georgopoulou, E.; Chatzigrigoriadis, C.; Velissaris, D.; Michailides, C. Heparin Binding Protein in Sepsis—A Comprehensive Overview of Pathophysiology, Clinical Usage and Utility as Biomarker. Biomedicines 2025, 13, 2315. https://doi.org/10.3390/biomedicines13092315
Tasouli F, Georgopoulou E, Chatzigrigoriadis C, Velissaris D, Michailides C. Heparin Binding Protein in Sepsis—A Comprehensive Overview of Pathophysiology, Clinical Usage and Utility as Biomarker. Biomedicines. 2025; 13(9):2315. https://doi.org/10.3390/biomedicines13092315
Chicago/Turabian StyleTasouli, Foteini, Eleni Georgopoulou, Christodoulos Chatzigrigoriadis, Dimitrios Velissaris, and Christos Michailides. 2025. "Heparin Binding Protein in Sepsis—A Comprehensive Overview of Pathophysiology, Clinical Usage and Utility as Biomarker" Biomedicines 13, no. 9: 2315. https://doi.org/10.3390/biomedicines13092315
APA StyleTasouli, F., Georgopoulou, E., Chatzigrigoriadis, C., Velissaris, D., & Michailides, C. (2025). Heparin Binding Protein in Sepsis—A Comprehensive Overview of Pathophysiology, Clinical Usage and Utility as Biomarker. Biomedicines, 13(9), 2315. https://doi.org/10.3390/biomedicines13092315






