The Usefulness of Peripheral and Organ Perfusion Monitoring in Predicting Mortality in Patients with Severe SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
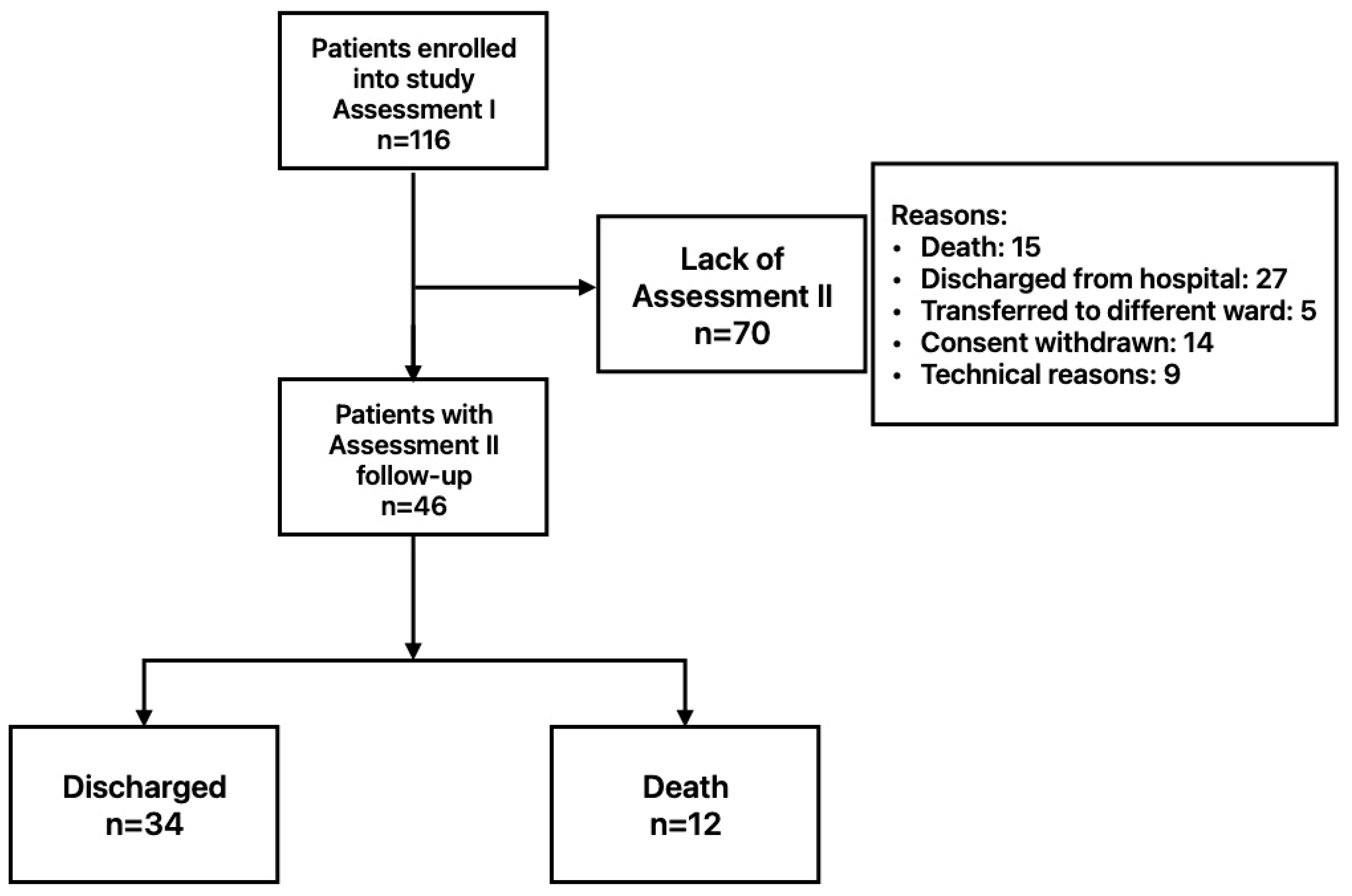
2.1. Patients
2.2. Measures
2.2.1. Peripheral Oxygen Saturation—SpO2
2.2.2. Capilary Refill Time—CRT
2.2.3. Fingertip Infrared Thermography—FIT
2.2.4. Oxygenation Ratio—PaO2/FiO2
2.2.5. Ultrasound Examination
2.3. Outcomes
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, Y.-S.; Lam, C.-Y.; Tan, P.-H.; Tsang, H.-F.; Wong, S.-C.C. Comprehensive Review of COVID-19: Epidemiology, Pathogenesis, Advancement in Diagnostic and Detection Techniques, and Post-Pandemic Treatment Strategies. Int. J. Mol. Sci. 2024, 25, 8155. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570371/ (accessed on 1 December 2023).
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary Manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Feng, X.; Li, P.; Ma, L.; Liang, H.; Lei, J.; Li, W.; Wang, K.; Song, Y.; Li, S.; Yang, W.; et al. Clinical Characteristics and Short-Term Outcomes of Severe Patients With COVID-19 in Wuhan, China. Front. Med. 2020, 7, 491. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Guo, S.; Zhou, Y.; Li, M.; Wang, M.; Ying, B. Applications of Laboratory Findings in the Prevention, Diagnosis, Treatment, and Monitoring of COVID-19. Signal Transduct. Target. Ther. 2021, 6, 316. [Google Scholar] [CrossRef]
- Landete, P.; Loaiza, C.A.Q.; Aldave-Orzaiz, B.; Muñiz, S.H.; Maldonado, A.; Zamora, E.; Cerna, A.C.S.; Cerro, E.D.; Alonso, R.C.; Couñago, F. Clinical Features and Radiological Manifestations of COVID-19 Disease. World J. Radiol. 2020, 12, 247–260. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical Characteristics of 113 Deceased Patients with Coronavirus Disease 2019: Retrospective Study. BMJ (Clin. Res. Ed.) 2020, 368, m1091. [Google Scholar] [CrossRef]
- Han, J.; Xue, J.; Ye, X.; Xu, W.; Jin, R.; Liu, W.; Meng, S.; Zhang, Y.; Hu, X.; Yang, X.; et al. Comparison of Ultrasound and CT Imaging for the Diagnosis of Coronavirus Disease and Influenza A Pneumonia. J. Ultrasound Med. 2023, 42, 2557–2566. [Google Scholar] [CrossRef]
- Francone, M.; Iafrate, F.; Masci, G.M.; Coco, S.; Cilia, F.; Manganaro, L.; Panebianco, V.; Andreoli, C.; Colaiacomo, M.C.; Zingaropoli, M.A.; et al. Chest CT Score in COVID-19 Patients: Correlation with Disease Severity and Short-Term Prognosis. Eur. Radiol. 2020, 30, 6808–6817. [Google Scholar] [CrossRef]
- Mejía, F.; Medina, C.; Cornejo, E.; Morello, E.; Vásquez, S.; Alave, J.; Schwalb, A.; Málaga, G. Oxygen Saturation as a Predictor of Mortality in Hospitalized Adult Patients with COVID-19 in a Public Hospital in Lima, Peru. PLoS ONE 2020, 15, e0244171. [Google Scholar] [CrossRef] [PubMed]
- Hariri, G.; Joffre, J.; Leblanc, G.; Bonsey, M.; Lavillegrand, J.-R.; Urbina, T.; Guidet, B.; Maury, E.; Bakker, J.; Ait-Oufella, H. Narrative Review: Clinical Assessment of Peripheral Tissue Perfusion in Septic Shock. Ann. Intensive Care 2019, 9, 37. [Google Scholar] [CrossRef]
- de Miranda, A.C.; de Menezes, I.A.C.; Junior, H.C.; Luy, A.M.; do Nascimento, M.M. Monitoring Peripheral Perfusion in Sepsis Associated Acute Kidney Injury: Analysis of Mortality. PLoS ONE 2020, 15, e0239770. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Zárate, D.; Rosas-Sánchez, K.; Zaragoza, J.J. Clinical Evaluation of Peripheral Tissue Perfusion as a Predictor of Mortality in Sepsis and Septic Shock in the Intensive Care Unit: Systematic Review and Meta-Analysis. Med. Intensiv. (Engl. Ed.) 2023, 47, 697–707. [Google Scholar] [CrossRef]
- Amson, H.; Vacheron, C.-H.; Thiolliere, F.; Piriou, V.; Magnin, M.; Allaouchiche, B. Core-to-Skin Temperature Gradient Measured by Thermography Predicts Day-8 Mortality in Septic Shock: A Prospective Observational Study. J. Crit. Care 2020, 60, 294–299. [Google Scholar] [CrossRef]
- Antúnez-Montes, O.Y.; Buonsenso, D. Routine Use of Point-of-Care Lung Ultrasound during the COVID-19 Pandemic. Med. Intensiv. 2020, 46, 42. [Google Scholar] [CrossRef] [PubMed]
- Tung-Chen, Y.; Martí de Gracia, M.; Díez-Tascón, A.; Alonso-González, R.; Agudo-Fernández, S.; Parra-Gordo, M.L.; Ossaba-Vélez, S.; Rodríguez-Fuertes, P.; Llamas-Fuentes, R. Correlation between Chest Computed Tomography and Lung Ultrasonography in Patients with Coronavirus Disease 2019 (COVID-19). Ultrasound Med. Biol. 2020, 46, 2918–2926. [Google Scholar] [CrossRef]
- Gargani, L.; Soliman-Aboumarie, H.; Volpicelli, G.; Corradi, F.; Pastore, M.C.; Cameli, M. Why, When, and How to Use Lung Ultrasound during the COVID-19 Pandemic: Enthusiasm and Caution. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 941–948. [Google Scholar] [CrossRef]
- Scholbach, T. Dynamic Tissue Perfusion Measurement—Basics and Applications. In Sonography; Thoirs, K., Ed.; InTech: London, UK, 2012; ISBN 978-953-307-947-9. [Google Scholar]
- Rosenbaum, C.; Wach, S.; Kunath, F.; Wullich, B.; Scholbach, T.; Engehausen, D.G. Dynamic Tissue Perfusion Measurement: A New Tool for Characterizing Renal Perfusion in Renal Cell Carcinoma Patients. Urol. Int. 2013, 90, 87–94. [Google Scholar] [CrossRef]
- Gutowski, M.; Klimkiewicz, J.; Rustecki, B.; Michałowski, A.; Paryż, K.; Lubas, A. Effect of Respiratory Failure on Peripheral and Organ Perfusion Markers in Severe COVID-19: A Prospective Cohort Study. J. Clin. Med. 2024, 13, 469. [Google Scholar] [CrossRef]
- Scholbach, T.M.; Vogel, C.; Bergner, N. Color Doppler Sonographic Dynamic Tissue Perfusion Measurement Demonstrates Significantly Reduced Cortical Perfusion in Children with Diabetes Mellitus Type 1 without Microalbuminuria and Apparently Healthy Kidneys. Ultraschall Der Med. 2014, 35, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Lubas, A.; Zegadło, A.; Frankowska, E.; Klimkiewicz, J.; Jędrych, E.; Niemczyk, S. Ultrasound Doppler Flow Parameters Are Independently Associated with Renal Cortex Contrast-Enhanced Multidetector Computed Tomography Perfusion and Kidney Function. J. Clin. Med. 2023, 12, 2111. [Google Scholar] [CrossRef] [PubMed]
- Harrois, A.; Grillot, N.; Figueiredo, S.; Duranteau, J. Acute Kidney Injury Is Associated with a Decrease in Cortical Renal Perfusion during Septic Shock. Crit. Care 2018, 22, 161. [Google Scholar] [CrossRef] [PubMed]
- Lubas, A.; Kade, G.; Saracyn, M.; Niemczyk, S.; Dyrla, P. Dynamic Tissue Perfusion Assessment Reflects Associations between Antihypertensive Treatment and Renal Cortical Perfusion in Patients with Chronic Kidney Disease and Hypertension. Int. Urol. Nephrol. 2018, 50, 509–516. [Google Scholar] [CrossRef]
- Chatterjee, N.A.; Jensen, P.N.; Harris, A.W.; Nguyen, D.D.; Huang, H.D.; Cheng, R.K.; Savla, J.J.; Larsen, T.R.; Gomez, J.M.D.; Du-Fay-de-Lavallaz, J.M.; et al. Admission Respiratory Status Predicts Mortality in COVID-19. Influenza Other Respir. Viruses 2021, 15, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Cruz, E.G.; Broca Garcia, B.E.; Sandoval, D.M.; Gopar-Nieto, R.; Gonzalez Ruiz, F.J.; Gallardo, L.D.; Ronco, C.; Madero, M.; Vasquez Jimenez, E. Renal Resistive Index as a Predictor of Acute Kidney Injury and Mortality in COVID-19 Critically Ill Patients. Blood Purif. 2022, 51, 309–316. [Google Scholar] [CrossRef]
- Gutowski, M.; Klimkiewicz, J.; Rustecki, B.; Michałowski, A.; Skalec, T.; Lubas, A. Peripheral and Organ Perfusion’s Role in Prognosis of Disease Severity and Mortality in Severe COVID-19 Patients: Prospective Cohort Study. J. Clin. Med. 2024, 13, 7520. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Unal, I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput. Math. Methods Med. 2017, 2017, e3762651. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Castro-Arellano, S.B.; Sandoval-Mosqueda, L.E.; Flores-Murrieta, F.J. Saturation index and fraction of inspired oxygen as a predictor in COVID-19. Rev. Medica Inst. Mex. Seguro Soc. 2023, 61, S416–S421. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J.; Lamprea, S.; Barrera, S.; Acevedo, L.; Duque, C.; Trujillo, M.; Aguirre, V.; Jimenez, C. The Association between Prolonged Capillary Refill Time and Microcirculation Changes in Children with Sepsis. BMC Pediatr. 2024, 24, 68. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Bige, N.; Boelle, P.Y.; Pichereau, C.; Alves, M.; Bertinchamp, R.; Baudel, J.L.; Galbois, A.; Maury, E.; Guidet, B. Capillary Refill Time Exploration during Septic Shock. Intensive Care Med. 2014, 40, 958–964. [Google Scholar] [CrossRef]
- Lara, B.; Enberg, L.; Ortega, M.; Leon, P.; Kripper, C.; Aguilera, P.; Kattan, E.; Castro, R.; Bakker, J.; Hernandez, G. Capillary Refill Time during Fluid Resuscitation in Patients with Sepsis-Related Hyperlactatemia at the Emergency Department Is Related to Mortality. PLoS ONE 2017, 12, e0188548. [Google Scholar] [CrossRef] [PubMed]
- de Maistre, E.; Savard, P.; Guinot, P.-G. COVID-19 and the Concept of Thrombo-Inflammation: Review of the Relationship between Immune Response, Endothelium and Coagulation. J. Clin. Med. 2023, 12, 7245. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Orbegozo Cortes, D.; Donadello, K.; Vincent, J.-L. Pathophysiology of Microcirculatory Dysfunction and the Pathogenesis of Septic Shock. Virulence 2014, 5, 73–79. [Google Scholar] [CrossRef]
- Miranda, M.; Balarini, M.; Caixeta, D.; Bouskela, E. Microcirculatory Dysfunction in Sepsis: Pathophysiology, Clinical Monitoring, and Potential Therapies. Am. J. Physiol.-Heart Circ. Physiol. 2016, 311, H24–H35. [Google Scholar] [CrossRef]
- Bourcier, S.; Pichereau, C.; Boelle, P.-Y.; Nemlaghi, S.; Dubée, V.; Lejour, G.; Baudel, J.-L.; Galbois, A.; Lavillegrand, J.-R.; Bigé, N.; et al. Toe-to-Room Temperature Gradient Correlates with Tissue Perfusion and Predicts Outcome in Selected Critically Ill Patients with Severe Infections. Ann. Intensive Care 2016, 6, 63. [Google Scholar] [CrossRef]
- Kruger, A.; Joffe, D.; Lloyd-Jones, G.; Khan, M.A.; Šalamon, Š.; Laubscher, G.J.; Putrino, D.; Kell, D.B.; Pretorius, E. Vascular Pathogenesis in Acute and Long COVID: Current Insights and Therapeutic Outlook. Semin. Thromb. Hemost. 2025, 51, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Mondini, L.; Confalonieri, P.; Pozzan, R.; Ruggero, L.; Trotta, L.; Lerda, S.; Hughes, M.; Bellan, M.; Confalonieri, M.; Ruaro, B.; et al. Microvascular Alteration in COVID-19 Documented by Nailfold Capillaroscopy. Diagnostics 2023, 13, 1905. [Google Scholar] [CrossRef]
- Di Nicolò, P.; Granata, A. Renal Intraparenchymal Resistive Index: The Ultrasonographic Answer to Many Clinical Questions. J. Nephrol. 2019, 32, 527–538. [Google Scholar] [CrossRef]
- O’Neill, W.C. Renal Resistive Index. Hypertension 2014, 64, 915–917. [Google Scholar] [CrossRef]
- Zaitoun, T.; Megahed, M.; Elghoneimy, H.; Emara, D.M.; Elsayed, I.; Ahmed, I. Renal Arterial Resistive Index versus Novel Biomarkers for the Early Prediction of Sepsis-Associated Acute Kidney Injury. Intern. Emerg. Med. 2024, 19, 971–981. [Google Scholar] [CrossRef]
- Rajangam, M.; Nallasamy, K.; Bhatia, A.; Kumar, V.; Kaur, P.; Angurana, S.K. Renal Resistive Index by Point of Care Ultrasound to Predict Sepsis Associated Acute Kidney Injury in Critically Ill Children. Pediatr. Nephrol. 2024, 39, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Boddi, M.; Bonizzoli, M.; Chiostri, M.; Begliomini, D.; Molinaro, A.; Tadini Buoninsegni, L.; Gensini, G.F.; Peris, A. Renal Resistive Index and Mortality in Critical Patients with Acute Kidney Injury. Eur. J. Clin. Investig. 2016, 46, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Rozemeijer, S.; Haitsma Mulier, J.L.G.; Röttgering, J.G.; Elbers, P.W.G.; Spoelstra-de Man, A.M.E.; Tuinman, P.R.; de Waard, M.C.; Oudemans-van Straaten, H.M. Renal Resistive Index: Response to Shock and Its Determinants in Critically Ill Patients. Shock 2019, 52, 43. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine Elevation in Severe and Critical COVID-19: A Rapid Systematic Review, Meta-Analysis, and Comparison with Other Inflammatory Syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Klimkiewicz, J.; Grzywacz, A.; Michałowski, A.; Gutowski, M.; Paryż, K.; Jędrych, E.; Lubas, A. Acute Kidney Injury and Chronic Kidney Disease and Their Impacts on Prognosis among Patients with Severe COVID-19 Pneumonia: An Expert Center Case–Cohort Study. J. Clin. Med. 2024, 13, 1486. [Google Scholar] [CrossRef]
- Bartoletti, M.; Giannella, M.; Scudeller, L.; Tedeschi, S.; Rinaldi, M.; Bussini, L.; Fornaro, G.; Pascale, R.; Pancaldi, L.; Pasquini, Z.; et al. Development and Validation of a Prediction Model for Severe Respiratory Failure in Hospitalized Patients with SARS-CoV-2 Infection: A Multicentre Cohort Study (PREDI-CO Study). Clin. Microbiol. Infect. 2020, 26, 1545–1553. [Google Scholar] [CrossRef]
- Shamsoddin, E. Can Medical Practitioners Rely on Prediction Models for COVID-19? A Systematic Review. Evid. Based Dent. 2020, 21, 84–86. [Google Scholar] [CrossRef]
- Luo, Y.; Mao, L.; Yuan, X.; Xue, Y.; Lin, Q.; Tang, G.; Song, H.; Wang, F.; Sun, Z. Prediction Model Based on the Combination of Cytokines and Lymphocyte Subsets for Prognosis of SARS-CoV-2 Infection. J. Clin. Immunol. 2020, 40, 960–969. [Google Scholar] [CrossRef]
- Schumann, C.; Heigl, F.; Rohrbach, I.J.; Sheriff, A.; Wagner, L.; Wagner, F.; Torzewski, J. A Report on the First 7 Sequential Patients Treated Within the C-Reactive Protein Apheresis in COVID (CACOV) Registry. Am. J. Case Rep. 2022, 23, e935263. [Google Scholar] [CrossRef]
- Matthay, M.A.; Arabi, Y.; Arroliga, A.C.; Bernard, G.; Bersten, A.D.; Brochard, L.J.; Calfee, C.S.; Combes, A.; Daniel, B.M.; Ferguson, N.D.; et al. A New Global Definition of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2024, 209, 37–47. [Google Scholar] [CrossRef]
- Hueda-Zavaleta, M.; Copaja-Corzo, C.; Miranda-Chávez, B.; Flores-Palacios, R.; Huanacuni-Ramos, J.; Mendoza-Laredo, J.; Minchón-Vizconde, D.; Gómez de la Torre, J.C.; Benites-Zapata, V.A. Determination of PaO2/FiO2 after 24 h of Invasive Mechanical Ventilation and ΔPaO2/FiO2 at 24 h as Predictors of Survival in Patients Diagnosed with ARDS Due to COVID-19. PeerJ 2022, 10, e14290. [Google Scholar] [CrossRef] [PubMed]
- Gianstefani, A.; Farina, G.; Salvatore, V.; Alvau, F.; Artesiani, M.L.; Bonfatti, S.; Campinoti, F.; Caramella, I.; Ciordinik, M.; Lorusso, A.; et al. Role of ROX Index in the First Assessment of COVID-19 Patients in the Emergency Department. Intern. Emerg. Med. 2021, 16, 1959–1965. [Google Scholar] [CrossRef]
- Candel, B.G.J.; de Groot, B.; Nissen, S.K.; Thijssen, W.A.M.H.; Lameijer, H.; Kellett, J. The Prediction of 24-h Mortality by the Respiratory Rate and Oxygenation Index Compared with National Early Warning Score in Emergency Department Patients: An Observational Study. Eur. J. Emerg. Med. 2023, 30, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Lin, J.; Su, Z.; Lin, T. Association between PaO2/(FiO2*PEEP) Ratio and in-Hospital Mortality in COVID-19 Patients: A Reanalysis of Published Data from Peru Using PaO2/(FiO2*PEEP) Ratio in Place of PaO2/FaO2 Ratio. Medicine 2024, 103, e39931. [Google Scholar] [CrossRef] [PubMed]
- Marcolino, M.S.; Pires, M.C.; Ramos, L.E.F.; Silva, R.T.; Oliveira, L.M.; Carvalho, R.L.R.; Mourato, R.L.S.; Sánchez-Montalvá, A.; Raventós, B.; Anschau, F.; et al. ABC2-SPH Risk Score for in-Hospital Mortality in COVID-19 Patients: Development, External Validation and Comparison with Other Available Scores. Int. J. Infect. Dis. 2021, 110, 281–308. [Google Scholar] [CrossRef]
- Chiumello, D.; Fioccola, A. Recent Advances in Cardiorespiratory Monitoring in Acute Respiratory Distress Syndrome Patients. J. Intensive Care 2024, 12, 17. [Google Scholar] [CrossRef]
- Kanoore Edul, V.S.; Caminos Eguillor, J.F.; Ferrara, G.; Estenssoro, E.; Siles, D.S.P.; Cesio, C.E.; Dubin, A. Microcirculation Alterations in Severe COVID-19 Pneumonia. J. Crit. Care 2021, 61, 73–75. [Google Scholar] [CrossRef]
| Variable | N | % | |
|---|---|---|---|
| Gender | Male | 30 | 65.2 |
| Female | 16 | 34.8 | |
| Ward Type | Intensive Care Unit | 20 | 43.5 |
| High-Dependency Unit | 26 | 56.5 | |
| Death | 12 | 26.1 | |
| Comorbidity | Malignancy | 5 | 10.1 |
| Obesity | 7 | 15.2 | |
| Chronic Kidney Disease | 3 | 6.5 | |
| Coronary Artery Disease | 7 | 15.2 | |
| Heart Failure | 2 | 4.3 | |
| Myocardial infarction | 1 | 2.2 | |
| Atrial fibrillation | 6 | 12.3 | |
| Hypertension | 10 | 21.7 | |
| Diabetes | 6 | 12.3 | |
| Asthma | 3 | 6.5 | |
| Chronic Obstructive Pulmonary Disease | 1 | 2.2 | |
| ARDS | No ARDS (PaO2/FiO2 > 300) | 8 | 17.4 |
| MILD (PaO2/FiO2 300 to 200) | 10 | 21.7 | |
| MODERATE (PaO2/FiO2 200 to 100) | 19 | 41.3 | |
| SEVERE (PaO2/FiO2 < 100) | 9 | 19.6 | |
| Variable | Assessment I Mean ± SD Median (IQR) | Assessment II Mean ± SD Median (IQR) | Significance (p-Value) |
|---|---|---|---|
| (a) | |||
| Study group (n = 46) | |||
| Peripheral and organ perfusion parameters | |||
| SpO2 [%] | 93 ± 3 | 96 (4) | <0.001 |
| CRT [s] | 3.0 (2.8) | 3.0 (1.5) | 0.695 |
| FIT [°C] | 33.0 (3.9) | 32.6 (4.5) | 0.702 |
| RCP [cm/s] | 0.144 (0.231) | 0.108 (0.129) | 0.971 |
| RCRI [ratio] | 0.822 (0.265) | 0.941 (0.179) | 0.040 |
| Blood test results | |||
| WBC [1 × 109/L] | 8.0 (5.6) | 9.7 (4.8) | 0.002 |
| Hgb [g/dL] | 14.0 (3.9) | 13.1 (2.3) | <0.001 |
| PLT [1 × 109/L] | 197 ± 78 | 287 ± 127 | <0.001 |
| CRP [mg/dL] | 8.1 (7.3) | 2.4 (9.8) | 0.004 |
| Ferritin [ng/dL] | 1040.0 (1614.0) | 1102.0 (1245.0) | 0.405 |
| Creatinine [mg/dL] | 0.9 (0.3) | 0.8 (0.3) | 0.001 |
| Urea [mg/dL] | 33.5 (22.0) | 43.0 (36.0) | <0.001 |
| AST [U/L] | 51 (38) | 32 (40) | 0.022 |
| ALT [U/L] | 35 (23) | 53 (52) | 0.006 |
| CK [U/L] | 206 (461) | 176 (320) | 0.013 |
| LDH [U/L] | 449 (239) | 406 (267) | 0.013 |
| SBP [mmHg] | 127 (13) | 121 ± 13 | 0.154 |
| DBP [mmHg] | 77 ± 10 | 73 ± 11 | 0.089 |
| PaO2/FiO2 | 121 (187) | 200 (139) | 0.136 |
| (b) | |||
| Deceased group (n = 12) | |||
| Peripheral and organ perfusion parameters | |||
| SpO2 [%] | 92 ± 2 | 94 (6) | 0.230 |
| CRT [s] | 4.0 (2.6) | 4.0 (1.6) | 0.625 |
| FIT [°C] | 33.4 (7.8) | 33.7 (10.3) | 0.919 |
| RCP [cm/s] | 0.054 (0.224) | 0.081 ± 0.076 | 0.263 |
| RCRI [ratio] | 0.784 (0.308) | 1 (0.12) | 0.063 |
| Blood test results | |||
| WBC [1 × 109/L] | 10.3 ± 5.5 | 13.5 ± 4.6 | 0.075 |
| Hgb [g/dL] | 13.3 ± 2.7 | 12.4 ± 2.1 | 0.047 |
| PLT [1 × 109/L] | 180 ± 75 | 219 ± 109 | 0.155 |
| CRP [mg/dL] | 10.2 ± 9.2 | 12.1 ± 8.7 | 0.617 |
| Ferritin [ng/dL] | 1927 (2450) | 2819 ± 1887 | 1.000 |
| Creatinine [mg/dL] | 0.95 (0.40) | 0.85 (0.70) | 0.433 |
| Urea [mg/dL] | 41 (31) | 67 (39) | 0.010 |
| AST [U/L] | 44 (40) | 31 (35) | 0.248 |
| ALT [U/L] | 35 (38) | 40 (71) | 0.308 |
| CK [U/L] | 91 (233) | 93 (190) | 0.075 |
| LDH [U/L] | 609.8 ± 230.1 | 435.0 ± 134.6 | 0.035 |
| SBP [mmHg] | 131 ± 16 | 121 ± 17 | 0.278 |
| DBP [mmHg] | 79 ± 10 | 73 ± 15 | 0.323 |
| PaO2/FiO2 | 90 (21) | 98 (50) | 0.899 |
| (c) | |||
| Survivor group (n = 34) | |||
| Peripheral and organ perfusion parameters | |||
| SpO2 [%] | 94 ± 3 | 96 (3) | <0.001 |
| CRT [s] | 3.0 (1.8) | 3.0 (1.5) | 0.422 |
| FIT [°C] | 32.8 (3.6) | 32.5 (3.8) | 0.754 |
| RCP [cm/s] | 0.181 (0.266) | 0.133 (0.146) | 0.387 |
| RCRI [ratio] | 0.822 ± 0.129 | 0.879 (0.230) | 0.309 |
| Blood test results | |||
| WBC [1 × 109/L] | 7.97 (4.90) | 9.08 (4.42) | 0.016 |
| Hgb [g/dL] | 14.4 (3.9) | 13.6 (2.4) | 0.004 |
| PLT [1 × 109/L] | 203 ± 79 | 318 (170) | <0.001 |
| CRP [mg/dL] | 8.1 (6.3) | 1.2 (3.5) | <0.001 |
| Ferritin [ng/dL] | 806 (925) | 674 (664) | 0.286 |
| Creatinine [mg/dL] | 0.9 (0.3) | 0.8 (0.3) | <0.001 |
| Urea [mg/dL] | 28.6 (26.0) | 39.0 (26.0) | 0.025 |
| AST [U/L] | 53 (38) | 34.5 (54) | 0.051 |
| ALT [U/L] | 36 (23) | 54 (48) | 0.012 |
| CK [U/L] | 285 (501) | 210 (304) | 0.071 |
| LDH [U/L] | 433 (175) | 404 (273) | 0.767 |
| SBP [mmHg] | 126 (13) | 121 ± 12 | 0.374 |
| DBP [mmHg] | 76 ± 9 | 73 ± 10 | 0.181 |
| PaO2/FiO2 | 192.5 (199) | 202 (162) | 0.192 |
| Variable | Deceased Mean ± SD Median (IQR) | Survivors Mean ± SD Median (IQR) | Significance (p-Value) |
|---|---|---|---|
| Peripheral and organ perfusion parameters | |||
| SpO2 [%] | 92 ± 2 | 96 (3) | 0.030 |
| CRT [s] | 4.0 (2.6) | 3.0 (1.5) | 0.006 |
| FIT [°C] | 33.4 (7.8) | 32.5 (3.8) | 0.550 |
| RCP [cm/s] | 0.054 (0.224) | 0.133 (0.146) | 0.154 |
| RCRI [ratio] | 0.784 (0.308) | 0.879 (0.23) | 0.135 |
| Blood tests results | |||
| WBC [1 × 109/L] | 10.30 ± 5.50 | 9.08 (4.42) | 0.038 |
| Hgb [g/dL] | 13.3 ± 2.7 | 13.6 (2.4) | 0.405 |
| PLT [1 × 109/L] | 180 ± 75 | 318 (170) | 0.041 |
| CRP [mg/dL] | 10.2 ± 9.2 | 1.2 (3.5) | 0.007 |
| Ferritin [ng/dL] | 1927 (2450) | 674 (664) | 0.001 |
| Creatinine [mg/dL] | 0.95 (0.40) | 0.80 (0.30) | 0.388 |
| Urea [mg/dL] | 41.0 (30.5) | 39.0 (26.0) | 0.010 |
| AST [U/L] | 44 (40) | 35 (54) | 0.881 |
| ALT [U/L] | 35 (38) | 54 (48) | 0.311 |
| CK [U/L] | 91 (233) | 210 (304) | 0.068 |
| LDH [U/L] | 609.8 ± 230.1 | 403.5 (273.0) | 0.822 |
| SBP [mmHg] | 131 ± 16 | 121 ± 12 | 0.993 |
| DBP [mmHg] | 79 ± 10 | 73 ± 10 | 0.966 |
| PaO2/FiO2 | 90 (21) | 202 (162) | <0.001 |
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | |
| SpO2 [%] | 0.686 | 0.512–0.919 | 0.012 | 0.665 | 0.472–0.938 | 0.020 |
| CRT [s] | 2.149 | 1.136–4.067 | 0.019 | 2.223 | 1.144–4.322 | 0.018 |
| FIT [°C] | 0.991 | 0.837–1.172 | 0.912 | - | - | - |
| RCP [cm/s] | 0.001 | 0.000–15.152 | 0.163 | - | - | |
| RCRI [ratio] | 799.7 | 0.182–3,520,549.7 | 0.118 | - | - | - |
| Variable | Cut-Off Value | Method | Sensitivity (%) | Specificity (%) | LR (−); LR (+) | AUC | Significance (p-Value) |
|---|---|---|---|---|---|---|---|
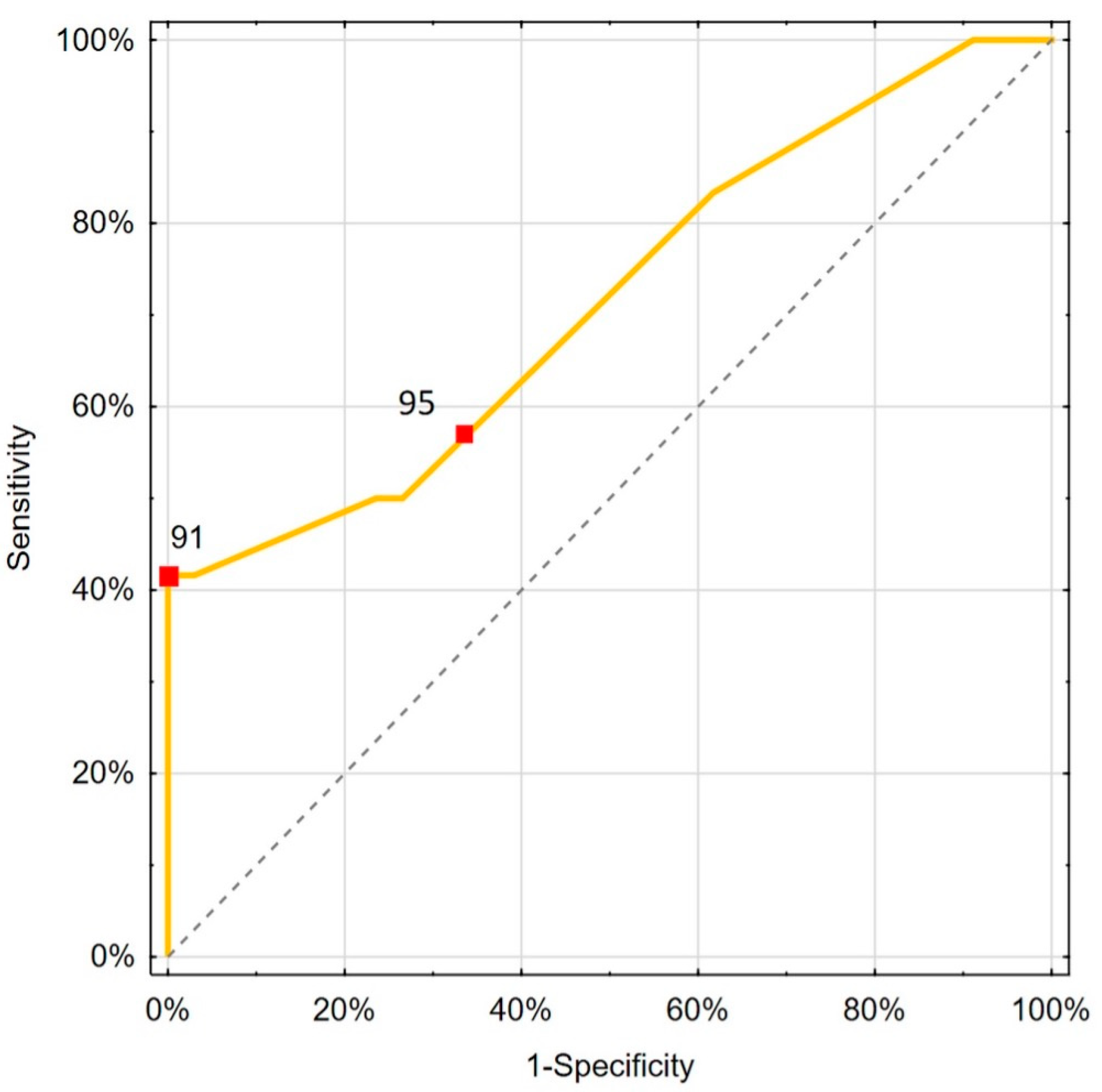
| SpO2 [%] | 95 | EH | 58.3 | 64.7 | 0.644; 1.653 0.583; NA | 0.714 | 0.021 |
| 91 | Youden | 41.7 | 100.0 | ||||
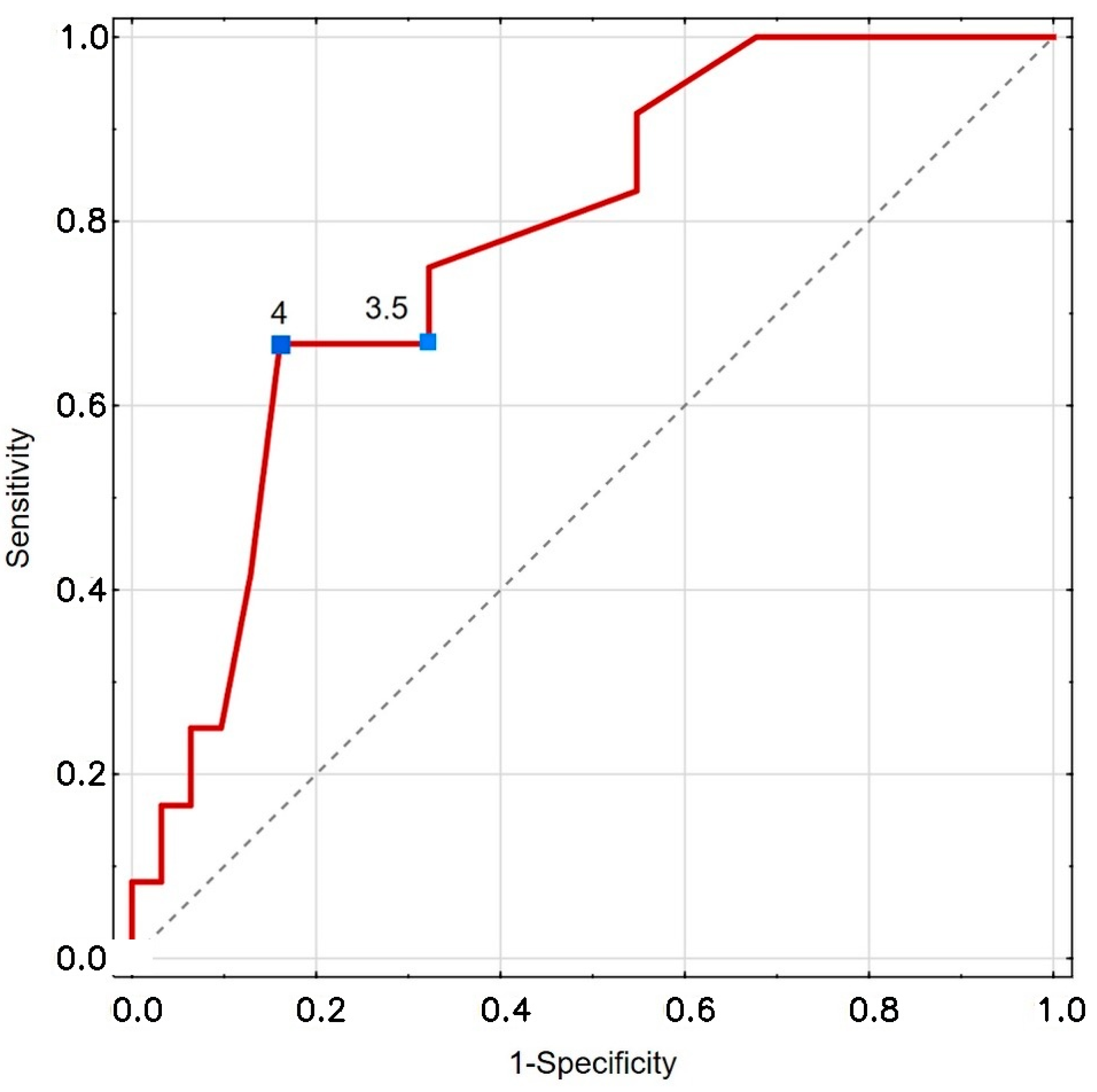
| CRT [s] | 3.5 | EH | 66.7 | 67.7 | 0.492; 2.067 0.397; 4.133 | 0.777 | <0.001 |
| 4 | Youden | 66.7 | 83.9 |
| Variable | Cut-Off Value | Net Benefit (Model) | Net Benefit (Treat All) | |
|---|---|---|---|---|
| Mean | 95%CI | Mean | ||
| SpO2 [%] | ≤95 | 0.087 | −0.022–0.207 | 0.076 |
| ≤91 | 0.109 | 0.022–0.196 | ||
| CRT [s] | ≥3.5 | 0.128 | 0.006–0.262 | 0.099 |
| ≥4 | 0.157 | 0.041–0.285 | ||
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | |
| WBC [1 × 109/L] | 1.134 | 0.991–1.297 | 0.068 | - | - | - |
| PLT [1 × 109/L] | 0.993 | 0.986–1.000 | 0.046 | - | - | - |
| CRP [mg/dL] | 1.081 | 1.002–1.187 | 0.044 | 1.252 | 1.023–1.532 | 0.029 |
| Ferritin [ng/dL] | 1.001 | 1.000–1.002 | 0.014 | 1.001 | 1.000–1.002 | 0.033 |
| Urea [mg/dL] | 1.026 | 1.003–1.049 | 0.027 | - | - | - |
| PaO2/FiO2 | 0.988 | 0.961–0.993 | 0.008 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutowski, M.; Lubas, A.; Rustecki, B.; Klimkiewicz, J. The Usefulness of Peripheral and Organ Perfusion Monitoring in Predicting Mortality in Patients with Severe SARS-CoV-2. Biomedicines 2025, 13, 2269. https://doi.org/10.3390/biomedicines13092269
Gutowski M, Lubas A, Rustecki B, Klimkiewicz J. The Usefulness of Peripheral and Organ Perfusion Monitoring in Predicting Mortality in Patients with Severe SARS-CoV-2. Biomedicines. 2025; 13(9):2269. https://doi.org/10.3390/biomedicines13092269
Chicago/Turabian StyleGutowski, Mateusz, Arkadiusz Lubas, Bartosz Rustecki, and Jakub Klimkiewicz. 2025. "The Usefulness of Peripheral and Organ Perfusion Monitoring in Predicting Mortality in Patients with Severe SARS-CoV-2" Biomedicines 13, no. 9: 2269. https://doi.org/10.3390/biomedicines13092269
APA StyleGutowski, M., Lubas, A., Rustecki, B., & Klimkiewicz, J. (2025). The Usefulness of Peripheral and Organ Perfusion Monitoring in Predicting Mortality in Patients with Severe SARS-CoV-2. Biomedicines, 13(9), 2269. https://doi.org/10.3390/biomedicines13092269





